Abstract
Real‐time functional magnetic resonance imaging (rtfMRI) has been proposed as a method of providing feedback to develop a participant's ability to control his or her own neuronal activity. However, this BOLD signal is vulnerable to contamination from nonneuronal sources that can also be shaped by the feedback provided. Here we illustrate an artifact found while training participants to control signal from an ROI in the insula. As the artifact was directly behind the eye and the experiment used an echo‐planar imaging (EPI) sequence with phase encoding direction that included the orbits and the insula in the same line, we hypothesized that the artifact was due to eye motion. We demonstrate a reduced training effect when eyeball signal is regressed out of the data and reproduce the artifact with block design voluntary eye movement. Further, using independent components analysis on historical data, we find the artifact is common in BOLD data, but typically not task‐correlated, even in tasks where one might expect differing amounts of eye movement in the active task blocks. The artifact, thus, does not significantly impact group results in typical fMRI experiments. Finally, we demonstrate this particular artifact can be avoided in rtfMRI experiments by ensuring that the phase encoding direction does not project any eye movement related artifact onto the ROI being used for feedback training. Our findings underscore the importance of taking great care in designing rtfMRI feedback procedures to avoid contamination with nonneuronal sources of BOLD signal alteration. Hum Brain Mapp, 2011. © 2010 Wiley‐Liss, Inc.
Keywords: real‐time feedback, fMRI, artifact, eyeball motion, individual data
INTRODUCTION
As defined by Heinrich et al. [ 2007], neurofeedback is “a form of behavioral training aimed at developing skills for self‐regulation of brain activity.” Within the past decade, a number of studies have been published that meet the above neurofeedback definition. After neurofeedback training, Vernon et al. [ 2003] reported that healthy adults could not only learn to increase their electroencephalogram (EEG) sensorimotor rhythm (12–15 Hz) activity while simultaneously inhibiting theta and beta activity but show improvement in a semantic working memory task. Egner and Gruzelier [ 2003] assessed the impact of alpha/theta neurofeedback training on subsequent musical performance in students. Stage performance improved in students only after alpha/theta training but not after other neurofeedback training or control training. In addition to healthy subjects, neurofeedback protocols have been conducted in some chronic diseases, e.g., ADHD [Heinrich et al., 2004], substance abuse [Sokhadze et al., 2008], and epilepsy [Ramaratnam et al., 2005]. For example, Heinrich et al. [ 2004] reported that children with ADHD who received neurofeedback training reduced their ADHD symptom rating compared to patients in an untreated group.
EEG was used in most of the above neurofeedback studies. More recently, real time feedback of the functional magnetic resonance imaging (rtfMRI) BOLD signal has been incorporated into this type of experimental design [reviewed by Weiskopf et al., 2007]. Compared to EEG, fMRI provides two significant advantages. First, in contrast to EEG, which is generally measured using 32–128 scalp electrodes yielding predominantly cortical signals, fMRI measures neuronally‐related activity across the entire brain with superior spatial resolution and localization fidelity [Weiskopf et al., 2007]. Second, fMRI can reliably measure deep cortical and/or subcortical brain regions [e.g., the insula; Caria et al., 2007], thus allowing new regional vistas in neurofeedback experiments.
In a ground breaking study, deCharms et al. [ 2005] reported that after a single session of rtfMRI feedback training using an anterior cingulate region of interest (ROI), chronic pain patients were better able to control their pain symptoms. However despite its important clinical promise, a clear association between EEG or fMRI feedback (i.e., neurofeedback) training and subsequent enhanced behavioral performance has yet to be reliably established. In some previous studies, neurofeedback training effects were found but subsequent behavioral performance was not enhanced [Sokhadze et al., 2008; Vernon, 2005], which may have been because of limited statistical power (e.g., the neurofeedback training effect size is small) or some systematic experimental error (e.g., the feedback signal was contaminated by nonneuronal artifacts). A nonneuronal artifact in a neurofeedback study not only influences group variance (e.g., reducing statistical power) but can also influence an individual's result by giving participants incorrect feedback, i.e., misleading the participant. Particularly, due to the feedback itself, the strength of an artifact can increase over time due to learning processes. It should be emphasized that the feedback signal relies upon individual data and not group‐averaged data. If an individual's neurofeedback signal was influenced by nonneuronal factors (e.g., task‐related head motion), changes in brain signals could be misinterpreted to reflect neuronal regulation when there was actually none, i.e., artifact‐influenced feedback can give participants the erroneous impression that their approach is successful and that erroneous approach would push participants to create more artifacts resulting in a positive feedback‐loop. Compared to EEG, this problem may be more serious in rtfMRI studies due to inherent magnet susceptibility properties of the BOLD signal [Federspiel et al., 2006]. Therefore, ruling out all nonneuronal artifacts at the individual level is particularly relevant in an rtfMRI study.
Recently, while trying to extend the results of Caria et al. [ 2007] using our own rtfMRI paradigm, we observed an unexpected task‐correlated artifact that covered not only the insula (the chosen feedback area), but also parts of the temporal and occipital lobes and the cerebellum in some individual subject data. Therefore, although subjects in this preliminary study appeared successful in controlling their BOLD signal from the insula at the group level, there remained the possibility that this artifact was from a nonneuronal source related to the rtfMRI paradigm specifically, and, thus, compromised the validity of the study outcome.
To explore this possibility, we employed an rtfMRI feedback task and several non‐rtfMRI tasks to explore the influence and the source(s) of the aforementioned artifact. Using two fMRI analysis approaches, the general linear model (GLM) and independent component analysis (ICA), we show that the signal artifact was present and task‐synchronized in the rtfMRI task and was present, but not task‐synchronized, in other tasks.
METHODS AND MATERIALS
Participants
In total, forty‐three participants took part in six imaging studies after providing written informed consent to protocols approved by the NIDA‐IRP Institutional Review Board. No participant had a history of neurological or psychiatric disorders, including use of psychotropic medications, substance abuse or dependence, except for nicotine. Each of the six studies (see below) was utilized to examine different aspects of either real time control and/or potential BOLD signal artifacts.
fMRI Data Acquisition
MRI data were acquired using a 3 Tesla Allegra scanner (Siemens Healthcare, Erlangen Germany). An echo‐planar imaging (EPI) sequence (TR = 2 s, TE = 27 ms, FA = 78°, Matrix = 64 × 64, FOV = 220 mm × 220 mm, slice thickness = 4–5 mm, gap = 0 mm, number of slices = 33–39) was modified from a standard EPI sequence to include sending the reconstructed data in real time to another computer for analysis and feedback to the participant [Yang et al., 2005]. Demographic and MRI parameters for each experiment are given in Table I. Data were acquired in the sagittal plane (phase direction A‐P) for all experiments; an in‐plane 40° rotation of the phase direction was used for two participants in Experiment A. A high‐resolution T1‐weighted structural scan of the whole brain was collected for each participant (MPRAGE, Matrix = 256 × 256, 1‐mm3 isotropic voxels, TR = 2.5 s, TE = 4.38 ms, FA = 8°) and used for superposition of functional maps. Foam cushion pads were used to minimize participant head motion. All visual stimuli were projected onto a screen within the bore of the magnet and viewed through a mirror attached to the head coil.
Table I.
Participant demographics and scan parameters
| Experiment | Number of participants (female)a | Age (mean ± SD) | Slice thickness (mm) | Slices/volume |
|---|---|---|---|---|
| A‐real time | 7 (4) | 29.6 ± 8.4 | 5 | 33 |
| B‐eye movement | 10 (5) | 29.2 ± 6.3 | 5 | 33 |
| C1‐craving report | 10 (6) | 31.4 ± 9.7 | 5 | 33 |
| C2‐executive function | 10 (6) | 29.6 ± 10.2 | 4 | 39 |
| C3‐olfactory | 10 (6) | 34.0 ± 7.1 | 4 | 39 |
| D‐resting state | 6 (5) | 29.2 ± 9.2 | 5 | 33 |
Six participants took part in two experiments and two participants took part in three experiments always in different scan sessions on different days.
Experimental Paradigms and Procedures
Experiment A
In a block design paradigm, participants were instructed to regulate the rtfMRI signal presented from a predetermined subregion of the insula (see Fig. 1) by trying to move a scrolling chart line as high above the x‐axis into a yellow colored zone as possible whenever the cue word (displayed below the signal line) was “INCREASE” (INCREASE block) (Fig. 2A). As an aid, participants were told that it might be helpful to recall fearful situations that they had experienced in the past; they generated a list of such memories just before entering the scanner. When the cue word was “DECREASE” (DECREASE block), participants were asked to try to move the line as far below the x‐axis as possible (again into a yellow zone), and were instructed that calming themselves might help. When the cue word was “COUNT” (COUNT block), participants had to count back from 100. A “STOP” cue (RESTING block) required participants to rest and neither regulate their signal nor count numbers. Importantly, participants were instructed to use the aforementioned advice as a guide during the increase and decrease blocks and were strongly encouraged to employ other strategies to regulate the signal that might be more effective for them. Participants were also informed that the signal was delayed about 5–7 s for technical reasons (1 s for data processing and 4–6 s for the hemodynamic response delay).
Figure 1.
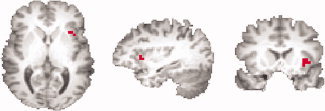
The location of the left anterior insula ROI used as the feedback source in one representative participant.
Figure 2.
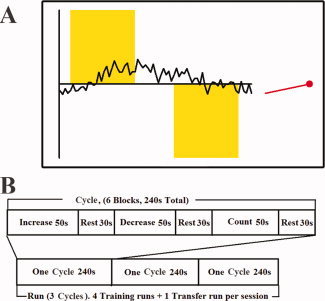
(A) Illustration of the experimental display and BOLD signal feedback viewed by subjects in Experiment A. The feedback consisted of a scrolling line chart, a red indicator, and a cue word. The red indicator showed the current feedback signal in the ROI. The scrolling line chart displayed the time‐course of the feedback signal in the ROI during the preceding 150‐s period was updated every TR (2 s). When the cue word was “INCREASE,” a yellow bar appeared in the upper part of the graph. When the cue word was “DECREASE,” the yellow bar was presented in the lower part of the graph. When the cue word was “COUNT,” a yellow bar was presented both above and below the x‐axis. No yellow bar was presented during the “STOP” cue. (B) Schematic diagram of Experiment A.
The left anterior insula was chosen as the feedback ROI and its anatomical location (see Fig. 1) was selected individually for each subject during a pilot scan with an emotion self‐regulating task (details of this scan session are given in the supplemental materials). The cluster in the left insula with the greatest positive activation in the pilot scan was selected as the feedback ROI and all remaining voxels in the brain were designated as the background ROI. Because participants did not show an identical activation pattern in the pilot scan, the number of contiguous voxels involved in the feedback ROI varied from 3 to 12 voxels.
Online rtfMRI data analysis was performed using AFNI [Cox, 1996] together with custom‐designed programs. Based on the strategy in deCharms et al., [ 2005], activation of the ROI was computed as the mean fMRI signal from voxels within the ROI at a given time point minus the mean fMRI signal from the same voxels over the entire scan run to that point, divided by the signal from the entire scan run to that point (i.e., 100% × (current signal – run average signal)/run average signal). This signal formula was applied to both the feedback and the background ROIs and the difference between these computed signals was displayed as a scrolling line chart and a red level indicator (Fig. 2A).
Three consecutive 240‐s task cycles (Fig. 2B) comprised each scan run (720 s/run). In a single scan session, participants performed four real‐time feedback runs (Fig. 2B). Each run consisted of three INCREASE blocks (50 s each), three DECREASE blocks (50 s each), and three COUNT blocks (50 s each) alternating with nine RESTING blocks (30 s each). Approximately 2–3 min separated each scan run to allow the participant to briefly relax during this cognitively demanding procedure.
Experiment B
As will be further described below, our results from Experiment A showed a signal artifact that always passed through the participant's eye and appeared similar to an eye‐motion artifact described previously [Chen and Zhu, 1997]. To ascertain the source, e.g., the eyeball motion, of this artifact, a block‐design eye‐rotation task was employed (Experiment B) wherein participants were instructed to move their eyeballs by looking around (i.e., clockwise or counterclockwise; the participants were instructed that they could change the direction anytime to make themselves comfortable) whenever they saw the cue word “MOVE” and to rest and maintain fixation to the center of the screen following a “STOP” cue. They were further instructed to always maintain their head as immobile as possible. Each scan session consisted of two scan runs, each with nine 30‐s blocks (four MOVE blocks alternating with five STOP blocks).
Experiments C1, C2, and C3
As our stated purpose for this article was to test whether the observed artifact was caused by ocular activity specifically related to the rtfMRI protocol, data from three tasks previously collected by our group and likely to have induced differing amounts of eyeball movement were reexamined for the signal artifact.
Experiment C1 was a craving self‐report task, wherein participants (all of whom were smokers) viewed smoking‐ and nonsmoking‐related pictures. They were instructed to report their cigarette craving level continuously by using a wheel manipulandum to move a tab along a visual analog rating scale placed below the picture. This block design paradigm was similar to that used in Hutcherson et al. [ 2005]. In this task, participants needed to move their eyes to view an entire image with the resultant potential for greater muscle tension during the drug‐related block. Three scan runs were performed in a single session with each run lasting 688 s.
Experiment C2 was an executive function task. During this task, small or large boxes were presented serially at the center of the screen and participants had to maintain separate counts of the large and small boxes in a manner similar to Garavan et al. [ 2000]. All stimuli were presented foveally in this event‐within‐block task design. Thus, minimal eye motion would be anticipated while performing this task, although additional attention to stimuli presentation could potentially lead to increased muscle tension around the eyes. One scan run, taking 728 s, was performed.
Experiment C3 was a classical conditioning reward task in which olfactory cues predict juice reward delivered directly into the subject's mouth similar to that of McClure et al. [ 2003]. Only a fixation cross was displayed on the screen during the scan session. Thus, minimal eye motion or eye‐muscle tension change during the experiment would be expected. In this event‐related experiment, 8–10 scan runs were performed, with each run between 320 and 440 s.
Experiment D
Finally, as will be further described, we found some evidence of the artifact in all tasks. To test whether a task was required and to test whether the source for the nontask‐related artifact is from the eyeball itself or from eye‐related activity during visual processing, resting‐state data were examined. Participants were instructed to lie in the scanner and rest with their eyes open during one scan run and closed during the other run. Each run took 270 s. The order of the eyes‐open and eyes‐closed runs was counterbalanced across participants.
Data Analysis
Off‐line image postprocessing and fMRI data analysis were performed using AFNI [Cox, 1996] and FSL [Smith et al., 2004]. Head motion and slice timing corrections were performed as preprocessing steps. Then, two fMRI analysis approaches, the general linear model (GLM) and spatial independent component analysis (ICA), were employed to examine different aspects of the BOLD signal. Activation derived from a GLM analysis shows the existence of a significant correlation between a paradigm design and the acquired time course, i.e., that the activity is task‐related [Friston et al., 1994]. In contrast, activation from an ICA analysis requires no hypothesis about the temporal pattern of the data [McKeown et al., 1998], and can be used to identify patterns in the data that may or may not be task‐specific.
In the GLM analysis for Experiment A, regressors of interest included INCREASE, DECREASE, and COUNT blocks. Head motion curves were also included into the analysis as regressors of no interest to help account for residual motion influence. Based on this GLM analysis, the signal intensity from the left anterior insula feedback ROI during INCREASE and DECREASE conditions was calculated for each run separately and then averaged across participants. In a separate analysis, a spatial probabilistic independent component analysis (PICA) was preformed to decompose the fMRI data into different spatial components using the MELODIC routine in FSL [Beckmann and Smith, 2004]. Each dataset was decomposed into 100 components. The ICA component maps were examined for the spatial signature of the artifact as described below. GLM (with regressors appropriate for each task) and PICA analysis were performed for Experiments A, B, C1, C2, and C3. As there was no task in Experiment D, only PICA analysis was performed.
To test our hypothesis that the artifact found in Experiments A and B using GLM is caused by eye movement‐related signals, a second GLM analysis was done wherein the time‐course from an orbit ROI was included as an extra regressor to determine if the eye signal could account for the BOLD results in the feedback region. Finally, the residual time‐course from a GLM analysis with only the orbital nuisance regressor was subjected to PICA analysis.
As our stated purpose for this article was to examine the signal artifact that we discovered in Experiment A, the following steps were established to assess for a potential brain artifact: first, an artifact ROI was drawn bilaterally just behind the eye covering the source of the potential noise and parallel to the phase‐encoding direction. Next, four bilateral reference ROIs were drawn, also parallel to the phase‐encoding direction: dorsal, ventral, medial, and lateral to the artifact ROI (center to center distance: about 2 cm; see Fig. 3). An artifact was considered present in a participant if two conditions were satisfied (in either or both hemispheres): The first condition was a conjunction test, requiring that the absolute value of the signal (the beta value from the GLM analysis or the weight of one component from the PICA analysis) in the artifact ROI was significantly larger (as determined by t‐test) than that in each reference ROI; the second condition was an ad‐hoc criteria requiring that the average of the data was not significantly different than that in any of the four reference ROIs. This later condition was included to avoid considering true positives artifactual, i.e., true insula regulation should manifest itself as a large positive signal in the insula, whereas the artifact is typically large positive and negative signals (see Fig. 4A for an exemplar). An alpha value of P < 0.05 was considered significant for the GLM analysis and P < 0.0005 (to correct for testing 100 components) for the PICA analysis.
Figure 3.
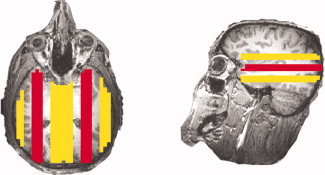
An example of the artifact ROIs (red) and the reference ROIs (yellow).
Figure 4.
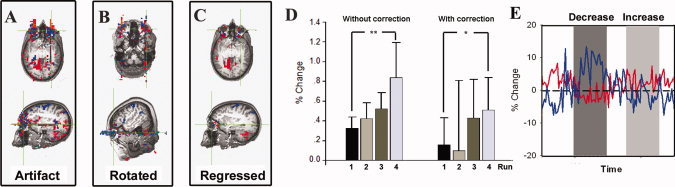
(A) Illustration of the BOLD signal artifact in one representative participant in Experiment A (following GLM analysis). The threshold in (A–C) is P uncorrected < 0.005 with a four voxel minimum volume. (B) Results from a representative participant in Experiment A where a 40° rotation of the phase encoding direction resulted in a corresponding rotation of the artifact. (C) Residual BOLD signal artifact after the BOLD time‐course from the orbit in (A) was included as a regressor during the GLM analysis. (D) Group average BOLD signal from the left insula ROI obtained during real‐time fMRI feedback training. **P < 0.02, *P < 0.05. (E) Partial time‐course from two voxels showing significant artifacts in (A). The blue line is from a blue voxel in (A) and shows significant negative activity. The red line is from a red voxel in (A) and shows significant positive activity.
RESULTS
Figure 4A shows the BOLD signal artifact from the INCREASE–DECREASE contrast activity map in a representative participant in Experiment A (referred to as “without correction” result). The orientation of the artifact was along the phase‐encoding direction as evidenced by the altered orientation of the artifact when the phase orientation was rotated (Fig. 4B). When the time‐course from the orbital ROI was applied as a regressor during the GLM analysis (referred to as “with correction” result), the magnitude of the artifact was greatly reduced (Fig. 4C).
The BOLD signal difference between the INCREASE and DECREASE blocks increased across the four scanning runs in both the “without correction” and “with correction” results (Fig. 4D), with the BOLD signal difference in Run 4 significantly larger than that in Run 1 (“without correction”: t = 3.097, P = 0.011; “with correction”: t = 1.993, P = 0.047). This signal enhancement across runs is what one would expect to see as individuals learn to control their BOLD signal. Note that the “training effect” (i.e., change from Run 1 to 4) trended toward being smaller in the “with correction” result (mean: 0.35 ± 0.30, % change) than in the “without correction” (mean: 0.51 ± 0.68, % change) result.
Figure 4E shows the time‐courses of two voxels significantly influenced by the artifact, one showing positive activity (the red line) and the other negative activity (the blue line), in Experiment A from the same participant as in Figure 4A.
Table II lists the number of participants who met our artifact‐detection criteria for the GLM and PICA results in each experiment. Using GLM, the greatest preponderance of task‐correlated artifact were in Experiment B (eyeball movement) and Run 4 in Experiment A (rtfMRI from insula), with little task‐correlated artifact seen in the historical tasks (Experiments C1/C2/C3). In particular, a trend of increased artifact production over time was observed in Experiment A, e.g., three out of five participants displayed the artifact in Run 4 but only one (out of five) and two (out of five) showed it in Runs 1, 2, and 3. After the time‐course from the orbit ROI was regressed out, no participants showed significant artifact in any scan session with GLM analysis in the real time feedback (Experiment A) and eyeball‐rotation (Experiment B) tasks. Furthermore, with the exception of the eyes‐closed run in Experiment D, the artifact was seen in almost all participants in all experiments using PICA. Because the PICA analysis is a data driven approach, this result suggests that the artifact is common and not task‐specific. After regressing out the time‐course from the orbit ROI, the artifact in the PICA result decreased (Table II).
Table II.
Participants with a BOLD signal artifact in each experiment after each analysis condition
| Experiment | Total number | Number with the artifact in GLM analysis | Number with the artifact in GLM analysis after orbit regressor | Number with the artifact in PICA analysis | Number with the artifact in PICA analysis after orbit regressor |
|---|---|---|---|---|---|
| A‐real time (Run 1) | 5a | 1 | 0 | 4 | 2 |
| A‐real time (Run 2) | 5 | 2 | 0 | 5 | 2 |
| A‐real time (Run 3) | 5 | 2 | 0 | 4 | 3 |
| A‐real time (Run 4) | 5 | 3 | 0 | 4 | 2 |
| B‐eyeball movement | 10 | 7 | 0 | 8 | 4 |
| C1‐craving report | 10 | 1 | † | 8 | † |
| C2‐executive function | 10 | 1 | † | 9 | † |
| C3‐olfactory | 10 | 0 | † | 9 | † |
| D‐resting state (eyes open) | 6 | † | † | 5 | † |
| D‐resting state (eyes closed) | 6 | † | † | 0 | † |
Excludes two participants with the phase‐encoding direction rotated.
: Not performed.
DISCUSSION
In Experiment A (rtfMRI from an insula ROI), a putative “training” effect was observed in that the signal difference from the feedback ROI increased across runs (Fig. 4D). However, an unexpected BOLD signal artifact that covered part of the insula ROI in that paradigm was found (Fig. 4A). Because the feedback signal is derived from an individual subject, if it is corrupted by task‐related nonneuronal factors (e.g., task‐related motion artifacts), it could lead both the researcher and critically, the participant, to the erroneous conclusion that active control of neuronal processes was achieved. In the following discussion, evidence will be discussed to suggest that the observed artifact contaminated the training effect, originated from nonneuronal factors, and is task‐synchronized only in the rtfMRI paradigm.
This Artifact Contaminates the Training Effect
In Experiment A, an artifact that covered part of an insula ROI was found in some runs and in some participants (Fig. 4A and Table II). GLM analysis showed that the time course of the artifact was correlated to the rtfMRI task. The brain areas influenced by this artifact included the insula, the area chosen for regulation. Additionally, three out of five participants displayed the artifact in Run 4 but only one (out of five) and two (out of five) showed it in Runs 1, 2, and 3, suggesting a potential increase in artifact production over time. Notably, when the time course within the orbit was included in the regression analyses, the presumptive training effect (the % signal difference between INCREASE and DECREASE blocks from Run 1 to 4) was reduced from 0.51 to 0.35, suggesting that this nonneuronal induced artifact may be increased due to a feedback learning procedure and further suggesting what we initially interpreted as a “training effect” in Experiment A may have resulted not only from emotional regulation of a neuronally derived BOLD signal but was also contaminated by this artifact.
The Artifact Originates From Nonneuronal Factors
A previous study [Chen and Zhu, 1997] reported an artifact similar to that seen in Experiment A in the present study, and consisted of large signal fluctuations located mainly near the eye and spreading along the phase‐encoding direction. By applying the time‐course extracted from an orbit ROI during the GLM and ICA analysis, we were able to significantly reduce the artifact, supporting the hypothesis that eye movement, at least in part, generated this artifact.
It is well known that orbital motion is coupled to conscious mental activity [Chen and Zhu, 1997; Previc and Murphy, 1997]. Several types of eye movements could have led to differences between INCREASE and DECREASE blocks, e.g., the frequency and magnitude of eye motion and the tension of the muscles around the eyes. Given our instructions to participants (i.e., relax during the DECREASE blocks and recall highly emotional events during the INCREASE blocks), it is likely that both greater eye motion and muscle tension occurred in the INCREASE condition due to the recall of emotional memories. We speculate that movement of the fluid‐filled eye within the magnetic field may have altered signal properties primarily along the phase encoding acquisition direction.
Although applying a time‐course extracted from an orbital ROI during the GLM and ICA analysis reduced much of this artifact (Table II), it remains a possibility that eye movement was not causative but rather was the result of neuronal activity. Therefore, an eyeball motion task was employed; the result showed the same pattern of artifacts as in the rtfMRI task, suggesting that the artifact originates from the nonneuronal eyeball motion, and not the cognitive task of modifying emotion. In addition, the ICA results showed that similar artifacts were found in almost all experiments and in almost all participants with the exception of the eyes‐closed resting scans, further suggesting that this artifact is related to vision but not cognition. Further evidence (e.g., eye tracking data or a pulse sequence with spatial suppression of the orbital signals) is needed to verify this conjecture.
This Artifact may be Task‐Synchronized Preferentially in the rtfMRI Paradigm
Since similar eye movement patterns can be expected while performing other cognitive tasks, we utilized data from a craving invocation task (Experiment C1), a central executive function task (Experiment C2), and a passive, classical‐conditioning reward task (Experiment C3) to assess whether this artifact might be caused by eye motion specifically related to the rtfMRI paradigm or if it generalizes to other tasks. The craving task required drug users to look at either drug‐related or control pictures and manually rate their response to them using a visual‐analog scale beneath the pictures. Thus considerable eye motion might be expected (i.e., scanning the picture for salient features and looking beneath them for the rating portion), with the potential for greater muscle tension during the drug‐related block (e.g., more visual attention and emotion to drug‐related cues). In contrast, minimal eye motion would be anticipated while performing the central executive task, as the stimuli were all small enough to be confined to the participants' foveal visual field, although additional attention to stimuli presentation could potentially lead to increased muscle tension around the eyes. Finally, we chose to examine a classical conditioning reward task, for which only passive fixation was required, thus minimal eye motion or eye‐muscle tension change during the experiment would be expected. In contrast to our expectations, results showed minimal task‐correlated EPI artifacts in any of the three experiments when examining single subject data using GLM analysis. Thus, neither eyeball motion itself nor the tension of muscles around the eyes appear to independently explain this artifact, suggesting that it may be uniquely synchronized to, and a result of, the rtfMRI paradigm.
ICA, a data‐driven approach, yielded results that showed similar artifacts in almost all experiments and in almost all participants, even in the eyes‐open resting scan runs. In contrast, ICA analysis found no artifacts in the eyes‐closed resting data, indicating that the artifact may be due to increased eye movements during task viewing, suggesting eye‐related activity during visual processing but not the eyeball itself was causative. Therefore, a neurofeedback paradigm that does not require visual process (e.g., auditory feedback) should not be affected by this artifact. Taken together, these results demonstrate that an orbital artifact exists widely across several types of fMRI paradigms but is uniquely synchronized to the rtfMRI task in some participants.
Limitations, Implications, and Recommendations
Although a significant insula signal training effect was found in Experiment A, suggesting successful neurofeedback learning, it appears premature to conclude that participants were able to voluntarily control an emotionally relevant brain region in the present study. The participants may have controlled the signal by controlling eye‐related activity because the non‐neuronal artifact appeared specifically synchronized to our rtfMRI paradigm and likely influenced the rtfMRI training effect.
It is important to note this conclusion should be considered preliminary because of the small number of participants in Experiment A. Another limitation of the present study is the absence of an acceptable method to normalize the training effect across participants. Thus, the signal effect size contaminated by this artifact is unknown. Nevertheless different scan parameters (e.g., an in‐plane 40° rotation of the phase direction) can be employed to avoid this artifact.
Eye‐movement related artifact is also a major contamination in EEG neurofeedback studies [Heinrich et al., 2007], although an auditory feedback protocol was employed to avoid this artifact [e.g., Peniston and Kulkosky, 1989]. Further, several commercial solutions have been developed for visual motion‐induced artifacts [e.g., the NF software package (http://www.brainmaster.com) or the EMSE software package (http://www.sourcesignal.com)]. The present result suggests such solutions may also be necessary in rtfMRI training procedures.
The artifact presented herein may not necessarily have been a problem in previous rtfMRI studies. For example, Caria et al. [ 2007] acquired data in the axial and not the sagittal plane employed herein and thus slice prescription or their choice of phase encoding direction may have precluded the artifact in the insula. Using an ROI in another brain area may also have avoided the artifact interference [deCharms et al., 2005]. Our goal in presenting this artifact is to alert future rtfMRI researchers of the disruptive potential of stray signals that can negatively affect study outcomes.
Future studies can easily avoid the influence of this particular artifact by rotating the phase‐encoding orientation or using another slice orientation (e.g., oblique axial) to place the artifact outside of the feedback ROI (see Fig. 4B), employing a special fMRI sequence [e.g., a slab presaturation sequence; Chen and Zhu, 1997] or orthogonalizing the feedback data to the orbital ROI as shown above, but prior to feedback. However, this latter solution is not perfect in practice because the orbit ROI correction will only remove linear and non‐delayed effects.
In addition to eye‐motion, other potential artifact sources (e.g., head motion, respiration and cardiac changes) might interfere with single subject task analysis [Hajnal et al., 1994]. It is entirely possible that changing neuronal activity in the feedback ROI is the hardest way for a participant to change the BOLD signal. Participants will naturally, subconsciously, gravitate toward the easiest solution (e.g., head or eye movements), leading to a false positive training effect. The present results underscore the importance of carefully reviewing single subject data and employing appropriate compensatory procedures during feedback, such as removing an orbit ROI signal (plus head motion‐related signals, respiration‐related signal, etc.) from data used as a feedback signal. Thus, caution should be exercised in designing and interpreting rtfMRI studies, as nonneuronal sources of training effects might serve as unexpected confounds.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information 1
Supporting Information 2
Acknowledgements
The authors thank Kimberly Modo and Loretta Spurgeon for their assistance in the conduct of some of the studies reported herein and Mary Pfeiffer for editing assistance.
The authors reported no financial interests or potential conflicts of interest.
REFERENCES
- Beckmann CF, Smith SM ( 2004): Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging 23: 137–152. [DOI] [PubMed] [Google Scholar]
- Caria A, Veit R, Sitaram R, et al. ( 2007): Regulation of anterior insular cortex activity using real‐time fMRI. Neuroimage 35: 1238–1246. [DOI] [PubMed] [Google Scholar]
- Chen W, Zhu XH ( 1997): Suppression of physiological eye movement artifacts in functional MRI using slab presaturation. Magn Reson Med 38: 546–550. [DOI] [PubMed] [Google Scholar]
- Cox RW ( 1996): AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29: 162–173. [DOI] [PubMed] [Google Scholar]
- deCharms RC, Maeda F, Glover GH, et al. ( 2005): Control over brain activation and pain learned by using real‐time functional MRI. Proc Natl Acad Sci USA 102: 18626–18631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner T, Gruzelier JH ( 2003): Ecological validity of neurofeedback: Modulation of slow wave EEG enhances musical performance. Neuroreport 14: 1221–1224. [DOI] [PubMed] [Google Scholar]
- Federspiel A, Muller TJ, Horn H, Kiefer C, Strik WK ( 2006): Comparison of spatial and temporal pattern for fMRI obtained with BOLD and arterial spin labeling. J Neural Transm 113: 1403–1415. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Jezzard P, Turner R ( 1994): Analysis of functional MRI time series. Hum Brain Mapp 1: 153–171. [Google Scholar]
- Garavan H, Ross TJ, Li SJ, Stein EA ( 2000): A parametric manipulation of central executive functioning. Cereb Cortex 10: 585–592. [DOI] [PubMed] [Google Scholar]
- Hajnal JV, Myers R, Oatridge A, Schwieso JE, Young IR, Bydder GM ( 1994): Artifacts due to stimulus correlated motion in functional imaging of the brain. Magn Reson Med 31: 283–291. [DOI] [PubMed] [Google Scholar]
- Heinrich H, Gevensleben H, Freisleder FJ, Moll GH, Rothenberger A ( 2004): Training of slow cortical potentials in attention‐deficit/hyperactivity disorder: Evidence for positive behavioral and neurophysiological effects. Biol Psychiatry 55: 772–775. [DOI] [PubMed] [Google Scholar]
- Heinrich H, Gevensleben H, Strehl U ( 2007): Annotation: neurofeedback—Train your brain to train behaviour. J Child Psychol Psychiatry 48: 3–16. [DOI] [PubMed] [Google Scholar]
- Hutcherson CA, Goldin PR, Ochsner KN, Gabrieli JD, Barrett LF, Gross JJ ( 2005): Attention and emotion: Does rating emotion alter neural responses to amusing and sad films? Neuroimage 27: 656–668. [DOI] [PubMed] [Google Scholar]
- McClure SM, Berns GS, Montague PR ( 2003): Temporal prediction errors in a passive learning task activate human striatum. Neuron 38: 339–346. [DOI] [PubMed] [Google Scholar]
- McKeown MJ, Makeig S, Brown GG, et al. ( 1998): Analysis of fMRI data by blind separation into independent spatial components. Hum Brain Mapp 6: 160–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peniston EG, Kulkosky PJ ( 1989): Alpha‐theta brainwave training and beta‐endorphin levels in alcoholics. Alcohol Clin Exp Res 13: 271–279. [DOI] [PubMed] [Google Scholar]
- Previc FH, Murphy SJ ( 1997): Vertical eye movements during mental tasks: A re‐examination and hypothesis. Percept Mot Skills 84: 835–847. [DOI] [PubMed] [Google Scholar]
- Ramaratnam S, Baker GA, Goldstein LH ( 2008): Psychological treatments for epilepsy. Cochrane Database Syst Rev (CD002029). DOI: 10.1002/14651858.CD002029.pub3. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, et al. ( 2004): Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 ( Suppl 1): S208–S219. [DOI] [PubMed] [Google Scholar]
- Sokhadze TM, Cannon RL, Trudeau DL ( 2008): EEG biofeedback as a treatment for substance use disorders: Review, rating of efficacy, and recommendations for further research. Appl Psychophysiol Biofeedback 33: 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon DJ ( 2005): Can neurofeedback training enhance performance? An evaluation of the evidence with implications for future research. Appl Psychophysiol Biofeedback 30: 347–364. [DOI] [PubMed] [Google Scholar]
- Vernon DJ, Egner T, Cooper N, et al. ( 2003): The effect of training distinct neurofeedback protocols on aspects of cognitive performance. Int J Psychophysiol 47: 75–85. [DOI] [PubMed] [Google Scholar]
- Weiskopf N, Sitaram R, Josephs O, et al. ( 2007): Real‐time functional magnetic resonance imaging: Methods and applications. Magn Reson Imaging 25: 989–1003. [DOI] [PubMed] [Google Scholar]
- Yang S, Ross TJ, Zhang Y, Stein EA, Yang Y ( 2005): Head motion suppression using real‐time feedback of motion information and its effects on task performance in fMRI. Neuroimage 27: 153–162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information 1
Supporting Information 2


