Abstract
Background
Men who have sex with men (MSM) are at risk for cancers attributable to human papillomavirus (HPV), including oropharyngeal cancer. HPV vaccination is recommended for U.S. MSM through age 26 years. Oral HPV infection is associated with oropharyngeal cancer. We determined oral HPV prevalence and risk factors among young MSM.
Methods
The Young Men’s HPV study enrolled MSM aged 18–26 years from clinics in Chicago and Los Angeles during 2012–2014. Participants self-reported demographics, sexual behaviors, vaccination and HIV status. Self-collected oral rinse specimens were tested for HPV DNA (37 types) by L1-consensus PCR. We calculated adjusted prevalence ratios (aPR) and 95% confidence intervals (CI) for risk factors associated with oral HPV among participants not previously vaccinated.
Results
Oral HPV was detected in 87/922 (9.4%); 9-valent vaccine (9vHPV) types were detected in 37/922 (4.0%). Among HIV-positive participants, 17/88 (19.3%) had oral HPV detected. Oral HPV was more prevalent among those reporting first sex at age ≤18 years (aPR:2.44; CI:1.16–5.12); HIV infection (aPR:1.99; CI:1.14–3.48); >5 sex partners within the past month (aPR:1.93; CI:1.13–3.31); performing oral sex on >5 partners within the last 3 months (aPR:1.87; CI:1.12–3.13); and having >5 male sex partners within the last 3 months (aPR:1.76; CI: 1.08–2.87). Only 454/922 (49.2%) were aware HPV can cause oropharyngeal cancers.
Conclusions
Many oral HPV infections were with types targeted by vaccination. Oral HPV infections were significantly associated with HIV and sexual behaviors. Fewer than half of participants were aware HPV could cause oropharyngeal cancer.
Keywords: Human papillomavirus, Public Health, Oral Health, Epidemiology, Sexual Minorities
INTRODUCTION
Human papillomavirus (HPV) is the most common sexually transmitted infection in the United States, with an estimated 14 million persons newly infected annually.1 Although most infections are asymptomatic and self-limited, persistent infections can lead to a variety of diseases, including genital warts and cancers. A causal link between oncogenic HPV types and cervical cancer has been established; knowledge is broadening to include associations between HPV and other anogenital cancers, as well as oropharyngeal cancers.2,3
Increases in HPV-associated oropharyngeal cancers have been observed over the last several decades, particularly in North America and Europe, with pronounced increases among males in developed countries.4,5 In the United States, the population-level incidence of HPV positive oropharyngeal cancer increased by 225% from 1988–2004, from 0.8 per 100,000 to 2.6 per 100,000;6 among U.S. men, oropharyngeal cancers accounted for 78% of HPV-associated cancers in 2009.7
Oral HPV infection has been associated with oropharyngeal cancer.8 Prevalence of oral HPV has been evaluated among various populations. A systematic review of 9 studies found a 7.5% oral HPV prevalence among cancer-free and HIV-negative individuals, with a 12-month cumulative incidence estimated at 4.8% (95% confidence interval [CI]: 3.2–7.3%).9 In 2009–2010, an estimated 10.1% (CI: 8.3–12.3%) of U.S. men aged 14–69 years had any of 37 types of HPV DNA detected in oral rinse specimens in a nationally representative study.10
Men who have sex with men (MSM) are at high risk for both HPV infections and HPV-associated diseases, with both a high incidence and prevalence of anal HPV infection and anal cancer rates among young MSM.11 Studies evaluating risk factors for oral HPV detection among MSM and the general male population identified associations with sexual behaviors, including oral sex frequency, and lifetime number of sex partners and kissing partners.12–15 However, studies have been conflicting regarding the association between HPV infections and lifetime or recent sexual partners among this population.
In the United States, two prophylactic HPV vaccines have been licensed for use among men.16 A quadrivalent HPV vaccine (4vHPV) (Gardasil, Merck and Co., Inc., Kenilworth, NJ) protecting against four HPV types (6, 11, 16 and 18) was used during 2006–2016. A 9-valent HPV vaccine (9vHPV) (Gardasil 9, Merck and Co., Inc.) protecting against 4vHPV types and an additional five oncogenic types (31, 33, 45, 52, and 58) has been used since 2015. In the United States, of 11,600 oropharyngeal cancers attributed to HPV annually, an estimated 9,900 (85.3%) are due to types HPV 16 and 18, the oncogenic types included in the quadrivalent HPV vaccine, and an additional 900 (7.8%) by the additional five oncogenic HPV types prevented by the 9-valent HPV vaccine.17 HPV vaccination is recommended for U.S. boys and girls aged 11–12 years (or starting at age 9 years), and through age 26 years for MSM and those with immunodeficiencies, including HIV infection, who have not been previously vaccinated.16,18
The purpose of this analysis was to evaluate oral HPV prevalence and risk factors among a population of young gay, bisexual and other MSM aged 18–26 years, a population eligible for HPV vaccination in the United States.
MATERIALS AND METHODS
Study Design and Population
The cross-sectional Young Men’s HPV study (YMHPV) enrolled gay, bisexual and other MSM, including transgender women, aged 18–26 years. Detailed study methods have been provided elsewhere.19–21 Briefly, enrollment was conducted at three community health clinics focused on providing sexual health services to lesbian, gay, bisexual and transgender populations in two U.S. cities (Chicago, Illinois and Los Angeles, California) from July 2012–August 2014. The study protocol was reviewed and approved by institutional review boards at the participating institutions. Eligible participants were aged 18–26 years, assigned male sex at birth and either (a) identified as gay, homosexual or bisexual, and/or (b) reported ever having oral or anal sex with a male partner. Participants who reported previous receipt of HPV vaccination were excluded from this analysis. Each consenting participant completed a 30-minute standardized computer-assisted interview; all participants included in this analysis completed the survey, but were not required to provide a response to all questions. The survey assessed demographic characteristics, sexual orientation, sexual behavior, and past medical history (including self-reported HIV status and HPV vaccination history), as well as knowledge, attitudes and practices regarding HPV infection and associated diseases and HPV vaccination. The survey assessed participant’s perception of the severity of HPV-associated oropharyngeal cancer in a 5-level scale (not very serious, slightly serious, moderately serious, very serious, or extremely serious). The perceived efficacy of the HPV vaccine in preventing oral and throat cancers was assessed on a 4-level scale (not at all effective, slightly effective, moderately effective, or extremely effective); responders could also answer they did not know or were not sure.
Laboratory Testing
Oral sampling and HPV testing was performed as previously described.22 Briefly, each participant provided a self-collected oral rinse specimen by swishing and gargling 10mL of sterile saline for 30 seconds. Extracted DNA was tested by using the Research Use Only Linear Array HPV Genotyping Test (Roche Molecular Diagnostics, Indianapolis, IN) with supplementary HPV-52 quantitative polymerase chain reaction. This standardized commercial research assay uses L1 consensus polymerase chain reaction followed by type-specific hybridization for qualitative detection of 37 HPV types (6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 63, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 83, 84, 89 or IS39) and β-globin (control for sample amplification). Specimens that tested negative for both HPV and β-globin were considered inadequate.
Data Analysis
This analysis was limited to vaccine-eligible participants, defined as those not reporting prior HPV vaccination, with survey information and adequate specimen results. Demographic and behavioral factors were analyzed for associations with detection of any of 37 HPV types, (“any HPV”) and any of the nine HPV types protected against by the 9-valent vaccine (“9vHPV types”) detected by Linear Array in the oral specimen.
We calculated descriptive statistics and assessed risk factors using chi-square tests, with a p-value <0.05 considered significant. We also used log-binomial models to report prevalence ratios (PR) and 95% confidence intervals (CI). For variables with a p-value <0.10 in bivariate analysis, we calculated adjusted prevalence ratios (aPR), adjusting for variables chosen a priori that could be associated with both sexual behavior and HPV prevalence. Separate models were built for each association. Each model was adjusted for age, race, smoking cigarettes and self-reported HIV status, unless one of these was the variable tested; for example, the final model for self-reported HIV status was adjusted only for age, race, and smoking. HIV status was categorized as positive for participants who self-reported their most recent HIV test result as positive (n=88) (“known HIV positive”), compared to all other participants, who reported their most recent HIV test result to be negative (n=712), indeterminate (n=11), unknown (n=26), or did not answer (n=85) (combined into “HIV negative or unknown”).
RESULTS
Overall, 1033 participants aged 18–26 years enrolled in the YMHPV study, completed the questionnaire, and provided specimens with adequate test results. Of those, 111 (10.7%) reported having previously received at least 1 dose of HPV vaccine and were excluded from this analysis; the remaining 922 were considered vaccine-eligible and included here. All participants were aged 18–26 years; mean participant age was 23 years and median age was 24 years. Most participants identified as male gender (853, 92.5%) and as homosexual or gay (643, 69.7%). Mean number of lifetime sex partners of any gender was 37 (median: 15 sex partners, range 0–2002, among 751 participants answering this question). There were 88 (9.5%) participants who self-reported their most recent HIV test result was positive.
In oral specimens from 922 vaccine-eligible participants, 87 (9.4%) had any HPV detected; 37 (4.0%) had at least one 9vHPV types, 29 (3.1%) had at least one of the 7 oncogenic 9vHPV types and 11 (1.2%) had HPV type 16 detected. Detection of any oral HPV by demographic characteristics is presented in Table 1. Characteristics associated with detection of any oral HPV included reporting a positive HIV test result, as well as sexual behaviors including age at first sex ≤18 years, having >20 lifetime sex partners, >5 partners of any gender within the last 1 month, >5 male sex partners within the last 3 months, performing oral sex on >5 partners within last 3 months, and performing oral sex on a male partner >5 times within the last 3 months. Characteristics associated with detection of any oral 9vHPV types were assessed and were similar to associations for any oral HPV. Although there was an observed trend with increased detection of oral HPV as the time since performing oral sex decreased, overall time since performing oral sex on a male partner did not reach statistical significance.
Table 1.
Oral HPV detection, by demographic, behavioral and sexual characteristics of 922 vaccine-eligible young men who have sex with men — Young Men’s HPV Study, 2012–2014
| Characteristic | Participants N* |
Any oral HPV detected n (%) |
p-value** |
|---|---|---|---|
|
| |||
| Total | 922 | 87 (9.4%) | |
|
| |||
| Age (years) | |||
| 18–21 | 245 | 24 (9.8) | 0.8 |
| 22–26 | 677 | 63 (9.3) | |
|
| |||
| Race/ethnicity | |||
| Non-Hispanic white | 221 | 19 (8.6) | 0.1 |
| Non-Hispanic black | 175 | 26 (14.9) | |
| Non-Hispanic Asian/Pacific Islander | 76 | 5 (6.6) | |
| Hispanic | 352 | 29 (8.2) | |
| Other | 98 | 8 (8.2) | |
|
| |||
| City | |||
| Chicago | 283 | 31 (11.0) | 0.3 |
| Los Angeles | 639 | 56 (8.8) | |
|
| |||
| Education | |||
| Up to high school only | 422 | 39 (9.2) | 0.08 |
| Some college or higher | 444 | 38 (8.6) | |
| Other | 56 | 10 (17.9) | |
|
| |||
| Current health insurance | |||
| No | 362 | 35 (9.7) | 0.8 |
| Yes | 469 | 42 (9.0) | |
| Don’t know/unsure | 91 | 10 (11.0) | |
|
| |||
| Smoking cigarettes | |||
| No | 259 | 19 (7.3) | 0.08 |
| Yes, ≤12 cigarettes in past year | 276 | 21 (7.6) | |
| Yes, >12 cigarettes in past year | 340 | 40 (11.8) | |
| Unsure | 44 | 7 (15.9) | |
|
| |||
| Marijuana use | |||
| No | 189 | 14 (7.4) | 0.2 |
| Yes, ≤12 times in past year | 304 | 35 (11.5) | |
| Yes, >12 times in past year | 360 | 29 (8.1) | |
| Unsure | 66 | 9 (13.6) | |
|
| |||
| Gender identity | |||
| Male | 853 | 81 (9.5) | 0.9 |
| Transgender female | 45 | 4 (8.9) | |
| Other | 24 | 2 (8.3) | |
|
| |||
| Sexual orientation | |||
| Heterosexual or straight | 26 | 3 (11.5) | 0.6 |
| Homosexual or gay | 643 | 63 (9.8) | |
| Bisexual | 199 | 15 (7.5) | |
|
| |||
| Self-reported recent HIV test results | |||
| Not Positive | 834 | 70 (8.4) | 0.0009 |
| Positive | 88 | 17 (19.3) | |
|
| |||
| Ever had sex with someone born male | |||
| No | 16 | 1 (6.3) | 0.7 |
| Yes | 901 | 85 (9.4) | |
|
| |||
| Ever had sex with someone born female | |||
| No | 515 | 43 (8.4) | 0.2 |
| Yes | 392 | 41 (10.5) | |
|
| |||
| Age at first sex (years) | |||
| ≤18 | 680 | 75 (11.0) | 0.004 |
| >18 | 194 | 8 (4.1) | |
|
| |||
| Number of lifetime sex partners | |||
| ≤20 | 468 | 34 (7.3) | 0.02 |
| >20 | 454 | 53 (11.7) | |
|
| |||
| Number of male partners, last 3 months | |||
| ≤5 | 617 | 49 (7.9) | 0.01 |
| >5 | 175 | 25 (14.3) | |
|
| |||
| Number of female partners, last 3 months | |||
| ≤5 | 320 | 29 (9.1) | 0.9 |
| >5 | 12 | 1 (8.3) | |
|
| |||
| Number of any sex partners, last 1 month | |||
| ≤5 | 662 | 53 (8.0) | 0.005 |
| >5 | 117 | 19 (16.2) | |
|
| |||
| Number of partners performed oral sex on, last 3 months | |||
| ≤5 | 661 | 54 (8.2) | 0.01 |
| >5 | 139 | 21 (15.1) | |
|
| |||
| Times performed oral sex with male partner, last 3 months | |||
| ≤5 | 409 | 29 (7.1) | 0.02 |
| >5 | 267 | 33 (12.4) | |
|
| |||
| Most recent time performed oral sex on a male partner | |||
| <48 hours | 211 | 27 (12.8) | 0.5 |
| 2–7 days | 244 | 23 (9.4) | |
| 1 week–1 month | 187 | 15 (8.0) | |
| >1 month ago | 157 | 11 (7.0) | |
| Never | 40 | 3 (7.5) | |
| Don’t know | 61 | 6 (9.8) | |
Numbers do not always sum to total due to skip patterns or participant non-response
p-values <0.05 are bolded
After adjusting for age, race, and smoking, HIV status remained a significant factor associated with the detection of any oral HPV (aPR: 1.99; CI: 1.14–3.48) (Table 2). After adjusting for age, race, smoking and self-reported HIV-status, age at first sex (aPR: 2.44; CI: 1.16–5.12), number of sex partners of any gender in the last 1 month (aPR: 1.93; CI: 1.13–3.31), numbers of partners performing oral sex on within the last 3 months (aPR: 1.87; CI: 1.12–3.13), times performed oral sex with a male partner in the last 3 months (aPR: 1.83; CI: 1.09–3.07), number of male partners in the last 3 months (aPR: 1.76; CI: 1.08–2.87) and number of lifetime partners (aPR: 1.61; CI: 1.04–2.50) were all significantly associated with oral HPV among study participants overall.
Table 2.
Unadjusted and adjusted prevalence ratios for detection of any oral HPV among 922 vaccine-eligible young men who have sex with men — Young Men’s HPV Study, 2012–2014
| CHARACTERISTIC | Unadjusted prevalence ratio |
95% CI | Adjusted prevalence ratio* |
95% CI |
|---|---|---|---|---|
|
| ||||
| Age (years) | ||||
| 18–21 | Ref | – | Ref | – |
| 22–26 | 0.95 | 0.59–1.52 | 0.94 | 0.58–1.51 |
|
| ||||
| Race/Ethnicity | ||||
| Non-Hispanic white | Ref | – | Ref | – |
| Non-Hispanic black | 1.73 | 0.95–3.12 | 1.43 | 0.77–2.64 |
| Non-Hispanic Asian/Pacific Islander | 0.77 | 0.29–2.05 | 0.82 | 0.31–2.23 |
| Hispanic | 0.96 | 0.53–1.71 | 0.91 | 0.51–1.63 |
| Other | 0.95 | 0.42–2.17 | 0.80 | 0.34–1.84 |
|
| ||||
| Smoking | ||||
| No | Ref | – | Ref | – |
| Yes, ≤12 cigarettes in past year | 1.04 | 0.56–1.92 | 1.07 | 0.57–2.00 |
| Yes, >12 cigarettes in past year | 1.60 | 0.93–2.77 | 1.48 | 0.85–2.57 |
|
| ||||
| Education | ||||
| Up to high school only | Ref | – | Ref | – |
| Some college or higher | 0.92 | 0.59–1.45 | 1.16 | 0.70–1.90 |
| Other | 1.93 | 0.96–3.87 | 1.96 | 0.97–3.94 |
|
| ||||
| Self-reported recent HIV test results | ||||
| Not Positive | Ref | – | Ref | – |
| Positive | 2.30 | 1.35–3.91 | 1.99 | 1.14–3.48 |
|
| ||||
| Age at first sex (years) | ||||
| ≤18 | 2.67 | 1.29–5.54 | 2.44 | 1.16–5.12 |
| >18 | Ref | Ref | Ref | – |
|
| ||||
| Number of lifetime sex partners | ||||
| ≤20 | Ref | – | Ref | – |
| >20 | 1.61 | 1.04–2.47 | 1.61 | 1.04–2.50 |
|
| ||||
| Number of male partners, last 3 months | ||||
| ≤5 | Ref | – | Ref | – |
| >5 | 1.80 | 1.11–2.91 | 1.76 | 1.08–2.87 |
|
| ||||
| Number of sex partners, last 1 month | ||||
| ≤5 | Ref | – | Ref | – |
| >5 | 2.03 | 1.20–3.43 | 1.93 | 1.13–3.31 |
|
| ||||
| Number of partners performed oral sex on, last 3 months | ||||
| ≤5 | Ref | – | Ref | – |
| >5 | 1.85 | 1.12–3.06 | 1.87 | 1.12–3.13 |
|
| ||||
| Times performed oral sex with male partner, last 3 months | ||||
| ≤5 | Ref | – | Ref | – |
| >5 | 1.74 | 1.06–2.87 | 1.83 | 1.09–3.07 |
Adjusted for age, race, smoking, and self-reported HIV test result, unless one of these was the variable tested (see Methods).
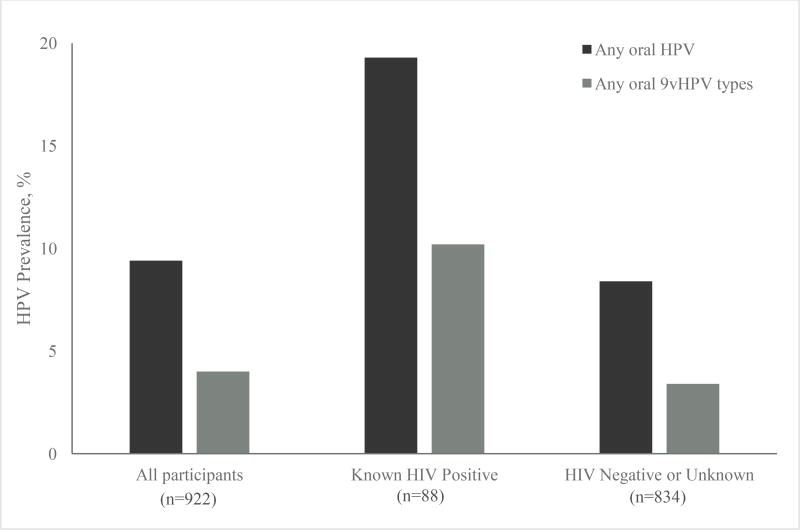
Oral HPV prevalence was assessed by self-reported HIV status (Figure 1). Prevalence of both any oral HPV and any 9vHPV type was significantly higher among participants who reported being HIV-positive versus among those with negative or unknown HIV status (any HPV: 19.3% versus 8.4%, p=0.004; 9vHPV: 10.2% versus 3.4%, p=0.004).
Figure 1.
Prevalence of any oral HPV and 9vHPV types among vaccine-eligible young men who have sex with men, overall and by self-reported HIV status — YMHPV Study, 2012–2014
9vHPV, 9-valent HPV vaccine; YMHPV, The Young Men’s HPV study
Participants were asked about their perception and understanding regarding HPV-associated diseases and HPV vaccines. Only 454 (49.4%) of participants knew that HPV can cause oropharyngeal cancer. Although 798 (86.6%) of participants thought a diagnosis of oropharyngeal cancer would be very or extremely serious, only 132 (14.3%) believed HPV vaccine would be moderately or extremely effective at preventing oropharyngeal cancer.
DISCUSSION
This study assesses prevalence of any oral HPV and 9-valent vaccine-type oral HPV specifically among MSM within the recommended target age range for HPV vaccine, through age 26 years, who reported not being previously vaccinated. Oral HPV was detected in almost 10% of this large group of 922 young gay, bisexual and other MSM, including transgender women, and in nearly 20% of those who reported being HIV-positive. A high proportion of infections detected were 9vHPV types; thus, many potentially could be prevented by pre-exposure HPV vaccination. At least one 9vHPV type was detected in 4.0% of unvaccinated participants and 10.2% of known HIV-positive participants. Sexual behaviors were significantly associated with oral HPV detection, including younger age at first sex, and more lifetime and recent sex partners, including oral sex partners. Concerningly, a majority of participants were unaware that HPV can cause oropharyngeal cancers, and few thought that HPV vaccination would effectively prevent oropharyngeal cancer.
HPV prevalence, including oral HPV, is common among MSM; in previous analyses of data from the YMHPV study, anal HPV was detected in 661 (71.7%) participants, and 70 (7.6%) had both oral and anal HPV detected, yet type-specific concordance between anal and oral HPV infectious was infrequent.19,21 Our findings, an oral HPV prevalence of 9.4% among all participants and 19.3% among known HIV-positive participants, are somewhat higher than among men in general in this age group, yet lower than reported in some previous studies of older MSM. A meta-analysis estimated pooled prevalence of any oral HPV DNA was 17.1% (95% CI: 7.3–26.8) among HIV-negative MSM of all ages (6 studies, total N=1329) and 28.9% (95% CI: 19.1–38.7) among HIV-positive MSM of all ages (11 studies, total N=1886).23 Importantly, median ages of participants in these studies were all ≥30 years of age. Furthermore, several studies found older age to be a significant predictor of oral HPV infection among MSM.10,14,15,23 Thus, the lower HPV prevalence we observed may be attributable to the younger age group studied by limiting to MSM 18–26 years of age. Additionally, a unique feature of our study is that it assessed oral HPV prevalence among transgender women; prevalence was similar among men and transgender women in this analysis.
This study specifically included MSM aged 18–26 years because they fall within the age group in which catch-up HPV vaccination is recommended by the Advisory Committee on Immunization Practice (ACIP).16,18 HPV vaccination coverage in this population remains low; in this study, only 10.7% of participants reported receiving any doses of HPV vaccine. This is a useful group in which to characterize HPV prevalence from multiple body sites, since this information could be used to assess vaccine effectiveness in this at-risk population in potential future post-licensure studies, especially as HPV vaccination coverage increases. Efficacy studies for the initial vaccine licensure evaluated cervical pre-cancer lesions, genital warts, or persistent cervico-vaginal infections in women.24,25 Additional studies then demonstrated efficacy for the prevention of external genital lesions in males and anal intraepithelial neoplasia among men who have sex with men.26,27 Information is emerging regarding efficacy of vaccination against oral HPV infection.28 However, lack of an identified precursor lesion for oropharyngeal cancer has prevented efficacy studies for this outcome to date. Data from this study may serve as a baseline for future monitoring of HPV vaccine impact on oral HPV prevalence in this population. As more boys and men receive HPV vaccination at the recommended age, oral HPV prevalence among vaccinated MSM can be compared to historical prevalence among unvaccinated MSM.
Our study included assessment of several other behavioral risk factors, including smoking cigarettes and smoking marijuana, yet only sexual behavior risk factors were found to be significantly associated with oral HPV prevalence. Other studies have found tobacco or marijuana use associated with oral HPV detection among both MSM and other men.10,12,15,29 However, as noted previously, these studies had broader age ranges, suggesting that sexual behavior could have more impact on oral HPV prevalence than other behaviors among this young population.
We also found that HIV was an important risk factor for oral HPV detection. This could be related to changes in immune function due to HIV infection, or sexual behaviors among this population. One study in the Netherlands found that several sexual behaviors were associated with oral HPV prevalence among HIV-negative MSM, but not among HIV-positive MSM14. Studies of HIV-positive men found HIV infection limited clearance of genital HPV infection; among HIV-positive MSM, immune function might be more important risk factor than sexual behavior30, or, some men might change their sexual behaviors once they are aware of their HIV-status. Additional research is needed to evaluate the effects of both HIV infection and sexual behaviors on oral HPV prevalence.
Our findings are subject to several limitations. First, the study population was from a limited geographic area and might not be representative of all young MSM across the United States. Second, medical record reviews were not performed as a part of this study; HIV test results, HPV vaccination eligibility and all behavioral information was based on self-report and could be subject to recall bias or social desirability bias. Third, this study assessed prevalence of oral HPV detection at one time point. The oral sample may not fully represent the HPV status of the oropharynx, and the cross-sectional nature of the study does not assess persistent infection or development of HPV-associated disease. Additionally, multiple sexual behaviors were assessed, but multiple comparisons were not accounted for in the bivariate analysis. Finally, many participants reported no female sex partners, and so were not asked about sexual behaviors with females.
Despite the available of safe and effective HPV vaccines, and national guidelines recommending vaccination for MSM through age 26 years, young MSM remain an at-risk population for HPV infections and HPV-associated diseases that are potentially preventable with vaccination. Many oral HPV infections among MSM could be prevented by pre-exposure vaccination, potentially preventing oropharyngeal cancers in the future. In addition, our results suggest that education regarding HPV-associated oropharyngeal cancers and HPV vaccination could be useful among this at-risk population. Raising awareness of oropharyngeal cancers among MSM might help increase HPV vaccination uptake.
Acknowledgments
Sources of funding: Centers for Disease Control and Prevention.
Cody Randel, Janell Moore, Steven Carrasco, Mark McGrath, Jim Braxton, Akbar Zaidi, Gitika Panicker, and staff at participating clinics.
Footnotes
The authors report no conflicts of interest.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Note: Part of these data were presented at the 2017 International Papillomavirus Conference in Cape Town, South Africa, on March 2, 2017 (Poster 432).
References
- 1.Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40(3):187–193. doi: 10.1097/OLQ.0b013e318286bb53. [DOI] [PubMed] [Google Scholar]
- 2.zur Hausen H. Papillomaviruses in the causation of human cancers - a brief historical account. Virology. 2009;384(2):260–265. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 3.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92(9):709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 4.Chaturvedi AK, Anderson WF, Lortet-Tieulent J, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31(36):4550–4559. doi: 10.1200/JCO.2013.50.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma. J Clin Oncol. 2015;33(29):3235–3242. doi: 10.1200/JCO.2015.61.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jemal A, Simard EP, Dorell C, et al. Annual Report to the Nation on the Status of Cancer, 1975–2009, featuring the burden and trends in human papillomavirus(HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst. 2013;105(3):175–201. doi: 10.1093/jnci/djs491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 9.Wood ZC, Bain CJ, Smith DD, Whiteman DC, Antonsson A. Oral human papillomavirus infection incidence and clearance: a systematic review of the literature. J Gen Virol. 2017;98(4):519–526. doi: 10.1099/jgv.0.000727. [DOI] [PubMed] [Google Scholar]
- 10.Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009–2010. JAMA. 2012;307(7):693–703. doi: 10.1001/jama.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Machalek DA, Poynten M, Jin F, et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol. 2012;13(5):487–500. doi: 10.1016/S1470-2045(12)70080-3. [DOI] [PubMed] [Google Scholar]
- 12.Chaturvedi AK, Graubard BI, Broutian T, et al. NHANES 2009–2012 Findings: Association of Sexual Behaviors with Higher Prevalence of Oral Oncogenic Human Papillomavirus Infections in U.S. Men. Cancer Res. 2015;75(12):2468–2477. doi: 10.1158/0008-5472.CAN-14-2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edelstein ZR, Schwartz SM, Hawes S, et al. Rates and determinants of oral human papillomavirus infection in young men. Sex Transm Dis. 2012;39(11):860–867. doi: 10.1097/OLQ.0b013e318269d098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mooij SH, Boot HJ, Speksnijder AG, et al. Oral human papillomavirus infection in HIV-negative and HIV-infected MSM. AIDS. 2013;27(13):2117–2128. doi: 10.1097/QAD.0b013e328362395c. [DOI] [PubMed] [Google Scholar]
- 15.Read TR, Hocking JS, Vodstrcil LA, et al. Oral human papillomavirus in men having sex with men: risk-factors and sampling. PloS one. 2012;7(11):e49324. doi: 10.1371/journal.pone.0049324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2014;63(RR-05):1–30. [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. [Accessed December 1, 2017];How Many Cancers Are Linked with HPV Each Year? https://www.cdc.gov/cancer/hpv/statistics/cases.htm.
- 18.Meites E, Kempe A, Markowitz LE. Use of a 2-Dose Schedule for Human Papillomavirus Vaccination - Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65(49):1405–1408. doi: 10.15585/mmwr.mm6549a5. [DOI] [PubMed] [Google Scholar]
- 19.Meites E, Gorbach PM, Gratzer B, et al. Monitoring for Human Papillomavirus Vaccine Impact Among Gay, Bisexual, and Other Men Who Have Sex With Men-United States, 2012–2014. J Infect Dis. 2016;214(5):689–696. doi: 10.1093/infdis/jiw232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorbach PM, Cook R, Gratzer B, et al. Human Papillomavirus Vaccination Among Young Men Who Have Sex With Men and Transgender Women in 2 US Cities, 2012–2014. Sex Transm Dis. 2017;44(7):436–441. doi: 10.1097/OLQ.0000000000000626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinau M, Gorbach P, Gratzer B, et al. Concordance Between Anal and Oral Human Papillomavirus Infections Among Young Men Who have Sex With Men. J Infect Dis. 2017;215(12):1832–1835. doi: 10.1093/infdis/jix232. [DOI] [PubMed] [Google Scholar]
- 22.Steinau M, Reddy D, Sumbry A, et al. Oral sampling and human papillomavirus genotyping in HIV-infected patients. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 2012;41(4):288–291. doi: 10.1111/j.1600-0714.2011.01093.x. [DOI] [PubMed] [Google Scholar]
- 23.King EM, Oomeer S, Gilson R, et al. Oral Human Papillomavirus Infection in Men Who Have Sex with Men: A Systematic Review and Meta-Analysis. PloS one. 2016;11(7):e0157976. doi: 10.1371/journal.pone.0157976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 25.Future II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915–1927. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 26.Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med. 2011;364(5):401–411. doi: 10.1056/NEJMoa0909537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365(17):1576–1585. doi: 10.1056/NEJMoa1010971. [DOI] [PubMed] [Google Scholar]
- 28.Herrero R, Quint W, Hildesheim A, et al. Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PloS one. 2013;8(7):e68329. doi: 10.1371/journal.pone.0068329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D'Souza G, Agrawal Y, Halpern J, Bodison S, Gillison ML. Oral sexual behaviors associated with prevalent oral human papillomavirus infection. J Infect Dis. 2009;199(9):1263–1269. doi: 10.1086/597755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tobian AA, Kigozi G, Gravitt PE, et al. Human papillomavirus incidence and clearance among HIV-positive and HIV-negative men in sub-Saharan Africa. AIDS. 2012;26(12):1555–1565. doi: 10.1097/QAD.0b013e328353b83c. [DOI] [PMC free article] [PubMed] [Google Scholar]