Abstract
Congenital infections with pathogens such as Zika virus, Toxoplasma gondii, Listeria monocytogenes, Treponema pallidium, parvovirus, HIV, varicella zoster virus, Rubella, Cytomegalovirus, and Herpesviruses are a major cause of morbidity and mortality worldwide. Despite the devastating impact of microbial infections on the developing fetus, relatively little is known about how pathogens associated with congenital disease breach the placental barrier to transit vertically during human pregnancy. In this review, we focus on transplacental transmission of pathogens during human gestation. We introduce the structure of the human placenta and describe the innate mechanisms by which the placenta restricts microbial access to the intrauterine compartment. Based on current knowledge, we also discuss the potential pathways employed by microorganisms to overcome the placental barrier and prospects for the future.
Introduction
During human pregnancy, the vertical transmission of infectious agents from an infected mother to her fetus can lead to devastating consequences in the developing fetus. Vertical transmission can be antenatal (before birth), perinatal (weeks immediately prior to or after birth) or postnatal (after birth). Antenatal fetal infections are major causes of global morbidity and mortality. In this review, we focus on proposed antenatal mechanisms of transplacental transmission of known “TORCH” pathogens, which includes Toxoplasma gondii, other (Listeria monocytogenes, Treponema pallidium, parvovirus, HIV, varicella zoster virus, amongst others), Rubella, Cytomegalovirus (CMV), and Herpesviruses (HSV) 1 and 2, and more recently Zika virus (ZIKV).
In the U.S., there are approximately 4 million deliveries each year, and in most of these cases, there is no evidence of in utero infection. However, infections with TORCH pathogens continue to occur. This issue came into focus last year when it became clear that ZIKV infection of pregnant women was associated with high rates of microcephaly and other congenital anomalies in their infants. Although ZIKV has received considerable attention since its recognition in the Western Hemisphere and is the newest mircroorganism classified as a TORCH pathogen (Schwartz, 2017), vertical transmission of other infectious agents is linked to adverse pregnancy outcomes worldwide, particularly in resource-limited countries where access to adequate prenatal care is scarce. Even in the United States, maternal infections with TORCH pathogens is associated with significant fetal disease. For example, between 400-4000 infants are affected with congenital toxoplasmosis annually. T. gondii infections in utero can be associated with pregnancy loss (miscarriage or stillbirth) or severe disease in the neonate, including blindness, developmental delay, or neurologic manifestations such as epilepsy. In many cases, neonates infected in utero display no obvious symptoms at birth but develop disabilities later in life, which are mostly ocular or neural in nature, including developmental delay and intellectual impairment (Faucher et al., 2012; Koppe et al., 1986; Lindsay and Dubey, 2011; Peyron et al., 2011). The magnitude of disability caused by congenital human CMV (HCMV) infection is immense. Approximately 40,000 children are born in the United States each year with congenital HCMV infection, and as many as 8000 of these require complex medical and surgical care (Cannon and Davis, 2005). These statistics highlight the need to better define the pathways by which infectious agents are vertically transmitted, which can be used to design therapeutic approaches to limit these events.
The placenta
To understand the mechanisms of vertical transmission, it is essential to define the structure of the human placenta, which forms the primary barrier between the maternal and fetal compartments throughout pregnancy. The human placenta is composed of fetal cells and is characterized by a close association between fetal-derived trophoblasts and the maternal tissues that they come into contact with. In humans and other eutherian organisms, the maternal-fetal interface consists of fetal-derived trophoblast progenitor cells which differentiate into cytotrophoblasts (CTBs) and have a proliferative capacity, and syncytiotrophoblasts, which are terminally differentiated, fused, multi-nucleated cells. In humans, the placenta begins to form within 5-6 days post fertilization, when a layer of syncytialized trophoblasts surrounds the blastocyst and begins to anchor into the maternal endometrium. By 10-12 weeks of gestation, the maternal circulation has been remodeled through the formation of spiral arteries and the human placenta becomes hemochorial, which indicates direct contact between maternal blood and the placenta. Together, trophoblasts form the placental villi and mediate the maternal–fetal exchange of gases, nutrients, and waste products (Figure 1A-C). (Cross et al., 1994; Hamilton and Boyd, 1960; Weisblum et al., 2011). The human placenta differentiates into two types of villi—floating and anchoring (Figure 1C). Floating villi are formed by an inner layer of CTBs that are covered by a layer of syncytiotrophoblasts, which are bathed in maternal blood flowing into the intervillous space (IVS). The IVS can contain as much as 500mL of maternal blood, exposing the villous surfaces to microbes that might be present in maternal blood. Unlike floating villi, anchoring villi are attached to maternal decidual tissue (termed the decidua basalis, Figure 1A) by highly invasive CTBs called extravillous trophoblasts (EVTs) that form cell columns at the distal ends of the anchoring villi. EVTs that directly invade the decidua basalis and myometrium are referred to as interstitial EVTs whereas those that invade the maternal vasculature (spiral arteries) are referred to as endovascular EVTs. The decidua capsularis, in turn, forms the endometrial layer in direct contact with the chorioamniotic sac and diminishes through gestation and the decidua parietalis lines the entire uterus (Figure 1A). EVTs directly invade the decidua basalis, anchoring the placenta into the uterine implantation site where the EVTs are directly juxtaposed to maternal immune cells. On both floating and anchoring villi, syncytiotrophoblasts, which are formed by the fusion of the underlying CTBs, form the outermost cell layer and thus compose the key interface between maternal and fetal blood (Figure 1D). The core of the chorionic villous consists of fetal macrophages (termed Hofbauer cells), placental fibroblasts, and fetal endothelial cells lining villous capillaries and their associated basement membranes, which are thereby protected by the trophoblast cell layers from microbes in maternal blood.
Figure 1. Structure and cellular composition of the human placenta.
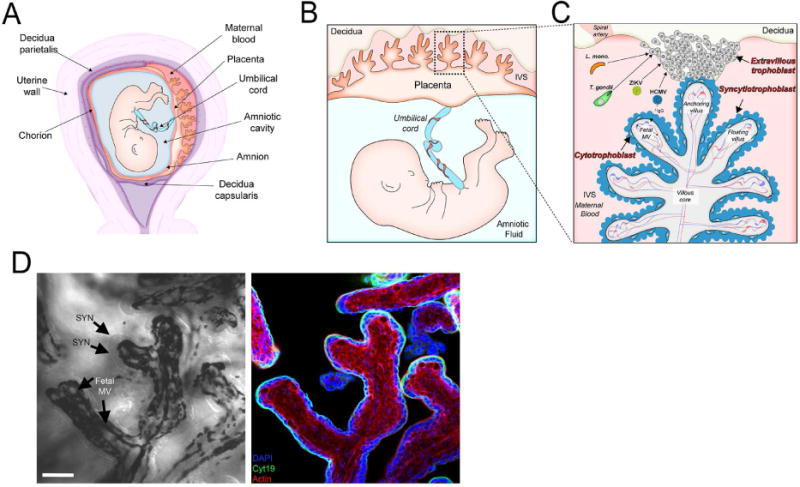
(A) Schematic of the uterine cavity during pregnancy. The developing fetus is encased within the amniotic cavity, surrounded by the chorion and amnion, and anchored to the maternal decidua by the placenta (at the site of attachment, the decidua basalis). (B) Maternal blood fills the intervillous space (IVS) via spiral arteries that bathe the surfaces of the placenta in maternal blood (once the maternal microvasculature has been established). (C) The human hemochorial placenta is formed by villous trees composed of both floating villi and anchoring villi, which attach directly to the decidua basalis by the invasion of extravillous trophoblasts (EVTs). The human placenta villous trees are covered by syncytiotrophoblasts, with a layer of cytotrophoblasts (which become discontinuous throughout pregnancy) below this layer. Several pathogens, including Listeria monocytogenes (L. mono), Toxoplasma gondii (T. gondii), human CMV (HCMV), and Zika virus (ZIKV), are thought to access the villous core following replication in EVTs. (D) Left, differential interference contrast (DIC) image of a floating villous from an ~ 20-week gestation placenta highlighting the syncytiotrophoblast (SYN) layer and underlying fetal microvasculature (MV). Right, confocal micrograph of cytokeratin-19-positive SYNs and the underlying cytokeratin-19-negative core of the villous trees. Scale bar, 50 μm.
Given its function as the sole interface between the maternal and fetal environments, the placenta protects the fetus from pathogens present in maternal blood. Yet, the defense mechanisms used by the placenta to limit microbial access to the fetus are largely undefined. Syncytiotrophoblasts provide potent protection against both viral and nonviral pathogens. In addition, CTBs also possess innate defense mechanisms against intracellular pathogens. Strategies used by pathogens to bypass these trophoblast-intrinsic defenses are varied, but common themes are emerging.
The maternal decidua is a specialized endometrium that makes up the implantation site. The decidua is a multicellular structure consisting of maternal cells and fetal interstitially invading extravillous CTBs. The decidua basalis consists of approximately 40% immune cells, 70% of which are unique decidual natural killer (NK) cells (dNK), 20 to 25% are macrophages, and 3 to 10% are T cells (Bulmer et al., 1988; King et al., 1997; Manaster and Mandelboim, 2010; Mor et al., 2006; Wicherek et al., 2009). The decidua basalis, apart from being a site of placental implantation, also functions in immunological tolerance of the semi-allogeneic fetal origin trophoblasts, which lie in direct contact with these maternal cells. The decidua is thought to maintain its immune privilege by virtue of its cell composition, which is characterized by limited lymphocyte access and control of cell trafficking by the tight regulation of local chemokine expression (Nancy et al., 2012; Red-Horse et al., 2004), which is maintained by adaptation of both fetal and maternal cells (Arck and Hecher, 2013; Erlebacher, 2013; Mor and Cardenas, 2010; Zenclussen, 2013). The expression of human leukocyte antigen (HLA)-G exclusively on extravillous trophoblasts (Chumbley et al., 1993; Proll et al., 1999; Yelavarthi et al., 1991) has been shown to play a role in maternal tolerance of the semiallogenic fetus. In addition, HLA-G expression on the stromal cells of the decidua may also play a direct role in maintaining fetal tolerance (Blanco et al., 2008).
Humans have a villous placenta with only one layer of syncytiotrophoblasts bathed in maternal blood. In contrast, mice have a labyrinthine placenta with two layers of syncytiotrophoblasts and one complete layer of mononucleated cytotrophoblasts, with the cytotrophoblast layer being in direct contact with maternal blood (Maltepe et al., 2010). Given the three layers of cells, the mouse placenta is referred to as a hemotrichorial placenta. These differences between mouse and human placental cellular structures may make it difficult to directly translate findings made using mouse models to studies of vertical transmission pathways in human pregnancy.
Syncytiotrophoblaste as a barrier to vertical transmission
The syncytiotrophoblast layer is highly resistant to infection by numerous pathogens, including Listeria monocytogenes, Toxoplasma gondii, and viruses such as HCMV, HSV1, and ZIKV (Bayer et al., 2015; Bayer et al., 2016; Delorme-Axford et al., 2013; Fisher et al., 2000; Koi et al., 2002; Maidji et al., 2006; Maidji et al., 2010; Robbins et al., 2010). Primary syncytiotrophoblasts isolated from full-term placentas potently resist infection by diverse viruses and confer broad antiviral resistance to non-trophoblast cells through effectors that operate in a paracrine manner (Bayer et al., 2015; Bayer et al., 2016; Delorme-Axford et al., 2013; Ouyang et al., 2016). Accordingly, the syncytiotrophoblast layer of first-trimester chorionic villi is largely resistant to HCMV infection, whereas CTBs and other cells of the villous core are susceptible (Fisher et al., 2000). Amniotic epithelium and CTBs of chorionic villi isolated from mid- and late-gestation placentas as well as explants from first-trimester chorionic villi are susceptible to ZIKV infection, whereas the syncytium is spared (Tabata et al., 2016). Interestingly, higher ZIKV titers are observed in amniotic epithelial cells from mid-gestation compared to late-gestation placentas, indicating a greater susceptibility to infection in the early placenta. Consistent with this, primitive trophoblasts, which represent the earliest phases of trophoblast development, are sensitive to ZIKV, but become increasingly resistant as the syncytium forms, suggesting that trophoblasts that form very early in gestation are sensitive to ZIKV (Sheridan et al., 2017).
Resistance of syncytiotrophoblasts to microbial infection is not specific to viruses. For example, T. gondii is reduced in partially syncitialized primary human trophoblasts relative to those with no syncytilization. (Abbasi et al., 2003). In addition, T.gondii infection across the syncytiotrophoblast layer appears to be rare in vivo (Buxton and Finlayson, 1986; Ferro et al., 2002; Shiono et al., 2007). Damage of the syncytium would allow parasite access to the villous core, which might increase parasite infection (Robbins et al., 2012). This process may occur in the context of a secondary infection and could be exacerbated by trauma. The effects may also depend on gestational time, as the layer of subsyncytial CTBs grows thinner and partially discontinuous after the first trimester (Jones et al., 2008; Mori et al., 2007). Remarkably, T.gondii is incapable of infecting syncytiotrophoblasts, but replicates well in underlying subsyncytial cytotrophoblasts (Robbins et al., 2012), suggesting that in the stages of pregnancy associated with an intact CTB layer, T. gondii targets these cells following its breach of the syncytium. The restriction of T. gondii replication in syncytiotrophoblasts is particularly striking given that the parasite is thought to replicate in all nucleated cells. Similarly, syncytiotrophoblasts also are highly resistant to L. monocytogenes infection in human first trimester placental explants by either internalin-mediated invasion or cell-to-cell spread (Robbins et al., 2010).
HCMV can infect early and late gestation placentas (McDonagh et al., 2004; Mostoufi-zadeh et al., 1984; Muhlemann et al., 1992; Pereira et al., 2005; Revello and Gerna, 2004; Trincado et al., 2005). Importantly, most of the HCMV nucleic acids are observed in CTBs and only rarely in syncytiotrophoblasts. Concordantly, HCMV can infect cultured CTBs and syncytiotrophoblasts, albeit at low efficiency (Fisher et al., 2000; Halwachs-Baumann et al., 2006; Halwachs-Baumann et al., 1998; Hemmings and Guilbert, 2002; Hemmings et al., 1998; Maidji et al., 2002; Pereira et al., 2005; Revello and Gerna, 2004; Schleiss et al., 2007). However, these studies used lab-adapted as opposed to clinical HCMV strains and obtained CTBs from full term placentas, which may account for the modest observed replication efficiency. Thus, studies of diverse microorganisms point to a barrier formed by syncytiotrophoblasts that limits vertical transmission of pathogens into the fetal compartment at multiple stages of gestation.
Syncytiotrophoblast defenses
What are the mechanisms by which syncytiotrophoblasts restrict the growth of diverse pathogens? In most cases, the mechanistic basis for this restriction is unclear, largely given the difficulties in working with trophoblasts at various stages of gestation and modeling the human placenta using small animals. In experiments with primary syncytiotrophoblasts from full term placentas, we discovered that these cells resist viral infections and transfer this resistance to non-placental cells in a paracrine manner through pathways involving placental-specific miRNAs packaged in exosomes as well as type III interferons (IFNs) (Bayer et al., 2015; Bayer et al., 2016; Delorme-Axford et al., 2013; Ouyang et al., 2016). Others have shown that the physical properties of the syncytium function in the restriction of microbial infections. The syncytial surface has unique physical properties, such as the presence of dense, branched microvilli at the apical surface and a complex cortical actin network that might limit microbial invasion (Cantle et al., 1987; Fisher et al., 2000; Koi et al., 2002; Maidji et al., 2010; McDonagh et al., 2006; Robbins et al., 2010; Zeldovich et al., 2013; Zeldovich et al., 2011). Indeed, disruption of the actin cytoskeleton slightly enhances invasion of L. monocytogenes (Zeldovich et al., 2013), suggesting that the syncytium employs direct physical barriers to restrict pathogen infections.
The human placenta also expresses high levels of other antimicrobial defense components, such as the antimicrobial peptides defensins (King et al., 2007; Svinarich et al., 1997), toll-like receptors (TLRs) (Ma et al., 2007; Patni et al., 2009; Pudney et al., 2016; Tangeras et al., 2014), and nucleotide-binding oligomerization domain (NOD) proteins (Costello et al., 2007). However, whether these components play active roles in defending the human placenta from microbial infections in vivo remains unclear. Importantly, the expression of these components differs between primary human trophoblasts and trophoblast cell lines (Pudney et al., 2016), which may further limit the usefulness of these models for the study of microbial vertical transmission.
Bypassing the syncytiotrophoblast layer
It is unclear how pathogens might breach the syncytiotrophoblast layer to reach the underlying villous core. Placental villous inflammation associated with multiple pathogens including T. gondii induces syncytial expression of ICAM-1 and subsequent monocyte binding to the syncytial surface, which might lead to inflammatory syncytial damage (Juliano et al., 2006). However, increased ICAM-1 expression likely occurs in response to infection rather than providing the cause. Therefore, infection with one pathogen could predispose to infection with another via immune-mediated breakdown of the syncytium, as has been suggested (Mor and Cardenas, 2010).
CTBs can differentiate along two pathways. In floating villi, CTBs differentiate into syncytiotrophoblasts, aiding in the expansion of this continuous and ever expanding layer. In anchoring villi, CTBs differentiate into invasive EVTs, which are specialized cells that invade the uterine implantation site and contact maternal cells. EVTs represent a possible pathway into the placenta and are targeted by several microbes to facilitate vertical transmission. Since the maternal decidua has a multicellular phenotype composed of EVTs, uterine epithelium, stroma, endothelial cells and a variety of immune cells, several cell types that comprise the decidua may serve as replication sites for vertically transmitted pathogens.
Unlike syncytiotrophoblasts, EVTs are susceptible to infection by all three strains T. gondii (Robbins et al., 2012). Remarkably, EVTs occupy less than 5% of the surface area of the first trimester placental explants, but they contained 80% of the T. gondii parasitophorous vacuoles. T. gondii also may enter into the villous core by first infecting maternal immune cells that are enriched in the decidua, which might then facilitate parasite transfer to EVTs following this initial infection. This mechanism is consistent with data from in vivo studies (Buxton and Finlayson, 1986; Ferro et al., 2002; Shiono et al., 2007) demonstrating that maternal leukocytes in the uterine decidua are initially infected with T. gondii. This infection is followed temporally by infection of the trophoblast giant cells (Ferro et al., 2002). There is an 8-day delay between initial detection of T. gondii in the mouse placenta and the fetus, suggesting that infection begins in the blood, perhaps in maternal immune cells that subsequently transfer the parasite to EVTs or other cell types (Shiono et al., 2007). T. gondii cannot be detected in the mouse uterus, placenta, or fetus until 7 days following inoculation, also supporting a delay (Pezerico et al., 2009). In sheep experimentally infected with T. gondii, necrotic foci are initially detected in the uterus and then later observed in the placental villi (Buxton and Finlayson, 1986). Thus, the syncytiotrophoblast layer is not a significant site of T. gondii vertical transmission. Instead, the parasite likely first infects the decidua and then passes to the EVTs and subsequently to the villous core and fetal vasculature, such that infection of this interface occurs at approximately the same time as fetal infection.
Studies of placental explant infection by L. monocytogenes also are consistent with the preferential targeting of EVTs to allow entry into the intrauterine space. L. monocytogenes can infect non-phagocytic cells by two means—direct invasion or cell-to-cell spread. Syncytiotrophoblasts in first-trimester explants are resistant to infection, whereas EVTs, which express E-cadherin, are the preferred site for bacterial infection (Robbins et al., 2010). EVTs eliminate 80% of the intracellular bacteria in 24 hours following inoculation via a mechanism that involves lysosomal-mediated degradation. Thus, although EVTs are a primary site of infection in the placenta, they also limit the spread of L. monocytogenes by inhibiting vacuolar escape (Zeldovich et al., 2011).
ZIKV appears to bypass the syncytium by virtue of its capacity to replicate in EVTs (Tabata et al., 2016), although other placental cell types also can support ZIKV replication. Therefore, in the case of ZIKV, multiple cell types might support viral replication, which could differ with gestational age, virus access to target cells, or both parameters. Given that maternal immune cells are likely to be targeted by ZIKV, the virus also might use these cells to reach EVTs to access the villous core. It is also important to note that ZIKV can be sexually transmitted, which would allow it to bypass the trophoblast layers entirely (D’Ortenzio et al., 2016; Hills et al., 2016).
For HCMV to reach CTBs, where replication occurs, the virus must first breach the syncytium. This breach could be facilitated by transcytosis of virions across the syncytium rather than by their direct infection. Syncytiotrophoblasts express the neonatal Fc receptor (FcRn), which transports IgG at high levels in the second half of pregnancy (Bright and Ockleford, 1995; Dancis et al., 1961; Garty et al., 1994; Gusdon, 1969; Malek et al., 1996). Immunohistochemical analysis of HCMV infection in human first-trimester floating and anchoring placental villi explants indicates that HCMV replicates in underlying villous CTBs, whereas the syncytiotrophoblasts are spared. Infection is detected in placentas with low-to-moderate CMV-specific neutralizing antibody titers, which is consistent with virion transcytosis across the syncytium via FcRn-mediated transcytosis (Fisher et al., 2000; Gabrielli et al., 2001; Maidji et al., 2007; Maidji et al., 2006; Pereira et al., 2005).
In addition to the antibody-mediated mechanisms described above, EVTs also might be targeted by HCMV to bypass the syncytial layer. Many cell types in ex vivo decidual organ cultures allow HCMV replication, including EVTs, microvasculature, and immune cells such as macrophages and dendritic cells (Weisblum et al., 2011). Therefore, in addition to EVTs, HCMV might target multiple cell types located in the decidua to reach the EVT layer. Concordantly, a wide range of cells in ex vivo human first-trimester decidual organ cultures are infected by both clinical and laboratory-adapted strains of HCMV (Weisblum et al., 2011). Notably, viral transmission in these cultures appears to be mediated by cell-to-cell spread. This pattern of tissue-associated spread, which is observed for low-passage clinical isolates as well as laboratory-adapted strains, closely mirrors the mode of HCMV spread in vivo and could confer a replication advantage within solid tissue, while potentially facilitating immune evasion (Weisblum et al., 2014).
Hofbauer cells are placenta-specific macrophages and are a potential target for ZIKV infection once it breaches the syncytium (Quicke et al., 2016). Once within the villous core where it replicates in Hofbauer cells, ZIKV may access the fetal compartment by spreading from the parietal decidua to the amniochorionic membrane (Tabata et al., 2016). Hofbauer cells also may facilitate HIV vertical transmission given that an HIV coreceptor, dendritic cell specific intercellular adhesion molecule-3-grabbing non integrin (DC-SIGN), is expressed on these cells, and DC-SIGN polymorphisms are associated with risk of mother-to-child HIV transmission (Boily-Larouche et al., 2012). However, studies of animal and human fetuses indicate that there is almost no HIV transmission during the first and second trimesters of pregnancy (Brossard et al., 1995; Van Dyke et al., 1999), suggesting that this process has a limited biological role.
Final thoughts
Despite the growing number of pathogens associated with in utero fetal disease, the mechanistic basis of vertical transmission of infection across the placental barrier is unclear. Undoubtedly, the difficulty in establishing laboratory models that recapitulate the complexities of vertical transmission in humans, particularly at all stages of gestation, is a major roadblock to understanding mechanisms of vertical transmission. The placentas of many small animals display significant anatomic differences relative to the human placenta, which complicates an assessment of whether findings made using these systems mirror the biology in humans. In addition, in vitro trophoblast cell lines, such as BeWo and JEG-3 cells, do not spontaneously fuse to form syncytia and do not recapitulate the resistance of the placenta to microbial infections (Bayer et al., 2016; McConkey et al., 2016). While many groups, including ours, use primary trophoblast models, these culture systems may not represent cells at all gestational ages and do not recapitulate the structure of placental villi or contain signals from maternal blood that might act on these cells in a paracrine manner. Although explant models maintain villous structure, these systems are limited in the capacity to recapitulate the gestational ages of human pregnancy and also lack exposure to maternal factors. Furthermore, both primary cells and explant models are not genetically tractable, which limits mechanistic studies. Thus, it is essential that we recognize the limitations of models used in studies of microbial vertical transmission in humans and interpret our findings accordingly.
The placenta is arguably the most critical barrier in human life, yet the precise mechanisms by which microbes breach this barrier remain largely unknown. While it is possible that pathogens associated with congenital disease employ a common mechanism, it is more likely that these microbes have evolved distinct strategies, which might vary throughout gestation or with the level of maternal infection or corresponding immune responses. This is particularly true for ZIKV, which seems to be uniquely capable of penetrating the placental barrier at a variety of gestational ages (Brasil et al., 2016). Although the ZIKV outbreak appears to be waning, the critical role of the placenta in restricting vertical transmission of microbes must not be overlooked, and the mechanisms by which this organ does so should remain the focus of future investigation. Defining the means by which the placenta limits fetal infections is likely to provide important insights about previously unknown host-defense pathways and enhance knowledge about how these pathways could be targeted therapeutically.
Acknowledgments
Our work on the human placenta is supported by NIH R01 HD075665 [C.B.C. and Y.S.] and a Burroughs Wellcome Investigators in the Pathogenesis of Infectious Disease Award [C.B.C]. Additional support comes from NIH R01 AI081759 [C.B.C.] and R01 AI123348 [T.S.D.].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- Abbasi M, Kowalewska-Grochowska K, Bahar MA, Kilani RT, Winkler-Lowen B, Guilbert LJ. Infection of placental trophoblasts by Toxoplasma gondii. J Infect Dis. 2003;188:608–616. doi: 10.1086/377132. [DOI] [PubMed] [Google Scholar]
- Arck PC, Hecher K. Fetomaternal immune cross-talk and its consequences for maternal and offspring’s health. Nat Med. 2013;19:548–556. doi: 10.1038/nm.3160. [DOI] [PubMed] [Google Scholar]
- Bayer A, Delorme-Axford E, Sleigher C, Frey TK, Trobaugh DW, Klimstra WB, Emert-Sedlak LA, Smithgall TE, Kinchington PR, Vadia S, et al. Human trophoblasts confer resistance to viruses implicated in perinatal infection. Am J Obstet Gynecol. 2015;212:71 e71–78. doi: 10.1016/j.ajog.2014.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer A, Lennemann NJ, Ouyang Y, Bramley JC, Morosky S, Marques ET, Jr, Cherry S, Sadovsky Y, Coyne CB. Type III Interferons Produced by Human Placental Trophoblasts Confer Protection against Zika Virus Infection. Cell Host Microbe. 2016;19:705–712. doi: 10.1016/j.chom.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco O, Tirado I, Munoz-Fernandez R, Abadia-Molina AC, Garcia-Pacheco JM, Pena J, Olivares EG. Human decidual stromal cells express HLA-G: Effects of cytokines and decidualization. Hum Reprod. 2008;23:144–152. doi: 10.1093/humrep/dem326. [DOI] [PubMed] [Google Scholar]
- Boily-Larouche G, Milev MP, Zijenah LS, Labbe AC, Zannou DM, Humphrey JH, Ward BJ, Poudrier J, Mouland AJ, Cohen EA, et al. Naturally-occurring genetic variants in human DC-SIGN increase HIV-1 capture, cell-transfer and risk of mother-to-child transmission. PLoS One. 2012;7:e40706. doi: 10.1371/journal.pone.0040706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil P, Pereira JP, Jr, Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, Rabello RS, Valderramos SG, Halai UA, Salles TS, et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro. N Engl J Med. 2016;375:2321–2334. doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright NA, Ockleford CD. Cytotrophoblast cells: a barrier to maternofetal transmission of passive immunity. J Histochem Cytochem. 1995;43:933–944. doi: 10.1177/43.9.7642966. [DOI] [PubMed] [Google Scholar]
- Brossard Y, Aubin JT, Mandelbrot L, Bignozzi C, Brand D, Chaput A, Roume J, Mulliez N, Mallet F, Agut H, et al. Frequency of early in utero HIV-1 infection: a blind DNA polymerase chain reaction study on 100 fetal thymuses. AIDS. 1995;9:359–366. [PubMed] [Google Scholar]
- Bulmer JN, Pace D, Ritson A. Immunoregulatory cells in human decidua: morphology, immunohistochemistry and function. Reprod Nutr Dev. 1988;28:1599–1613. doi: 10.1051/rnd:19881006. [DOI] [PubMed] [Google Scholar]
- Buxton D, Finlayson J. Experimental infection of pregnant sheep with Toxoplasma gondii: pathological and immunological observations on the placenta and foetus. J Comp Pathol. 1986;96:319–333. doi: 10.1016/0021-9975(86)90052-6. [DOI] [PubMed] [Google Scholar]
- Cannon MJ, Davis KF. Washing our hands of the congenital cytomegalovirus disease epidemic. BMC Public Health. 2005;5:70. doi: 10.1186/1471-2458-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantle SJ, Kaufmann P, Luckhardt M, Schweikhart G. Interpretation of syncytial sprouts and bridges in the human placenta. Placenta. 1987;8:221–234. doi: 10.1016/0143-4004(87)90046-4. [DOI] [PubMed] [Google Scholar]
- Chumbley G, King A, Holmes N, Loke YW. In situ hybridization and northern blot demonstration of HLA-G mRNA in human trophoblast populations by locus-specific oligonucleotide. Hum Immunol. 1993;37:17–22. doi: 10.1016/0198-8859(93)90138-q. [DOI] [PubMed] [Google Scholar]
- Costello MJ, Joyce SK, Abrahams VM. NOD protein expression and function in first trimester trophoblast cells. Am J Reprod Immunol. 2007;57:67–80. doi: 10.1111/j.1600-0897.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- Cross JC, Werb Z, Fisher SJ. Implantation and the placenta: key pieces of the development puzzle. Science. 1994;266:1508–1518. doi: 10.1126/science.7985020. [DOI] [PubMed] [Google Scholar]
- D’Ortenzio E, Matheron S, Yazdanpanah Y, de Lamballerie X, Hubert B, Piorkowski G, Maquart M, Descamps D, Damond F, Leparc-Goffart I. Evidence of Sexual Transmission of Zika Virus. N Engl J Med. 2016;374:2195–2198. doi: 10.1056/NEJMc1604449. [DOI] [PubMed] [Google Scholar]
- Dancis J, Lind J, Oratz M, Smolens J, Vara P. Placental transfer of proteins in human gestation. Am J Obstet Gynecol. 1961;82:167–171. doi: 10.1016/s0002-9378(16)36111-7. [DOI] [PubMed] [Google Scholar]
- Delorme-Axford E, Donker RB, Mouillet JF, Chu T, Bayer A, Ouyang Y, Wang T, Stolz DB, Sarkar SN, Morelli AE, et al. Human placental trophoblasts confer viral resistance to recipient cells. Proc Natl Acad Sci U S A. 2013;110:12048–12053. doi: 10.1073/pnas.1304718110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlebacher A. Immunology of the maternal-fetal interface. Annu Rev Immunol. 2013;31:387–411. doi: 10.1146/annurev-immunol-032712-100003. [DOI] [PubMed] [Google Scholar]
- Faucher B, Garcia-Meric P, Franck J, Minodier P, Francois P, Gonnet S, L’Ollivier C, Piarroux R. Long-term ocular outcome in congenital toxoplasmosis: a prospective cohort of treated children. J Infect. 2012;64:104–109. doi: 10.1016/j.jinf.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Ferro EA, Silva DA, Bevilacqua E, Mineo JR. Effect of Toxoplasma gondii infection kinetics on trophoblast cell population in Calomys callosus, a model of congenital toxoplasmosis. Infect Immun. 2002;70:7089–7094. doi: 10.1128/IAI.70.12.7089-7094.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher S, Genbacev O, Maidji E, Pereira L. Human cytomegalovirus infection of placental cytotrophoblasts in vitro and in utero: implications for transmission and pathogenesis. J Virol. 2000;74:6808–6820. doi: 10.1128/jvi.74.15.6808-6820.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielli L, Losi L, Varani S, Lazzarotto T, Eusebi V, Landini MP. Complete replication of human cytomegalovirus in explants of first trimester human placenta. J Med Virol. 2001;64:499–504. doi: 10.1002/jmv.1077. [DOI] [PubMed] [Google Scholar]
- Garty BZ, Ludomirsky A, Danon YL, Peter JB, Douglas SD. Placental transfer of immunoglobulin G subclasses. Clin Diagn Lab Immunol. 1994;1:667–669. doi: 10.1128/cdli.1.6.667-669.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusdon JP., Jr Fetal and maternal immunoglobulin levels during pregnancy. Am J Obstet Gynecol. 1969;103:895–900. doi: 10.1016/s0002-9378(16)34434-9. [DOI] [PubMed] [Google Scholar]
- Halwachs-Baumann G, Weihrauch G, Gruber HJ, Desoye G, Sinzger C. hCMV induced IL-6 release in trophoblast and trophoblast like cells. J Clin Virol. 2006;37:91–97. doi: 10.1016/j.jcv.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Halwachs-Baumann G, Wilders-Truschnig M, Desoye G, Hahn T, Kiesel L, Klingel K, Rieger P, Jahn G, Sinzger C. Human trophoblast cells are permissive to the complete replicative cycle of human cytomegalovirus. J Virol. 1998;72:7598–7602. doi: 10.1128/jvi.72.9.7598-7602.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton WJ, Boyd JD. Development of the human placenta in the first three months of gestation. J Anat. 1960;94:297–328. [PMC free article] [PubMed] [Google Scholar]
- Hemmings DG, Guilbert LJ. Polarized release of human cytomegalovirus from placental trophoblasts. J Virol. 2002;76:6710–6717. doi: 10.1128/JVI.76.13.6710-6717.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings DG, Kilani R, Nykiforuk C, Preiksaitis J, Guilbert LJ. Permissive cytomegalovirus infection of primary villous term and first trimester trophoblasts. J Virol. 1998;72:4970–4979. doi: 10.1128/jvi.72.6.4970-4979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills SL, Russell K, Hennessey M, Williams C, Oster AM, Fischer M, Mead P. Transmission of Zika Virus Through Sexual Contact with Travelers to Areas of Ongoing Transmission - Continental United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:215–216. doi: 10.15585/mmwr.mm6508e2. [DOI] [PubMed] [Google Scholar]
- Jones CJ, Harris LK, Whittingham J, Aplin JD, Mayhew TM. A reappraisal of the morphophenotype and basal lamina coverage of cytotrophoblasts in human term placenta. Placenta. 2008;29:215–219. doi: 10.1016/j.placenta.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Juliano PB, Blotta MH, Altemani AM. ICAM-1 is overexpressed by villous trophoblasts in placentitis. Placenta. 2006;27:750–757. doi: 10.1016/j.placenta.2005.07.008. [DOI] [PubMed] [Google Scholar]
- King A, Loke YW, Chaouat G. NK cells and reproduction. Immunol Today. 1997;18:64–66. doi: 10.1016/s0167-5699(97)01001-3. [DOI] [PubMed] [Google Scholar]
- King AE, Paltoo A, Kelly RW, Sallenave JM, Bocking AD, Challis JR. Expression of natural antimicrobials by human placenta and fetal membranes. Placenta. 2007;28:161–169. doi: 10.1016/j.placenta.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Koi H, Zhang J, Makrigiannakis A, Getsios S, MacCalman CD, Strauss JF, 3rd, Parry S. Syncytiotrophoblast is a barrier to maternal-fetal transmission of herpes simplex virus. Biol Reprod. 2002;67:1572–1579. doi: 10.1095/biolreprod.102.004325. [DOI] [PubMed] [Google Scholar]
- Koppe JG, Loewer-Sieger DH, de Roever-Bonnet H. Results of 20-year follow-up of congenital toxoplasmosis. Lancet. 1986;1:254–256. doi: 10.1016/s0140-6736(86)90785-3. [DOI] [PubMed] [Google Scholar]
- Lindsay DS, Dubey JP. Toxoplasma gondii: the changing paradigm of congenital toxoplasmosis. Parasitology. 2011;138:1829–1831. doi: 10.1017/S0031182011001478. [DOI] [PubMed] [Google Scholar]
- Ma Y, Krikun G, Abrahams VM, Mor G, Guller S. Cell type-specific expression and function of toll-like receptors 2 and 4 in human placenta: implications in fetal infection. Placenta. 2007;28:1024–1031. doi: 10.1016/j.placenta.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidji E, Genbacev O, Chang HT, Pereira L. Developmental regulation of human cytomegalovirus receptors in cytotrophoblasts correlates with distinct replication sites in the placenta. J Virol. 2007;81:4701–4712. doi: 10.1128/JVI.02748-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidji E, McDonagh S, Genbacev O, Tabata T, Pereira L. Maternal antibodies enhance or prevent cytomegalovirus infection in the placenta by neonatal Fc receptor-mediated transcytosis. Am J Pathol. 2006;168:1210–1226. doi: 10.2353/ajpath.2006.050482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidji E, Nigro G, Tabata T, McDonagh S, Nozawa N, Shiboski S, Muci S, Anceschi MM, Aziz N, Adler SP, et al. Antibody treatment promotes compensation for human cytomegalovirus-induced pathogenesis and a hypoxia-like condition in placentas with congenital infection. Am J Pathol. 2010;177:1298–1310. doi: 10.2353/ajpath.2010.091210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidji E, Percivalle E, Gerna G, Fisher S, Pereira L. Transmission of human cytomegalovirus from infected uterine microvascular endothelial cells to differentiating/invasive placental cytotrophoblasts. Virology. 2002;304:53–69. doi: 10.1006/viro.2002.1661. [DOI] [PubMed] [Google Scholar]
- Malek A, Sager R, Kuhn P, Nicolaides KH, Schneider H. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am J Reprod Immunol. 1996;36:248–255. doi: 10.1111/j.1600-0897.1996.tb00172.x. [DOI] [PubMed] [Google Scholar]
- Maltepe E, Bakardjiev AI, Fisher SJ. The placenta: transcriptional, epigenetic, and physiological integration during development. J Clin Invest. 2010;120:1016–1025. doi: 10.1172/JCI41211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manaster I, Mandelboim O. The unique properties of uterine NK cells. Am J Reprod Immunol. 2010;63:434–444. doi: 10.1111/j.1600-0897.2009.00794.x. [DOI] [PubMed] [Google Scholar]
- McConkey CA, Delorme-Axford E, Nickerson CA, Kim KS, Sadovsky Y, Boyle JP, Coyne CB. A three-dimensional culture system recapitulates placental syncytiotrophoblast development and microbial resistance. Sci Adv. 2016;2:e1501462. doi: 10.1126/sciadv.1501462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonagh S, Maidji E, Chang HT, Pereira L. Patterns of human cytomegalovirus infection in term placentas: a preliminary analysis. J Clin Virol. 2006;35:210–215. doi: 10.1016/j.jcv.2005.08.011. [DOI] [PubMed] [Google Scholar]
- McDonagh S, Maidji E, Ma W, Chang HT, Fisher S, Pereira L. Viral and bacterial pathogens at the maternal-fetal interface. J Infect Dis. 2004;190:826–834. doi: 10.1086/422330. [DOI] [PubMed] [Google Scholar]
- Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol. 2010;63:425–433. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor G, Straszewski-Chavez SL, Abrahams VM. Macrophage-trophoblast interactions. Methods Mol Med. 2006;122:149–163. doi: 10.1385/1-59259-989-3:149. [DOI] [PubMed] [Google Scholar]
- Mori M, Ishikawa G, Luo SS, Mishima T, Goto T, Robinson JM, Matsubara S, Takeshita T, Kataoka H, Takizawa T. The cytotrophoblast layer of human chorionic villi becomes thinner but maintains its structural integrity during gestation. Biol Reprod. 2007;76:164–172. doi: 10.1095/biolreprod.106.056127. [DOI] [PubMed] [Google Scholar]
- Mostoufi-zadeh M, Driscoll SG, Biano SA, Kundsin RB. Placental evidence of cytomegalovirus infection of the fetus and neonate. Arch Pathol Lab Med. 1984;108:403–406. [PubMed] [Google Scholar]
- Muhlemann K, Miller RK, Metlay L, Menegus MA. Cytomegalovirus infection of the human placenta: an immunocytochemical study. Hum Pathol. 1992;23:1234–1237. doi: 10.1016/0046-8177(92)90290-j. [DOI] [PubMed] [Google Scholar]
- Nancy P, Tagliani E, Tay CS, Asp P, Levy DE, Erlebacher A. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science. 2012;336:1317–1321. doi: 10.1126/science.1220030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Y, Bayer A, Chu T, Tyurin VA, Kagan VE, Morelli AE, Coyne CB, Sadovsky Y. Isolation of human trophoblastic extracellular vesicles and characterization of their cargo and antiviral activity. Placenta. 2016;47:86–95. doi: 10.1016/j.placenta.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patni S, Wynen LP, Seager AL, Morgan G, White JO, Thornton CA. Expression and activity of Toll-like receptors 1-9 in the human term placenta and changes associated with labor at term. Biol Reprod. 2009;80:243–248. doi: 10.1095/biolreprod.108.069252. [DOI] [PubMed] [Google Scholar]
- Pereira L, Maidji E, McDonagh S, Tabata T. Insights into viral transmission at the uterine-placental interface. Trends Microbiol. 2005;13:164–174. doi: 10.1016/j.tim.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Peyron F, Garweg JG, Wallon M, Descloux E, Rolland M, Barth J. Long-term impact of treated congenital toxoplasmosis on quality of life and visual performance. Pediatr Infect Dis J. 2011;30:597–600. doi: 10.1097/INF.0b013e31820bb5f3. [DOI] [PubMed] [Google Scholar]
- Pezerico SB, Langoni H, Da Silva AV, Da Silva RC. Evaluation of Toxoplasma gondii placental transmission in BALB/c mice model. Exp Parasitol. 2009;123:168–172. doi: 10.1016/j.exppara.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Proll J, Blaschitz A, Hutter H, Dohr G. First trimester human endovascular trophoblast cells express both HLA-C and HLA-G. Am J Reprod Immunol. 1999;42:30–36. doi: 10.1111/j.1600-0897.1999.tb00462.x. [DOI] [PubMed] [Google Scholar]
- Pudney J, He X, Masheeb Z, Kindelberger DW, Kuohung W, Ingalls RR. Differential expression of toll-like receptors in the human placenta across early gestation. Placenta. 2016;46:1–10. doi: 10.1016/j.placenta.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quicke KM, Bowen JR, Johnson EL, McDonald CE, Ma H, O’Neal JT, Rajakumar A, Wrammert J, Rimawi BH, Pulendran B, et al. Zika Virus Infects Human Placental Macrophages. Cell Host Microbe. 2016;20:83–90. doi: 10.1016/j.chom.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Red-Horse K, Drake PM, Fisher SJ. Human pregnancy: the role of chemokine networks at the fetal-maternal interface. Expert Rev Mol Med. 2004;6:1–14. doi: 10.1017/S1462399404007720. [DOI] [PubMed] [Google Scholar]
- Revello MG, Gerna G. Pathogenesis and prenatal diagnosis of human cytomegalovirus infection. J Clin Virol. 2004;29:71–83. doi: 10.1016/j.jcv.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Robbins JR, Skrzypczynska KM, Zeldovich VB, Kapidzic M, Bakardjiev AI. Placental syncytiotrophoblast constitutes a major barrier to vertical transmission of Listeria monocytogenes. PLoS Pathog. 2010;6:e1000732. doi: 10.1371/journal.ppat.1000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins JR, Zeldovich VB, Poukchanski A, Boothroyd JC, Bakardjiev AI. Tissue barriers of the human placenta to infection with Toxoplasma gondii. Infect Immun. 2012;80:418–428. doi: 10.1128/IAI.05899-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleiss MR, Aronow BJ, Handwerger S. Cytomegalovirus infection of human syncytiotrophoblast cells strongly interferes with expression of genes involved in placental differentiation and tissue integrity. Pediatr Res. 2007;61:565–571. doi: 10.1203/pdr.0b013e318045be6d. [DOI] [PubMed] [Google Scholar]
- Schwartz DA. The Origins and Emergence of Zika Virus, the Newest TORCH Infection: What’s Old Is New Again. Arch Pathol Lab Med. 2017;141:18–25. doi: 10.5858/arpa.2016-0429-ED. [DOI] [PubMed] [Google Scholar]
- Sheridan MA, Yunusov D, Balaraman V, Alexenko AP, Yabe S, Verjovski-Almeida S, Schust DJ, Franz AW, Sadovsky Y, Ezashi T, et al. Vulnerability of primitive human placental trophoblast to Zika virus. Proc Natl Acad Sci U S A. 2017;114:E1587–E1596. doi: 10.1073/pnas.1616097114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiono Y, Mun HS, He N, Nakazaki Y, Fang H, Furuya M, Aosai F, Yano A. Maternal-fetal transmission of Toxoplasma gondii in interferon-gamma deficient pregnant mice. Parasitol Int. 2007;56:141–148. doi: 10.1016/j.parint.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Svinarich DM, Gomez R, Romero R. Detection of human defensins in the placenta. Am J Reprod Immunol. 1997;38:252–255. doi: 10.1111/j.1600-0897.1997.tb00511.x. [DOI] [PubMed] [Google Scholar]
- Tabata T, Petitt M, Puerta-Guardo H, Michlmayr D, Wang C, Fang-Hoover J, Harris E, Pereira L. Zika Virus Targets Different Primary Human Placental Cells, Suggesting Two Routes for Vertical Transmission. Cell Host Microbe. 2016;20:155–166. doi: 10.1016/j.chom.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangeras LH, Stodle GS, Olsen GD, Leknes AH, Gundersen AS, Skei B, Vikdal AJ, Ryan L, Steinkjer B, Myklebost MF, et al. Functional Toll-like receptors in primary first-trimester trophoblasts. J Reprod Immunol. 2014;106:89–99. doi: 10.1016/j.jri.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Trincado DE, Munro SC, Camaris C, Rawlinson WD. Highly sensitive detection and localization of maternally acquired human cytomegalovirus in placental tissue by in situ polymerase chain reaction. J Infect Dis. 2005;192:650–657. doi: 10.1086/431999. [DOI] [PubMed] [Google Scholar]
- Van Dyke RB, Korber BT, Popek E, Macken C, Widmayer SM, Bardeguez A, Hanson IC, Wiznia A, Luzuriaga K, Viscarello RR, et al. The Ariel Project: A prospective cohort study of maternal-child transmission of human immunodeficiency virus type 1 in the era of maternal antiretroviral therapy. J Infect Dis. 1999;179:319–328. doi: 10.1086/314580. [DOI] [PubMed] [Google Scholar]
- Weisblum Y, Panet A, Haimov-Kochman R, Wolf DG. Models of vertical cytomegalovirus (CMV) transmission and pathogenesis. Semin Immunopathol. 2014;36:615–625. doi: 10.1007/s00281-014-0449-1. [DOI] [PubMed] [Google Scholar]
- Weisblum Y, Panet A, Zakay-Rones Z, Haimov-Kochman R, Goldman-Wohl D, Ariel I, Falk H, Natanson-Yaron S, Goldberg MD, Gilad R, et al. Modeling of human cytomegalovirus maternal-fetal transmission in a novel decidual organ culture. J Virol. 2011;85:13204–13213. doi: 10.1128/JVI.05749-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicherek L, Basta P, Pitynski K, Marianowski P, Kijowski J, Wiatr J, Majka M. The characterization of the subpopulation of suppressive B7H4(+) macrophages and the subpopulation of CD25(+) CD4(+) and FOXP3(+) regulatory T-cells in decidua during the secretory cycle phase, Arias Stella reaction, and spontaneous abortion - a preliminary report. Am J Reprod Immunol. 2009;61:303–312. doi: 10.1111/j.1600-0897.2009.00696.x. [DOI] [PubMed] [Google Scholar]
- Yelavarthi KK, Fishback JL, Hunt JS. Analysis of HLA-G mRNA in human placental and extraplacental membrane cells by in situ hybridization. J Immunol. 1991;146:2847–2854. [PubMed] [Google Scholar]
- Zeldovich VB, Clausen CH, Bradford E, Fletcher DA, Maltepe E, Robbins JR, Bakardjiev AI. Placental syncytium forms a biophysical barrier against pathogen invasion. PLoS Pathog. 2013;9:e1003821. doi: 10.1371/journal.ppat.1003821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeldovich VB, Robbins JR, Kapidzic M, Lauer P, Bakardjiev AI. Invasive extravillous trophoblasts restrict intracellular growth and spread of Listeria monocytogenes. PLoS Pathog. 2011;7:e1002005. doi: 10.1371/journal.ppat.1002005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenclussen AC. Adaptive immune responses during pregnancy. Am J Reprod Immunol. 2013;69:291–303. doi: 10.1111/aji.12097. [DOI] [PubMed] [Google Scholar]


