Abstract
Purpose:
Fatigue is the most common and distressing symptom experienced by cancer survivors. This study sought to determine the prevalence and risk factors for fatigue among breast cancer (BC) survivors receiving aromatase inhibitors (AIs).
Material and Methods:
We conducted a cross-sectional survey study among postmenopausal women with stage 0 to III BC receiving adjuvant AI therapy at the outpatient breast oncology clinic of a large university hospital. Participants with a score ≥4 on the “worst fatigue” item of the Brief Fatigue Inventory (BFI) were classified as having moderate or severe fatigue. Multivariate logistic regression analyses were performed to evaluate risk factors.
Results:
Among 1,103 participants, 616 (55.8%) had moderate or severe fatigue. In the multivariate logistic regression model, women younger than 55 years were significantly more likely to report moderate-severe fatigue than women older than 65 years (adjusted odds ratio (AOR), 1.58, 95% confidence interval (CI) 1.07–2.35; p=0.023). Compared to women with high school or less education, women with college or more education were significantly more likely to report moderate-severe fatigue (AOR, 1.40, 95% CI 1.02–1.91; p=0.037). Increasing body mass index (BMI) was significantly associated with increased risk of experiencing moderate-severe fatigue (overweight: AOR, 1.37, 95% CI 1.01–1.84; p=0.042; obesity: AOR, 2.08, 95% CI 1.53–2.81; p<0.001). Fatigue was significantly correlated with pain severity (r=0.48, p<0.001) and insomnia (r=0.62, p<0.001).
Conclusion:
Moderate-severe fatigue complaints exceed 50% among AI users. Fatigue is highly related to younger age, higher education level, higher BMI, pain severity, and insomnia.
Keywords: Breast cancer, fatigue, aromatase inhibitor
INTRODUCTION
Cancer-related fatigue (CRF) is the most common and distressing symptom experienced by cancer patients. It is widely prevalent among breast cancer (BC) survivors, with a range of 27% to 96% depending on the stage or type of treatment received and method of assessment (1, 2). Fatigue is also the most uncomfortable symptom for BC survivors (3), impairing function and overall quality of life among this population(4, 5).
In the past decade, aromatase inhibitors (AIs) have been the recommended first-line adjuvant endocrine therapy in postmenopausal women with hormone-receptor-positive breast cancer (6); they are associated with improved disease free survival and overall survival (7). With an increase in AI use, BC survivors taking this class of medication are suffering from several troublesome AI-associated symptoms, including arthralgia, hot flashes, and mood disorders (8). Recent data indicates that arthralgia and insomnia related to AIs have a high prevalence and impact on BC survivors (9, 10). Research studies have shown a significant link between these AI-related symptoms and fatigue in BC survivors (11–13). Despite the wide use of AIs and evidence to indicate that their associated side effects may increase the risk of fatigue in BC survivors, to our knowledge, no data has been published to determinate the prevalence and risk factors for fatigue among this population.
Because fatigue may be particularly impacted by arthralgia and insomnia among postmenopausal breast cancer survivors receiving AIs, in the present study, we sought to determine its prevalence and risk factors. This information will help develop targeted interventions for addressing the overall fatigue burden in this population. Thus, the specific aims of this study were to: 1) define the prevalence of fatigue among postmenopausal breast cancer survivors on AIs, 2) identify socio-demographic and clinical risk factors for fatigue, and 3) evaluate the relationship between fatigue and comorbid symptoms (i.e. pain, insomnia) in this population.
MATERIAL AND METHODS
Study Design and Patient Population
Between November 2011 and April 2015, we conducted a cross-sectional survey study among BC survivors receiving care at the Rowan Breast Cancer Center at the Abramson Cancer Center of the University of the Pennsylvania (Philadelphia, PA). Potential participants were postmenopausal women with histologically confirmed stage 0 to III hormone receptor-positive breast cancer who were currently taking a third generation AI; had completed chemotherapy, radiotherapy, or surgery at least one month prior to enrollment; and had the ability to understand and provide informed consent in English. Research assistants obtained permission from the treating oncologist, screened medical records, and approached potential participants for recruitment at their regular follow-up appointments. All participants provided written informed consent. They were then given a self-administered survey to complete. For those participants who could not complete the survey in time, we gave them a stamped envelope with our return address to mail the survey back to us. The Institutional Review Board of the University of Pennsylvania approved the study.
Outcome Measurement
The primary outcome was participants’ self-reported fatigue as measured by the Brief Fatigue Inventory (BFI). This 9-item instrument was designed to assess fatigue severity in cancer and non-cancer populations on a numerical rating scale ranging from 0 to 10. Among these nine items, the “worst fatigue” item has been validated as a single-item dichotomous variable, with a cut point ≥4 indicating moderate-severe fatigue (14). The scale score has excellent internal consistency of 0.96 (14).
Participants completed questionnaires to assess the severity of comorbid symptoms (i.e. pain, insomnia). We assessed pain severity by using the pain severity score, which is the average of the first four items of the Brief Pain Inventory (BPI) (15). A cutoff point of ≥4 has been validated as clinically significant pain. We assessed participants’ sleep disturbance using the Insomnia Severity Index (ISI). The ISI is a 7-item instrument with scores ranging from 0 to 28. The validated cutoff scores are 0–6 (no clinically significant sleep difficulties), 7–14 (mild insomnia), and 15+ (presence of clinically significant insomnia) (16). For the purpose of analysis, we used a cutoff point of ≥15 to indicate clinically significant insomnia.
We collected information on covariates including age, race, education, and employment status. Clinical and treatment variables including body mass index (BMI), stage of cancer, time since cancer diagnosis, and previous and current cancer treatments were assessed by self-report and medical chart abstraction.
Statistical Analysis
Data analysis was performed using STATA 12 for Windows (STATA Corporation, College Station, TX). Descriptive statistics were used to report the demographic variables of the study participants. Bivariate analyses using chi-square tests were then conducted to identify the factors associated with fatigue among breast cancer survivors on AIs. We then developed a multivariate logistic regression model to identify independent risk factors associated with the presence of moderate-severe fatigue. Variables with p-values of <0.1 in the bivariate analyses were included in the multivariate analysis. To evaluate the relationship between fatigue and comorbid pain and insomnia, we first used a Venn diagram to describe the overlap of symptoms by proportion. We then calculated Pearson’s correlation coefficients among fatigue, pain, and insomnia. Statistical tests were 2-sided, and p values of <0.05 indicated statistical significance.
RESULTS
Participant Characteristics
Of the 1,518 consecutive BC survivors we screened, 1,321 (87.0%) agreed to participate and provided consent. Among the 197 who declined (13.0%), the main reasons were: lack of time to complete the survey (n=62, 31.5%), did not want to participate in research (n=85, 43.1%), and ineligible (n=50, 25.4%). Additionally, 15 (1.1%) subjects withdrew consent from the study and 26 (2.0%) subjects did not return their survey, resulting in the final sample of 1,280. This population reflects an 87.0% response rate among all initially approached subjects. Additionally, 177 subjects discontinued AIs due to various reasons. For this study, we restricted analysis to the 1,103 subjects who were on AIs at the time of enrollment.
Among these 1,103 participants, the mean age was 63.2 years (SD=9.8; range=20.0–92.0 years). Although the majority (82.7%) were non-Hispanic white, a substantial proportion (15.3%) were non-Hispanic black. For the purpose of analysis, we combined the race categories into white and nonwhite. Characteristics of the study population are listed in Table 1.
Table 1.
Demographic and Clinical Characteristic of Participants
| Variables | N (%) | No-Mild Fatigue |
Mod-Severe Fatigue |
P-value |
|---|---|---|---|---|
| Total | 1,103 | 487 (44.15) | 616 (55.85) | |
| Age, years | 0.021 | |||
| <55 | 212 (19.22) | 76 (35.85) | 136 (64.15) | |
| 55–65 | 515 (46.69) | 233 (45.24) | 282 (54.76) | |
| >65 | 376 (34.09) | 178 (47.34) | 198 (52.66) | |
| Race/Ethnicity | 0.033 | |||
| White | 912 (82.68) | 416 (45.61) | 496 (54.39) | |
| Non-white | 191 (17.32) | 71 (37.17) | 120 (62.83) | |
| Employment (n=1 missing data) | 0.120 | |||
| Full-time | 423 (38.38) | 189 (44.68) | 234 (55.32) | |
| Part-time | 151 (13.70) | 77 (50.99) | 74 (49.01) | |
| Not employed | 528 (47.91) | 220 (41.67) | 308 (58.33) | |
| Education (n=1 missing data) | 0.059 | |||
| High school or less | 225 (20.42) | 112 (49.78) | 113 (50.22) | |
| College or more | 877 (79.58) | 375 (42.76) | 502 (57.24) | |
| Body Mass Index (Kg/m2) | <0.001 | |||
| <25 | 426 (38.62) | 222 (52.11) | 204 (47.89) | |
| 25–30 | 325 (29.47) | 143 (44.00) | 182 (56.00) | |
| >30 | 352 (31.91) | 122 (34.66) | 230 (65.34) | |
| Cancer Stage (n=12 missing data) | 0.085 | |||
| 0 & I | 568 (52.06) | 260 (45.77) | 308 (54.23) | |
| II | 382 (35.01) | 170 (44.50) | 212 (55.50) | |
| III | 141 (12.92) | 50 (35.46) | 91 (64.54) | |
| Years since breast cancer diagnosis | 0.045 | |||
| >5 | 190 (17.23) | 91 (47.89) | 99 (52.11) | |
| 2–5 | 423 (38.35) | 200 (47.28) | 223 (52.72) | |
| <2 | 490 (44.42) | 196 (40.00) | 294 (60.00) | |
| Chemotherapy | 0.045 | |||
| None | 534 (48.41) | 250 (46.82) | 284 (53.18) | |
| Chemo without Taxane | 105 (9.52) | 52 (49.52) | 53 (50.48) | |
| Chemo with Taxane | 464 (42.07) | 185 (39.87) | 279 (60.13) | |
| Radiotherapy | 0.474 | |||
| None | 311 (28.20) | 132 (42.44) | 179 (57.56) | |
| Yes | 792 (71.80) | 355 (44.82) | 437 (55.18) | |
| Surgery (n=1 missing data) | 0.011 | |||
| Lumpectomy | 638 (57.89) | 302 (47.34) | 336 (52.66) | |
| Mastectomy | 464 (42.11) | 184 (39.66) | 280 (60.34) | |
| Aromatase Inhibitors, current (n=6 missing data) | 0.721 | |||
| Anastrozole (Arimidex) | 884 (80.58) | 384 (43.44) | 500 (56.56) | |
| Exemestane (Aromasin) | 61 (5.56) | 28 (45.90) | 33 (54.10) | |
| Letrozole (Femara) | 152 (13.86) | 71 (46.71) | 81 (53.29) | |
| Duration of AI use, years | 0.059 | |||
| >3 | 272 (24.66) | 136 (50.00) | 136 (50.00) | |
| 1–3 | 565 (51.22) | 244 (43.19) | 321 (56.81) | |
| <1 | 266 (24.12) | 107 (40.23) | 159 (59.77) |
Prevalence and Severity of Fatigue Among Women on AIs
Among the 1,103 participants, 151 (13.7%) reported no fatigue, 336 (30.5%) had mild fatigue (“worst fatigue” score=1–3), 278 (25.2%) had moderate fatigue (“worst fatigue” score=4–6), and 338 (30.6 %) had severe fatigue (“worst fatigue” score=7–10). For the purpose of further analysis, we merged those four groups into two groups: no-mild fatigue (n=487, 44.2%) and moderate-severe fatigue (n=616, 55.8%).
Socio-demographic and Clinical Factors Associated with Fatigue Among Women on AIs
In bivariate analyses, we found statistically significant differences between BC survivors with moderate-severe fatigue and no-mild fatigue by age, race/ethnicity, education level, BMI, stage of cancer, time since breast cancer diagnosis, chemotherapy, surgery type, and duration of AIs (Table 1). Non-white participants, age younger than 55 years, and those with a college degree or more were more likely to report moderate-severe fatigue. Clinical risk factors for reporting moderate-severe fatigue included being overweight /obese, having stage III disease, being less than two years from diagnosis, having received chemotherapy with Taxane or having had mastectomy surgery, and having been on an AI for less than one year (Table 2).
Table 2.
Multivariate Logistic Regression Model
| Variables | Bivariable Analysis OR (95% CI) |
P-value | Multivariate Analysis AOR (95% CI) |
P-value | |
|---|---|---|---|---|---|
| Age, years | |||||
| >65 | 1 | 1 | |||
| 55–65 | 1.09 (0.83–1.42) | 0.535 | 1.03 (0.77–1.37) | 0.839 | |
| <55 | 1.61 (1.14–2.27) | 0.007 | 1.58 (1.07–2.35) | 0.023 | |
| Race/Ethnicity | |||||
| White | 1 | 1 | |||
| Non-white | 1.42 (1.03–1.95) | 0.033 | 1.32 (0.93–1.86) | 0.116 | |
| Education | |||||
| High school or less | 1 | 1 | |||
| College or more | 1.33 (0.99–1.78) | 0.059 | 1.40 (1.02–1.91) | 0.037 | |
| Body Mass Index (Kg/m2) | |||||
| <25 | 1 | 1 | |||
| 25–30 | 1.39 (1.04–1.85) | 0.028 | 1.37 (1.01–1.84) | 0.042 | |
| >30 | 2.05 (1.53–2.74) | <0.001 | 2.08 (1.53–2.81) | <0.001 | |
| Stage | |||||
| 0 & I | 1 | 1 | |||
| II | 1.05 (0.81–1.37) | 0.699 | 0.95 (0.70–1.28) | 0.720 | |
| III | 1.54 (1.05–2.25) | 0.028 | 1.21 (0.77–1.89) | 0.411 | |
| Years since breast cancer diagnosis | |||||
| >5 | 1 | 1 | |||
| 2–5 | 1.02 (0.73–1.44) | 0.888 | 0.96 (0.66–1.39) | 0.818 | |
| <2 | 1.38 (0.98–1.93) | 0.062 | 1.27 (0.83–1.94) | 0.275 | |
| Chemotherapy | |||||
| None | 1 | 1 | |||
| Chemo without | |||||
| Taxane | 0.90 (0.59–1.36) | 0.612 | 0.88 (0.54–1.41) | 0.582 | |
| Chemo with Taxane | 1.33 (1.03–1.71) | 0.027 | 1.18 (0.86–1.63) | 0.300 | |
| Surgery | |||||
| Lumpectomy | 1 | 1 | |||
| Mastectomy | 1.37 (1.07–1.74) | 0.011 | 1.25 (0.96–1.63) | 0.093 | |
| Duration of AI use, years | |||||
| >3 | 1 | 1 | |||
| 1–3 | 1.32 (0.98–1.76) | 0.064 | 1.10 (0.78–1.54) | 0.588 | |
| <1 | 1.49 (1.06–2.09) | 0.023 | 1.06 (0.68–1.65) | 0.786 |
In the multivariate logistic regression model (see Table 2), women younger than 55 years were significantly more likely to report moderate-severe fatigue than women older than 65 years (AOR, 1.58, 95% CI 1.07–2.35; p=0.023). Compared to women with high school or less education, women with college or more education were significantly more likely to report moderate-severe fatigue (AOR, 1.40, 95% CI 1.02–1.91; p=0.037). Also, higher BMIs were significantly associated with increased risk (up to a two-fold higher odds) of experiencing moderate-severe fatigue (overweight: AOR, 1.37, 95%1.01–1.84; p=0.042; obesity: AOR, 2.08, 95% CI 1.53–2.81; p<0.001). Clinical risk factors such as prior chemotherapy and mastectomy were no longer significant when we adjusted for other co-variates.
Relationship Between the Comorbid Symptoms and Fatigue Among Women on AIs
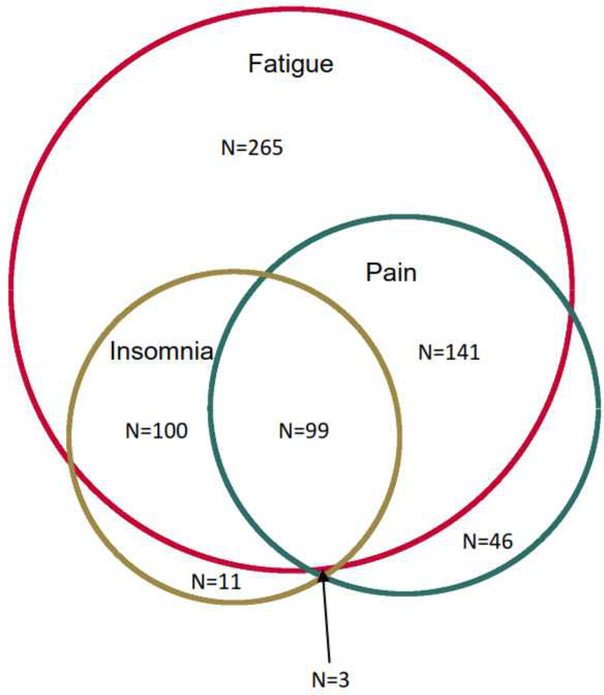
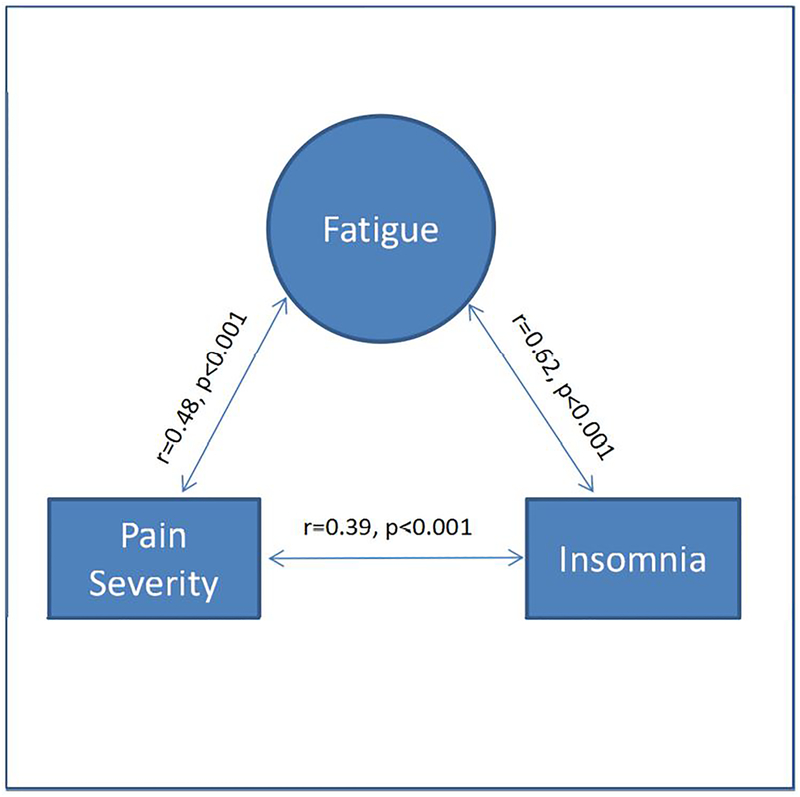
Of the 605 subjects who experienced moderate-severe fatigue and also provided data on insomnia and pain, 340 (52.6%) had clinically significant insomnia and/or pain. Among the 289 subjects who experienced clinically significant pain, the majority (83.0%) had moderate to severe fatigue. Similarly, among the 213 subjects who experienced clinically significant insomnia, the majority (93.4%) had moderate to severe fatigue. Furthermore, among the 102 participants who had both clinically significant pain and insomnia, almost all (97.1%) experienced moderate-severe fatigue (Figure 1). The fatigue score was significantly correlated with pain severity (r=0.48, p<0.001) and insomnia (r=0.62, p<0.001). There was also a significant correlation between pain severity and insomnia (r=0.39, p<0.001) (Figure 2).
Figure 1.
Venn Diagram of Fatigue, Pain Severity, and Insomnia
Figure 2.
Correlation of Fatigue, Pain Severity, and Insomnia
DISCUSSION
Fatigue is one of the most common and distressing symptoms affecting cancer survivors. In this study, we found that among breast cancer survivors receiving adjuvant AI therapy, more than four in five AIs users (86.3%) reported current fatigue and more than one in two (55.8%) experienced moderate to severe fatigue. Younger age, high education level, and obesity were also associated with increased risk for patient-reported moderate to severe fatigue. As expected, we found that fatigue, pain, and insomnia had substantial overlaps and were significantly correlated. These findings advance the current understanding of fatigue symptoms as experienced by breast cancer survivors on AIs and also highlight the need for targeted and effective treatments to manage fatigue in this population.
Among breast cancer patients, estimates of the prevalence of fatigue from prior data range from 27% to 96% (1, 2). Fatigue generally increases during chemotherapy (80% to 96%) (17) and radiotherapy (60% to 93%) (18). Studies indicate that at least 25% of cancer survivors continue to experience fatigue after completion of active treatment (19). Data about the prevalence of fatigue among BC survivors undergoing hormonal therapies is scarce; however, Schmidt et al. found that aromatase inhibitors were associated with long-term fatigue (20) and Haghighat et al. found that using Tamoxifen predicted fatigue (21). In our study, using a validated instrument, we found a high prevalence of fatigue among BC survivors on AIs, highlighting the magnitude of this clinical challenge for this population.
The etiology and pathogenesis of cancer-related fatigue in BC survivors is complex and multi-causal. Many prior studies have determined that fatigue often co-occurs with other symptom clusters such as sleeping disturbances, mood disorders, and pain (22). Accumulated evidence indicates that inflammation appears to be a key biological mechanism underlying this symptom cluster (23–26). The AI-induced decrease in estrogen levels may impact inflammation (27). Our prior study found that the coexistence of arthralgia, fatigue, and insomnia was associated with increased levels of inflammatory biomarkers among women on AIs (11). In this study, we found that for the majority of participants, clinically significant pain (83%) or insomnia (94%) was associated with moderate-severe fatigue. But approximately half (47.4%) of the participants who experienced moderate-severe fatigue did not have clinically significant pain or insomnia. Therefore our findings support the idea that pain and insomnia contribute to fatigue. Of note, our data is cross-sectional in nature and will require a prospective data set and experimental design to enhance our understanding of the interplays of fatigue and comorbid symptoms.
Apart from comorbid symptoms, obesity is the only clinical variable that was related to an increased risk for moderate-severe fatigue. Our findings are consistent with prior data suggesting that BMI and fatigue are positively correlated (20). To date, in studies of female sex hormone concentration in postmenopausal women, BMI has been shown to be a meaningful proxy for directly measuring obesity (28). Excess android (abdominal and upper-body) adipose accumulation is associated with increased levels of inflammatory activity driven by macrophages resident in adipose tissue (29–31). Among breast cancer patients, obesity has been independently associated with inflammation in adjacent normal tissue (32). Indeed, a recent study found that anti-inflammatory foods should be promoted for prevention of obesity and related diseases (33). Therefore, inflammation may be a key biological mechanism underlying fatigue among obese postmenopausal BC survivors on AIs.
We also found that younger age (<55 years) and higher education level were significantly associated with fatigue. Some prior data has shown that younger age (34–36) and higher education level (37) are significantly correlated with fatigue. Conversely, others have shown a significant link between older age (37), lower education level (20), and fatigue. There are a number of reasons that may account for greater fatigue among younger postmenopausal BC survivors: First, when treated with AIs, younger survivors have a more absolute drop in their estrogen levels. Biologically, this can result in greater symptoms related to estrogen withdrawal. Second, previous literature has shown that younger survivors are more likely to report psychosocial problems (38) compared with older survivors, which are linked to greater fatigue (39). Third, younger people are often struck by a cancer diagnosis when they are in a state of “perfect health.” Their own interpretation of their energy level is related to their recall prior to the cancer diagnosis and in reference to people around their age. In contrast, older people may have experienced other chronic illnesses or seen their peers dealing with illness or death, and therefore, have likely developed coping strategies to better mitigate the impact of a cancer diagnosis and treatment (40). Lastly, younger BC survivors may experience higher demands from raising a family or from professional challenges such as work and school. To help younger survivors manage CRF likely requires a multi-disciplinary approach targeting both physical and psychological causes of fatigue.
We need to acknowledge several limitations. First, because our study relied on patient self-report, some degree of recall bias exists; however, for subjective symptoms like fatigue, patient-reported outcomes are considered to be the gold standard. Second, our study focused on the prevalence of fatigue among breast cancer survivors taking AIs, so we cannot compare our findings to breast cancer survivors who were not taking AIs. Finally, as the study is cross-sectional in nature, it is challenging to dissect the causal relationship between fatigue and comorbid symptoms.
To our knowledge, this study is the first and largest (n=1,103) cross-sectional survey study to focus on evaluating the prevalence of and risk factors for fatigue among breast cancer survivors taking AIs. We found more than half of BC survivors experienced moderate to severe fatigue, which relates to comorbid symptoms like insomnia and pain. Obesity, younger age, and higher education were associated with increased rates of fatigue. Developing and testing effective interventions for fatigue in this population represents an important unmet need. It is likely that one size does not fit all. Interventions targeting multiple symptoms and obesity are needed to improve fatigue management for this population.
Highlights for “Prevalence and Risk Factors Among Breast Cancer Survivors on Aromatase Inhibitors”.
Moderate-severe fatigue complaints exceed 50% among AI users.
Obesity, younger age, and higher education level were significantly associated with fatigue.
The majority who experienced either pain or insomnia reported moderate to severe fatigue.
Acknowledgments:
This research is funded in part by grants from the National Cancer Institute / National Institutes of Health (R01 CA158243, P30-CA008748), the Byrne Fund, and the Translational Research and Integrative Medicine Fund at the Memorial Sloan Kettering Cancer Center. Dr. H. Mao is supported by the National Natural Science Foundation of China (81603703). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: None declared.
REFERENCES
- 1.Goedendorp MM, Jacobsen PB, Andrykowski MA. Fatigue screening in breast cancer patients: identifying likely cases of cancer-related fatigue. Psychooncology 2016;25(3):275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Jong N, Candel MJ, Schouten HC, Abu-Saad HH, Courtens AM. Prevalence and course of fatigue in breast cancer patients receiving adjuvant chemotherapy. Ann Oncol 2004;15(6):896–905. [DOI] [PubMed] [Google Scholar]
- 3.Minton O, Stone P. How common is fatigue in disease-free breast cancer survivors? A systematic review of the literature. Breast Cancer Res Treat 2008;112(1):5–13. [DOI] [PubMed] [Google Scholar]
- 4.Curt GA. Impact of fatigue on quality of life in oncology patients. Semin Hematol 2000;37(4 Suppl 6):14–7. [DOI] [PubMed] [Google Scholar]
- 5.Montazeri A Health-related quality of life in breast cancer patients: a bibliographic review of the literature from 1974 to 2007. J Exp Clin Cancer Res 2008;27:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burstein HJ, Temin S, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: american society of clinical oncology clinical practice guideline focused update. J Clin Oncol 2014;32(21):2255–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chlebowski RT. Aromatase inhibitor-associated arthralgias. J Clin Oncol 2009;27(30):4932–4. [DOI] [PubMed] [Google Scholar]
- 8.Santen RJ, Stuenkel CA, Davis SR, Pinkerton JV, Gompel A, Lumsden MA. Managing menopausal symptoms and associated clinical issues in breast cancer survivors. J Clin Endocrinol Metab 2017. [DOI] [PubMed] [Google Scholar]
- 9.Mao JJ, Stricker C, Bruner D, Xie S, Bowman MA, Farrar JT, et al. Patterns and risk factors associated with aromatase inhibitor-related arthralgia among breast cancer survivors. Cancer 2009;115(16):3631–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desai K, Mao JJ, Su I, Demichele A, Li Q, Xie SX, et al. Prevalence and risk factors for insomnia among breast cancer patients on aromatase inhibitors. Support Care Cancer 2013;21(1):43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauml J, Chen L, Chen J, Boyer J, Kalos M, Li SQ, et al. Arthralgia among women taking aromatase inhibitors: is there a shared inflammatory mechanism with co-morbid fatigue and insomnia? Breast Cancer Res 2015;17:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mao JJ, Farrar JT, Bruner D, Zee J, Bowman M, Seluzicki C, et al. Electroacupuncture for fatigue, sleep, and psychological distress in breast cancer patients with aromatase inhibitor-related arthralgia: a randomized trial. Cancer 2014;120(23):3744–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiorentino L, Rissling M, Liu L, Ancoli-Israel S. The Symptom Cluster of Sleep, Fatigue and Depressive Symptoms in Breast Cancer Patients: Severity of the Problem and Treatment Options. Drug Discov Today Dis Models 2011;8(4):167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer 1999;85(5):1186–96. [DOI] [PubMed] [Google Scholar]
- 15.Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain 1983;17(2):197–210. [DOI] [PubMed] [Google Scholar]
- 16.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med 2001;2(4):297–307. [DOI] [PubMed] [Google Scholar]
- 17.Jacobsen PB, Hann DM, Azzarello LM, Horton J, Balducci L, Lyman GH. Fatigue in women receiving adjuvant chemotherapy for breast cancer: characteristics, course, and correlates. J Pain Symptom Manage 1999;18(4):233–42. [DOI] [PubMed] [Google Scholar]
- 18.Irvine DM, Vincent L, Graydon JE, Bubela N. Fatigue in women with breast cancer receiving radiation therapy. Cancer Nurs 1998;21(2):127–35. [DOI] [PubMed] [Google Scholar]
- 19.Prue G, Rankin J, Allen J, Gracey J, Cramp F. Cancer-related fatigue: A critical appraisal. Eur J Cancer 2006;42(7):846–63. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt ME, Chang-Claude J, Seibold P, Vrieling A, Heinz J, Flesch-Janys D, et al. Determinants of long-term fatigue in breast cancer survivors: results of a prospective patient cohort study. Psychooncology 2015;24(1):40–6. [DOI] [PubMed] [Google Scholar]
- 21.Haghighat S, Akbari ME, Holakouei K, Rahimi A, Montazeri A. Factors predicting fatigue in breast cancer patients. Support Care Cancer 2003;11(8):533–8. [DOI] [PubMed] [Google Scholar]
- 22.Kenne Sarenmalm E, Browall M, Gaston-Johansson F. Symptom burden clusters: a challenge for targeted symptom management. A longitudinal study examining symptom burden clusters in breast cancer. J Pain Symptom Manage 2014;47(4):731–41. [DOI] [PubMed] [Google Scholar]
- 23.Dantzer R, Meagher MW, Cleeland CS. Translational approaches to treatment-induced symptoms in cancer patients. Nat Rev Clin Oncol 2012;9(7):414–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wood LJ, Weymann K. Inflammation and neural signaling: etiologic mechanisms of the cancer treatment-related symptom cluster. Curr Opin Support Palliat Care 2013;7(1):54–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thornton LM, Andersen BL, Blakely WP. The pain, depression, and fatigue symptom cluster in advanced breast cancer: covariation with the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system. Health Psychol 2010;29(3):333–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bower JE, Lamkin DM. Inflammation and cancer-related fatigue: mechanisms, contributing factors, and treatment implications. Brain Behav Immun 2013;30 Suppl:S48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Straub RH. The complex role of estrogens in inflammation. Endocr Rev 2007;28(5):521–74. [DOI] [PubMed] [Google Scholar]
- 28.Mahabir S, Baer DJ, Johnson LL, Hartman TJ, Dorgan JF, Campbell WS, et al. Usefulness of body mass index as a sufficient adiposity measurement for sex hormone concentration associations in postmenopausal women. Cancer Epidemiol Biomarkers Prev 2006;15(12):2502–7. [DOI] [PubMed] [Google Scholar]
- 29.Sun X, Casbas-Hernandez P, Bigelow C, Makowski L, Joseph Jerry D, Smith Schneider S, et al. Normal breast tissue of obese women is enriched for macrophage markers and macrophage-associated gene expression. Breast Cancer Res Treat 2012;131(3):1003–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol 2004;15(11):2792–800. [DOI] [PubMed] [Google Scholar]
- 31.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112(12):1796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quigley DA, Tahiri A, Luders T, Riis MH, Balmain A, Borresen-Dale AL, et al. Age, estrogen, and immune response in breast adenocarcinoma and adjacent normal tissue. Oncoimmunology 2017;6(11):e1356142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.San KMM, Fahmida U, Wijaksono F, Lin H, Zaw KK, Htet MK. Chronic low grade inflammation measured by dietary inflammatory index and its association with obesity among school teachers in Yangon, Myanmar. Asia Pac J Clin Nutr 2018;27(1):92–98. [DOI] [PubMed] [Google Scholar]
- 34.Okuyama T, Akechi T, Kugaya A, Okamura H, Imoto S, Nakano T, et al. Factors correlated with fatigue in disease-free breast cancer patients: application of the Cancer Fatigue Scale. Support Care Cancer 2000;8(3):215–22. [DOI] [PubMed] [Google Scholar]
- 35.Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol 2000;18(4):743–53. [DOI] [PubMed] [Google Scholar]
- 36.Reuter K, Classen CC, Roscoe JA, Morrow GR, Kirshner JJ, Rosenbluth R, et al. Association of coping style, pain, age and depression with fatigue in women with primary breast cancer. Psychooncology 2006;15(9):772–9. [DOI] [PubMed] [Google Scholar]
- 37.Tabrizi FM, Alizadeh S. Cancer Related Fatigue in Breast Cancer Survivors: in Correlation to Demographic Factors. Maedica (Buchar) 2017;12(2):106–111. [PMC free article] [PubMed] [Google Scholar]
- 38.Baker F, Denniston M, Smith T, West MM. Adult cancer survivors: how are they faring? Cancer 2005;104(11 Suppl):2565–76. [DOI] [PubMed] [Google Scholar]
- 39.Matulonis UA, Kornblith A, Lee H, Bryan J, Gibson C, Wells C, et al. Long-term adjustment of early-stage ovarian cancer survivors. Int J Gynecol Cancer 2008;18(6):1183–93. [DOI] [PubMed] [Google Scholar]
- 40.Mao JJ, Armstrong K, Bowman MA, Xie SX, Kadakia R, Farrar JT. Symptom burden among cancer survivors: impact of age and comorbidity. J Am Board Fam Med 2007;20(5):434–43. [DOI] [PubMed] [Google Scholar]