Abstract
Spatial neglect commonly follows right hemisphere stroke. It is defined as impaired contralesional stimulus detection, response, or action, causing functional disability. While prism adaptation treatment is highly promising to promote functional recovery of spatial neglect, not all individuals respond. Consistent with a primary effect of prism adaptation on spatial movements, we previously demonstrated that functional improvement after prism adaptation treatment is linked to frontal lobe lesions (Chen, Goedert, Shah, Foundas, & Barrett, 2014). However, that study was a treatment-only study with no randomized control group. The current study randomized individuals with spatial neglect to receive ten days of prism adaptation treatment, or to receive only standard care (control group). Replicating our earlier results, we found that the presence of frontal lesions moderated response to prism adaptation treatment: Among prism-treated patients, only those with frontal lesions demonstrated functional improvements in their neglect symptoms. Conversely, among individuals in the standard-care control, the presence of frontal lesions did not modify recovery. These results suggest that further research is needed on how frontal lesions may predict response to prism adaptation treatment. Additionally, the results help elucidate the neural network involved in spatial movement and could be used to aid decisions about treatment.
Keywords: spatial neglect, rehabilitation, lesion mapping, prism adaptation, stroke
Spatial neglect after right hemisphere stroke is defined by pathologically asymmetric attention, perception, spatial knowledge, or action, causing functional disability (Adair & Barrett, 2008; Barrett & Burkholder, 2006; Heilman, 2004; Heilman, Watson, & Valenstein, 2011). The presence of spatial neglect is associated with poorer rehabilitation outcomes and longer hospital stays (Chen, Hreha, Kong, & Barrett, 2015; Gillen, Tennen, & McKee, 2005; Kalra, Perez, Gupta, & Wittink, 1997; Nijboer, Kollen, & Kwakkel, 2014; Stone, Halligan, & Greenwood, 1993). Despite the prevalence and impact of this disorder, unfortunately many patients still do not receive treatments demonstrated to have lasting functional impact (Bowen, Hazelton, Pollock, & Lincoln, 2013; Kerkhoff & Schenk, 2012).
In recent years, prism adaptation has emerged as a promising therapeutic approach for spatial neglect (Barrett, Goedert & Basso, 2012; Yang, Zhou, Chung, Li-Tsang & Fong, 2013). During prism adaptation treatment, individuals don goggles with prism lenses that displace viewed objects 5 to 12º rightward (depending on the diopter used in a given study), and repeatedly perform a goal-directed visuomotor task (Rossetti et al., 1998). After the period of task training, the prisms are removed. Patients then characteristically make errors by reaching to the left of the target, in the opposite direction of prismatic displacement. This inaccurate performance is called the “aftereffect”, and it demonstrates that adaptive learning has taken place. Prism adaptation and its aftereffect occur naturally during prism adaptation treatment, requiring no effortful top-down strategy or conscious self-monitoring. In healthy subjects, spatial behavioral changes associated with prism adaptation are implicit – i.e., happen outside of conscious awareness (Michel, Pisella, Prablanc, Rode, & Rossetti, 2007), and are associated with parietal activation (Clower et al., 1996; Danckert, Ferber, & Goodale, 2008).
Prism adaptation treatment can have long-term functional benefits for individuals with spatial neglect (Fortis et al., 2010; Mizuno et al., 2011; Shiraishi, Muraki, Itou, & Hirayama, 2010). However, this benefit appears to require a sufficient dosing regimen of at least 10° of visual shift (c.f., Mancuso et al., 2012; Turton, O’Leary, Gabb, Woodward, & Gilchrist, 2010) and multiple days of daily treatment sessions (Goedert, Zhang, & Barrett, 2015). Even given these conditions, there are reports that prism adaptation does not produce a benefit in all individuals, nor for all symptoms (Priftis, Passarini, Pilosio, Meneghello, & Pitteri, 2013; Rousseaux, Bernati, Saj, & Kozlowski, 2006; Ten Brink et al., 2017). This heterogeneity in response to prism adaptation treatment led our group, and others, to suggest that prism adaptation may have selective direct impacts on spatio-motor or visuo-motor systems (Barrett, Goedert, & Basso, 2012; Striemer & Danckert, 2010a, 2010b, respectively).
In particular, spatial Aiming networks specialized for motor-exploratory action may be the primary systems responding to prism adaptation and accounting for spatial neglect improvement (Barrett, Goedert, & Basso, 2012; Fortis, Chen, Goedert, & Barrett, 2011; Fortis, Goedert, & Barrett, 2011; Goedert et al., 2014). We demonstrated that after prism adaptation with visuomotor training, both healthy individuals and stroke patients with neglect show specific alterations in spatial-motor Aiming bias (Fortis, Chen, et al., 2011; Fortis, Goedert, et al., 2011). Others have shown prism adaptation to improve the rightward bisection error of neglect patients on a standard line bisection task, which has both perceptual and motor components, but not on a Landmark version of the task, which has only perceptual components (Striemer & Danckert, 2010a). Research with neurologically-unimpaired individuals suggests that prism adaptation may stimulate lateralized motor-related spatial systems and increase the speed, magnitude, force, or duration of movements (Kitazawa, Kimura, & Uka, 1997). Such effects on the motor system may directly reduce pathological asymmetry in posture, movements of arms, eyes and body, exploratory and orienting behaviors, and may improve the functional performance of individuals with spatial neglect (e.g., Ferber, Danckert, Joanisse, Goltz, & Goodale, 2003; Nijboer, Olthoff, Van der Stigchel, & Visser-Meily, 2014).
Given that prism adaptation can strongly alter motor-intentional, spatial Aiming bias, we have suggested that therapy for spatial neglect could be improved by matching treatment mechanisms to the specific spatial mechanisms impaired within a given individual (Eskes and Barrett, 2009; Barrett et al., 2012; Riestra & Barrett, 2013). Matching treatment mechanisms to individuals requires finding appropriate treatment moderators (Kraemer, 2016). Methods to fractionate and quantify spatial-motor Aiming deficits are well established in the spatial neglect literature (Bisiach et al., 1978; Halligan & Marshall, 1989; Na et al., 1998; see Robertson & Halligan, 1999 for a review). Using a computerized line bisection task (Garza et al., 2008), we demonstrated that classifying patients on the basis of their rightward spatial-motor Aiming bias predicted response to prism adaptation (Goedert et al., 2014). In that study, we determined whether patients had spatial-motor Aiming bias, spatial perceptual-attentional Where bias, or both types of bias. Only patients with isolated spatial perceptual-attentional Where bias, who also lacked spatial motor-intentional Aiming errors, did not respond to prism adaptation over a seven week period of study. If prism adaptation treatment targets the spatial-motor system, this could also explain why some patients with spatial neglect do not improve after prism adaptation (Morris et al., 2004; Rousseaux, Bernati, Saj, & Kozlowski, 2006). Rather than being determined by random heterogeneity, response to prism adaptation might be systematically dependent on engaging spatial-motor Aiming recovery.
Recently, we attempted to identify potential neuroanatomical moderators of the prism treatment effect (Chen et al., 2014). Spatial perceptual-attentional Where systems and spatial motor-intentional Aiming systems may rely upon different neural networks (Buxbaum et al., 2004; Ghacibeh, Shenker, Winter, Triggs, & Heilman, 2007; Riestra & Barrett, 2013; Verdon, Schwartz, Lovblad, Hauert, & Vuilleumier, 2010). Because a disinclination to move in one direction (directional hypokinesia; Heilman, 2004) may result from frontal lesions as part of Aiming spatial bias (e.g., Ghacibeh et al., 2007; Na et al., 1998; Verdon et al., 2010), we hypothesized the presence of a frontal lesion may serve as an effect moderator, with prism adaptation treatment possibly most effective among individuals with frontal lesions. This possibility is of both theoretical and practical interest, because patients with stroke almost universally undergo brain imaging, and treatment assignment could be based upon the presence or absence of frontal brain injury.
To test the frontal lesion hypothesis of prism adaptation training response (Chen et al., 2014), we administered 10, once-daily prism adaptation sessions over two weeks to 21 individuals with right-hemisphere stroke and left neglect. Consistent with our hypothesis, the group of individuals with frontal lesions (n = 13) demonstrated functional improvement in their neglect symptoms, as assessed by the Catherine Bergego Scale (Azouvi et al., 1996). The group who did not have frontal lesions (n = 8), did not respond to prism adaptation treatment. Because we were unsure of how a brain lesion might mediate or support treatment response, we then looked for intact brain regions in the treatment responders that were lesioned in non-responders. We observed greater preservation of medial cortical and subcortical regions of the temporal lobe among responders. Thus, a post-hoc hypothesis resulting from Chen et al. (2014) was that intact medial temporal areas may mediate the positive effects of prism adaptation, at least in patients with frontal lesions.
Two important questions follow from the Chen et al. (2014) work, which we addressed in the current study. First, Chen et al. was a treatment-only study with no randomized control group. Thus, even if unlikely (cf. Farne et al., 2004), it leaves open the possibility that the improvement observed in patients with frontal lesions was not related to prism adaptation per se, but rather to improved outcomes generally in patients with frontal versus other lesions. Second, we wished to test the post-hoc hypothesis generated in Chen et al. prospectively, to seek confirmation that intact medial temporal regions predict response to prism adaptation therapy among patients with frontal lesions.
Current Study
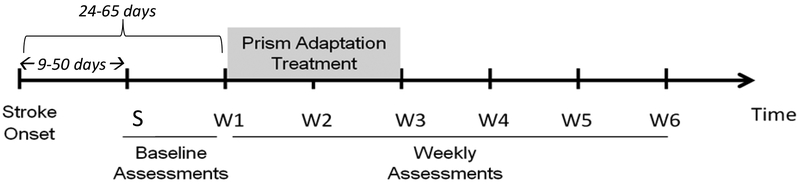
We investigated brain-based predictors of prism adaptation response in a randomized controlled study to determine whether lesioning or sparing of frontal or medial temporal regions is a critical indicator of response to prism treatment. Individuals with right hemisphere stroke and left spatial neglect were randomly assigned to a once-daily, ten-day course of prism adaptation treatment or to a standard care control group. We chose a standard care control group rather than a placebo control because we were determining whether these patient groups showed a different pattern of response to two treatment alternatives now in use in practice (prism adaptation and usual and standard care), rather than performing a basic evaluation of the mechanism of prism adaptation in people with frontal versus other brain lesions. We assessed spatial neglect on nine occasions: at study enrollment (baseline), just prior to the start of prism adaptation treatment, and weekly thereafter for five weeks, with additional follow-ups at 12 and 24 weeks. Assessment included the Behavioral Inattention Test-conventional (BIT) which measures severity of spatial neglect on paper-and-pencil tests (Halligan, Cockburn, & Wilson, 1991). Assessment also included the Catherine Bergego Scale, administered via the Kessler Foundation Neglect Assessment Process (CBS via KF-NAP; Chen, Chen, Hreha, Goedert, & Barrett, 2015). This test assesses symptoms of spatial neglect during activities of daily living. We chose the two assessments because of their demonstrated external validity (i.e., ability to predict real-world disability). To preview the results, in this randomized controlled study, we confirmed that prism adaptation’s benefit to functional activities was specific to individuals with frontal cortical lesions.
Methods
Participants
All participants gave informed consent, and the research was carried out as formally approved by the local institutional review board. Nineteen participants (10 male, 9 female) admitted to an inpatient rehabilitation facility were screened for spatial neglect between 9 and 50 days post-stroke and met the following inclusion criteria at screening: less than 60 days post-stroke, first clinical stroke, unilateral right brain event as confirmed by clinical CT or MRI, and BIT score at screening indicative of neglect (< 129; Halligan et al., 1991). These participants were randomly assigned to either a 10-day course of once-daily prism adaptation treatment (i.e., the prism group) or to a control group receiving standard care (see Appendix A for a detailed description of the randomization procedure). We excluded from the analysis two participants who were randomized to the prism group, but who received only four days of prism adaptation treatment, which left 8 participants in the prism and 9 in the control group. Of these 17 remaining participants, 15 were pre-morbidly right-handed (via a 17-item handedness questionnaire adapted from Raczkowski, Kalat, & Nebes, 1974). Table 1 depicts the participants’ characteristics at screening, including the CBS and the BIT. Table 1 also summarizes admission FIM, an assessment of the burden of care due to functional disability (Uniform Data Set for Medical Rehabilitation, 1996), and admission Mini Mental State Examination (MMSE), a brief dementia screening (Folstein, Folstein, & McHugh, 1975).
Table 1.
Participant characteristics at screening.
| Characteristic | Control Group | Prism Group | two sample Wilcoxon rank sum p-value |
|---|---|---|---|
| Sex (M/F) | 5/4 | 4/4 | |
| Age | 65.6 (12.5) | 61.8 (11.7) | p = .500 |
| MMSE | 24.7 (3.0) | 25.4 (3.0) | p = .770 |
| Admit FIM | 40.6 (15.8) | 36.8 (8.1) | p = .531 |
| Days Post Stroke (at week 1) | 36.6 (5.04) | 51.6 (13.3) | p = .021 |
| BIT (range 0 −146; lower = greater impairment) | 66.0 (34.5) | 61.25 (43.3) | p = .700 |
| CBS (range 0–30; higher = greater impairment) | 15.6 (5.4) | 12.4 (4.0) | p = .228 |
| Lesion Volume (cc) | 99.0 (38.9) | 71.2 (30.1) | p = .564 |
Note: Standard deviations in parentheses. MMSE = Mini-Mental State Exam; FIM = Functional Independence Measure; BIT = Behavioral Inattention Test-conventional, CBS = Catherine Bergego Scale.
Procedure
Treatment.
Both the prism and control groups received standard inpatient rehabilitation facility care, defined as three hours of occupational, physical and speech-language therapy daily, as well as recreational therapy, psychology, and social work consultation, rehabilitation-certified nursing care, and medical care by rehabilitation, medicine, and other physicians as needed. Those randomized to the prism group also received prism adaptation treatment once a day for ten days (five days per week for two weeks). Prism adaptation sessions were timed and lasted approximately 15 to 20 minutes each. During each session, participants wore 20-diopter right-shifting, goggle-mounted, wedge prism lenses (shifting the visual field 11.4 degrees of visual angle). Figure 1 depicts the prism adaptation apparatus. On each of 60 trials, participants used a pen in the right hand to mark a singly-presented stimulus, which was either the center of a line (line bisection: 24.1-cm line), or a 1.0-cm diameter circle (circle crossing). Lines and circles were printed at the center of a horizontally-oriented 8.5 × 11 inch sheet of paper and placed in one of three white placeholders, either at body midline or in left or right space (32.1 cm to the side of body midline). Line bisection and circle crossing alternated every 6 trials (two rounds of three locations in a pseudorandom order), with 30 trials each. Participants sat at a table for the task, and performed line bisections and circle crossing beneath an occluding shelf, which blocked their view of the initial part of their arm movement, but allowed them to view the stimulus and approximately the latter third of their hand-path.
Figure 1.
Prism adaptation treatment administration using the Kessler Foundation Prism Adaptation Treatment (KF-PAT) Portable Kit, patent pending.
Assessment of Prism Adaptation Aftereffects.
While our primary interest was in the therapeutic effect of prism adaptation, one can also assess whether individuals successfully adapt to the prisms with tests of aftereffects (Redding & Wallace, 2006). We assessed prism adaptation aftereffects with tests of proprioceptive straight-ahead pointing and visuo-proprioception immediately prior to and after each prism adaptation session using the procedure described in Fortis, Chen, et al. (2011).
Assessment for Spatial Neglect.
Figure 2 depicts the study timeline. The BIT and CBS were administered a total of nine times: at screening (S on the timeline), just prior to the start of the first prism adaptation session (W1 on the timeline), and weekly thereafter for five weeks, with additional assessments at 12 and 24 weeks after the start of prism adaptation. The BIT-Conventional is a paper-and-pencil test with six sub-tests: line crossing, letter cancellation, star cancellation, figure/shape copying, line bisection and representational drawing (Wilson, Cockburn, & Halligan, 1987). Lower scores on the BIT indicate more profound neglect (range 0 to 146).
Figure 2.
Enrollment, treatment, and weekly assessment timeline. S = screening.
The CBS assesses neglect-specific functional impairment (e.g., ineffective or incomplete performance in dressing the left side of the body or eating from the left side of a plate; Azouvi et al., 1996). It captures additional performance relevant to functional disability as compared with paper-and-pencil assessment (Goedert et al., 2012). Participants’ occupational therapists administered the CBS via the KF-NAP (Kessler Foundation Neglect Assessment Process; Chen, Chen, Hreha, Goedert, & Barrett, 2015) after completing reliability training on its administration. The KF-NAP involves direct observation and reflects the therapist’s ratings of the participant’s performance for stimuli and actions to the left on 10 items: limb awareness, personal belongings, dressing, grooming, gaze orientation, auditory attention, navigation, collisions, eating, and cleaning after a meal. Items were scored on a 0 to 3 scale of severity, with 0 indicating no neglect and 3 indicating severe neglect. Higher scores on the CBS indicate more profound neglect (range 0 to 30).
Lesion Mapping.
We performed a “double-strain” lesion mapping method as previously reported in Chen et al. (2014). Trained technicians, blinded to patients’ behavioral symptoms, manually mapped individual lesions on the axial plane of T1-weighted template brain images using MRIcron software (Rorden, Karnath, & Bonilha, 2007). The MRIcron package was used to calculate lesion volume (cm3) and to create overlap images of participant’s lesions. Meetings with a blinded neurologist ensured accuracy of lesion maps. The blinded neurologist served as an independent rater, inspected all the lesion maps, and determined whether a patient’s lesion involved brain areas anterior to the central sulcus. If so, the patient was marked as having frontal lesions. In addition, the independent rater determined if a patient’s lesion involved the medial temporal area marked in our previous study (Figure 4 of Chen et al., 2014).
Figure 4.
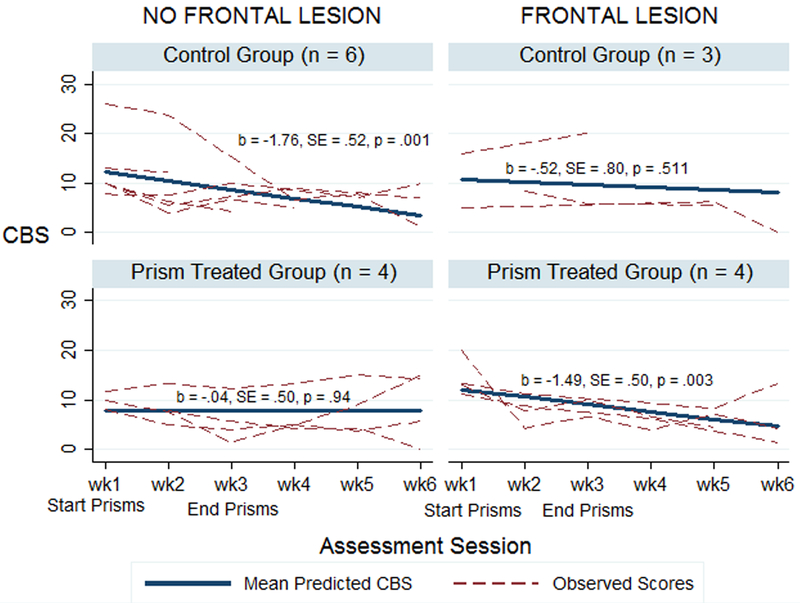
CBS recovery trajectories, including influential case indicated by * in control + no frontal lesion group (upper left panel). Removal of influential case leads to a flat slope in that group, b = −0.43, SE = 0.57, p = .453.
Data Analysis.
We used mixed linear modeling (MLM), a statistical approach particularly well-suited to longitudinal rehabilitation datasets (see Goedert, Boston, & Barrett, 2013 and Goedert et al., 2014, for additional information on MLM for rehabilitation studies). Our primary dependent measures of interest were participants’ scores on the BIT and CBS over time. For each, we performed MLM with the full factorial of frontal lesion (yes, no) by treatment condition (control, prisms) by time (Wk 1 – Wk 6, as a continuous variable) modeled as fixed effects. Note, a high drop-out rate at follow-up weeks 12 and 24 precluded us from including those time-points in the analyses. Thus, all reported results are for weeks one through six. The fixed effects assess whether participants’ recovery trajectories vary as a function of treatment condition and frontal lesion. Participants’ intercepts and slopes were modeled as random effects (see Goedert, Boston, & Barrett, 2013, and Goedert et al., 2014). Prior to each MLM analysis, we tested whether age, days post-stroke at week one, burden of care due to functional disability (admission FIM), or screening scores on the CBS or BIT predicted participants’ average performance for weeks two through six. Those variables emerging as significant predictors were included as covariates in the subsequent MLMs, provided they explained additional variance.
Furthermore, because of the potential vulnerability of the analyses to influential cases, we estimated the influence of each case on the analysis by calculating DFBETA (i.e., difference in beta). This measure is the difference between the regression coefficient when all of the data is included in the analysis and that when the case is removed, divided by the standard error of the coefficient for when the case is removed. We used a criterion of DFBETA > 2 for the removal of influential cases from the analysis, which is a conservative criterion appropriate for small samples, and it reflects a 0.05 confidence level (Hair, Black, Babin, & Anderson, 2009).
Results and Discussion
Lesion Mapping
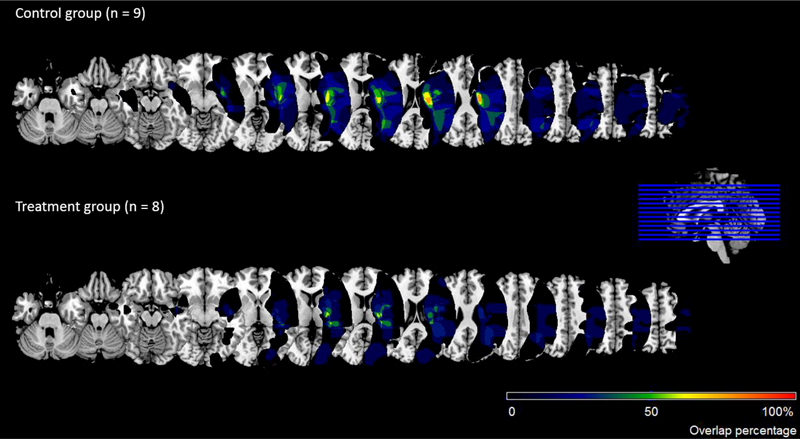
Image overlaps for the control and prism-treated groups appear in Figure 3. The lesion mapping procedure identified 7 participants with frontal lesions and 10 participants without frontal lesions, and separately, 4 participants with temporal lesions and 13 without temporal lesions. A Wilcoxon rank sum test revealed that the prism-treated and control groups did not significantly differ in their lesion volumes (see last row of Table 1). However, participants with frontal lesions had greater lesion volume (M = 123.29, SD = 114.04, med = 71.12) than did those without frontal lesions (M = 44.62, SD = 45.39, med = 20.61), although this difference did not reach significance, p = .205.
Figure 3.
Image overlaps for the control and prism-treated groups. Images are displayed in radiological convention (the left side of the image shows the right side of the brain).
Did Participants Adapt during Prism Adaptation Treatment?
The aftereffects tests were consistent with adaptive learning in participants treated with prism adaptation (n = 8). To analyze the aftereffects, we subtracted pre-prism performance from post-prism performance separately for each participant, session, and test (proprioceptive and visuo-proprioceptive). Negative values on this measure would indicate the expected leftward pre to post shift. We then averaged the shift values across sessions for each participant and test. Finally, we tested the group average against zero. Participants showed the expected leftward pre to post shift in both proprioceptive and visuo-proprioceptive pointing [M = −3.40, SE = .65, t(7) = −5.25, p = .001, for proprioceptive and M = −3.34, SE = .40, t(7) = −8.46, p < .001, for visuo-propriceptive]. Furthermore, the magnitude of this adaptation did not differ as a function of whether they had frontal lesions [independent-sample t-tests: t(6) = 0.78, SE = 1.36, p = 0.687, and t(6) = −1.09, SE = .73, p = 0.181, for proprioceptive straight-ahead and visuo-proprioceptive pointing, respectively].
Therapeutic Effects of Prism Adaptation Treatment?
CBS.
Preliminary analyses assessing potential covariates revealed that both the FIM (p = .002) and CBS screening scores (p < .001) predicted participants’ average CBS scores over weeks two through six, while participants’ age (p = .778) and the number of days post stroke at week 1 (p = .480) did not. However, when FIM and screening CBS were simultaneously used to predict the average CBS score at weeks two through six, only the screening CBS emerged as a significant predictor. Thus, the CBS screening score was the only covariate added to the MLM analysis.
A summary of the coefficients for the fixed effects of the MLM analysis appear in Table 2. Of primary relevance to our predictions is the significant three-way interaction among the presence of frontal lesions, treatment condition, and assessment session. Figure 4 depicts this interaction. Looking at the bottom row of the figure, we see results consistent with our prediction, and with our previous study (Chen et al., 2014). Over the course of the study, spatial neglect improved significantly in participants who received prism adaptation treatment and had frontal cortical lesions (bottom-right panel). However, participants who received prism adaptation treatment and did not have frontal lesions did not improve (bottom-left panel).
Table 2.
Results of MLM analysis on the CBS scores.
| Model Term | Coefficient | SE | p | 95% Confidence Interval |
|---|---|---|---|---|
| CBS screening score | 0.32 | 0.22 | .176 | −0.16, 0.80 |
| Frontal lesion | −5.37 | 4.21 | .226 | −14.53, 3.80 |
| Treatment condition | −7.05 | 3.24 | .050 | −14.12, 0.01 |
| Time | −1.76 | 0.52 | .001 | −2.80, −0.73 |
| Frontal × time | 1.24 | 0.95 | .196 | −0.66, 3.14 |
| Treatment × time | 1.73 | 0.72 | .020 | 0.29, 3.17 |
| Frontal × treatment × time | −2.69 | 1.18 | .027 | −5.07, −0.32 |
We observed the opposite pattern in the control group. Participants in the control group who did not have frontal lesions (upper-left panel of Figure 4) demonstrated significantly improved CBS scores over the course of the study, while those who did have frontal lesions did not (upper-right panel). However, the recovery trajectory in patients without frontal lesions was driven by a single outlier in the control + no frontal lesion group, who was much more impaired than other participants at baseline. Indeed, the influence analysis identified this participant as the sole observation in the dataset with a DFBETA greater than the exclusion cut-off of 2 (Hair et al., 2009). Removing this case from the control + no frontal lesion group resulted in a flat recovery trajectory for that group, while leaving the estimated recovery trajectories for the other three groups qualitatively unchanged.
Eliminating an Alternative Effect Moderator – Insula Damage
Inspection of the lesion overlaps depicted in Figure 3 suggests that the incidence of insula lesions was greater among members of the control versus the prism adaptation group. Indeed, insula lesions appeared in 7 of 9 participants (78%) in the control group, but in only 4 of 8 participants (50%) in the prism group. Because right insula damage is associated with impairments of updating behavior in response to a changing environment (Danckert, Stöttinger, Quehl, & Anderson, 2012), we performed an exploratory post-hoc analysis to determine whether the differential incidence of insula lesions could explain our results. We added the presence or absence of insula lesions to the model predicting performance on the CBS (without the outlier identified above). Doing so did not change the nature of the results. Although the number of participants in each cell of the analysis was small with this additional factor, the presence of frontal lesions continued to predict response to the prism adaptation treatment, even among individuals with insula lesions (b = −1.36, p = .014, n = 3, for prism-treated individuals with both insula and frontal lesions, and b= −0.30, p = .675, n = 3, for control group individuals with both insula and frontal lesions). We direct the reader to Appendix B for the slope of the recovery trajectories for all cells of this analysis, but we also suggest caution in interpreting associations with insula lesions in a patient group mostly composed of middle cerebral artery stroke survivors, because the insula is very commonly lesioned in MCA events (Fink et al., 2005).
In sum, the interaction between the presence of frontal lesions and recovery with prism adaptation replicates our prior work, in which we demonstrated that among individuals with spatial neglect, only those with frontal lesions benefited from prism adaptation treatment. The present study extends Chen et al. (2014) in a randomized control study, by showing that prism adaptation treatment is the key factor, and not just spontaneous recovery, in people with frontal lesions. A control group with frontal lesions recovered similarly to a control group without frontal lesions. The control group with frontal lesions also recovered similarly to the prism-treated group without frontal lesions.
In our prior work, lesion analyses had demonstrated that, compared to participants without frontal lesions, those with frontal lesions had greater volume of intact medial temporal cortex. In the current study, we did not observe such an association. Indeed, in the current study, participants without frontal lesions were more likely to have intact medial temporal regions (of the 9 participants without frontal lesions, 8 did not have medial temporal lesions). In contrast, among participants with frontal lesions, three also had lesions in medial temporal cortex, while four had spared medial temporal cortex. Thus, we did not replicate the association we reported in Chen et al. between spared medial temporal cortex and prism adaptation treatment response in patients with frontal lesions.
BIT.
Analogous to what we observed for the CBS, preliminary analyses assessing potential covariates revealed that both FIM (p = .001) and BIT screening scores (p < .001) predicted participants’ average BIT scores over weeks two through six, while age (p = .543) and the number of days post stroke at week 1 (p = .566) did not. However, when FIM and screening BIT were simultaneously used to predict average BIT score at weeks two through six, only the screening BIT emerged as a significant predictor. Thus, the BIT screening score was the only covariate added to the MLM analysis.
In the overall MLM assessing whether the slopes of the recovery trajectories varied as a function of treatment condition and frontal lesion, the only significant effect to emerge was a main effect of BIT screening (b = 0.68, SE = 0.19, p = .003; all other ps ≥ .178). Influence analysis did not identify any overly-influential case for removal from this MLM analysis.
Follow-up analyses looking at just week 1, before the start of prisms, and week 6, at the end of the study, revealed an overall improvement in BIT scores across the course of the study (b = 38.83, SE = 7.42, p = .001). However, this improvement did not vary with treatment condition (prism vs control), nor with lesion location (all ps > .53). In particular, while the prism group demonstrated directionally greater improvement between weeks one and six (Mdiff = 36.18, SD = 36.05) than the control group (Mdiff = 28.33, SD = 26.81), this effect was small (Cohen’s d = 0.26) and did not reach significance, t(15) = 7.85, p = .613.
We suspect that the lack of treatment effects in the BIT analysis, as in our prior study, relate to the different properties of this outcome measure as compared with the CBS (Goedert et al., 2014; Goedert et al., 2012). It is likely that the BIT may disproportionately assess spatial perceptual-attentional Where errors, or representational Where errors, rather than the errors that are likely to change with prism adaptation therapy, which are those related to spatial-motor performance (Fortis, Chen et al., 2011).
General Discussion
Our current study observed a beneficial effect of prism adaptation therapy on functional performance in spatial neglect as assessed by the Catherine Bergego Scale. Our results add a critical theoretical dimension to previous prism adaptation treatment studies. The goal of the current work was to replicate and extend the results of our previous study, Chen et al. (2014), demonstrating an association between the presence of frontal lesions and response to prism adaptation treatment in spatial neglect. In the current study we employed a randomized control group and demonstrated that the moderating effect of frontal lesions on recovery after prism adaptation treatment was not the result of differences in spontaneous recovery between patients with and without frontal brain lesions. Specifically, we demonstrated that frontal lesions were associated with greater functional improvement among those receiving prism adaptation treatment, but not among those randomized to receive standard care. Thus, consistent with Farne et al. (2004), having a frontal lesion was not associated with more rapid spontaneous recovery of spatial neglect. However, the presence of a frontal lesion did moderate the effectiveness of prism adaptation treatment for functional symptoms of spatial neglect.
Dysfunctional frontal cortical, premotor systems and their white matter connections have been linked to spatial-motor errors (Heilman & Valenstein, 1972; Valenstein & Heilman, 1981; Watson, Miller, & Heilman, 1978). In previous studies, we observed that prism adaptation treatment reduced rightward spatial motor-intentional Aiming errors. We hypothesized that this was due to a direct effect of this therapy on leftward directional hypokinesia (Fortis, Chen, et al., 2011), and demonstrated that patients who had directional hypokinesia, a spatial-motor reduction in the ability to generate movements in a contralesional direction, responded better to prism adaptation treatment (Goedert et al., 2014).
What is the Mechanism of Improvement?
In the current study, we replicated our previous observation that response to prism adaptation depends on frontal lesions (Chen et al., 2014). We did not, however, observe mediating effects of medial temporal lesions, as previously suggested in the same work. In the current study, only a small number of participants had medial temporal lesions (n=4). Furthermore, in contrast to the previous study, which found greater sparing of medial temporal cortex among those with frontal lesions, here we found that medial temporal lesions occurred more frequently in those with frontal lesions (3 of 7) compared to those without frontal lesions (1 of 9). Thus, spared medial temporal regions could not mediate the positive effects of prism adaptation that we observed here.
Similar to what we have argued (Barrett et al., 2012; Fortis, Chen, Goedert, & Barrett, 2011; Goedert, Chen, Boston, Foundas, & Barrett, 2014), others have argued that prism adaptation treatment may selectively improve motor rather than perceptual errors (Striemer & Danckert, 2010a, 2010b), or that spatial-motor treatments may be of specific benefit in the treatment of neglect (Robertson & North, 1993). Nonetheless, the precise mechanism of these effects remain unknown. Striemer and Dankcert (2010b) suggested that prisms may selectively operate on the dorsal stream of visual perception. Robertson and North suggested that a spatial-motor treatment may directly activate portions of the body schema that are poorly attended due to neglect and that this activation indirectly increases attention to corresponding locations in extrapersonal space. Future studies that directly assess both spatial motor Aiming bias, and functional improvement, may clarify the effect of prism adaptation in brain-lesioned patient subgroups.
Existing functional imaging evidence does little to clarify the potential mechanisms. Functional imaging of brain activity pre- and post- prism adaptation in spatial neglect does not identify a set of consistent brain regions associated with neglect improvement. Improvement in neglect symptoms has been associated with activation increases in temporal cortex (Crottaz-Herbette et al., 2017), cerebellar, occipital, and temporal areas (Luaute et al., 2006), and in frontal and parietal areas (Saj, Cojan, Vocat, Luaute, & Vuilleumier, 2013; Taniguchi, Hiyamizu, Tominaga, & Morioka, 2012). The heterogeneity of the imaging results may reflect underlying heterogeneity in pre-treatment neglect symptoms or potential heterogeneity in the prism adaptation procedure.
Heterogeneity of Neglect Symptoms and the Prism Adaptation Procedure
We have argued that prism adaptation has a direct, selective effect on spatial-motor systems and hence is most effective in alleviating spatial-motor neglect symptoms. If so, why do some studies find effects of prism adaptation treatment on purely perceptual tasks such as neglect dyslexia and visual search (e.g., (Làdavas, Bonifazi, Catena, & Serino, 2011; Saj et al., 2013)? There are several possible explanations, which are not mutually exclusive: One explanation is that the beneficial effects observed for perceptual tasks result from frontal, spatial-motor systems controlling eye movement. Indeed, directional hypokinesia of the eyes improves as a result of prism adaptation (Làdavas et al., 2011). Another alternative is that prism adaptation exerts direct effects on spatial-motor representations, but as a result, additional neural reorganization occurs supporting indirect improvements in performance on perceptual tasks (similar to mechanisms proposed by (Redding & Wallace, 2006; Robertson & North, 1993).
A final alternative – one that is supported by mounting evidence, is that different prism adaptation procedures differentially act on the Where, perceptual-attentional, versus the Aiming, motor-intentional, spatial systems, producing heterogeneity in the observed effects of prism adaptation. In particular, the prism adaptation procedure that we used in the current and previous studies (Chen et al., 2014; Goedert et al., 2014) may induce stronger effects on proprioceptive and spatial-motor systems than do other prism adaptation methods. In our studies of prism adaptation, participants make goal-directed arm movements while the latter third of their movement path is visible (i.e., concurrent exposure), with the first two-thirds of the movement path occluded from view. In work with healthy young individuals, Redding and Wallace (1990) demonstrated that during prism adaptation, visual and proprioceptive adaptation occur independently, with greater visual adaptation when individuals see less of their movement path (a terminal exposure condition) and greater proprioceptive adaptation when individuals can see more of their movement path (a concurrent exposure condition). Consistent with this work, in a sample of healthy young adults, Herlihey, Black, & Ferber (2012) demonstrated that a terminal exposure condition produced both visual adaptation and a post-prism shift in the perceptual line bisection task. Conversely, a concurrent exposure condition produced both proprioceptive adaptation and a post-prism shift in the manual line bisection task. Thus, the amount of movement path visible during prism adaptation may determine the degree to which spatial-motor bias, motor-intentional Aiming, and frontal cortical lesions determine recovery, and furthermore, which types of symptoms recover. Future studies of different prism adaptation procedures, including Where and Aiming spatial bias assessment, and both brain lesion subgroups, would address this question.
While different prism adaptation procedures may target different underlying spatial-attentional systems, these procedures may also differentially rely upon explicit strategies for error correction during exposure to the prisms. In comparing the terminal and concurrent prism adaptation procedures for rehabilitating spatial neglect, Làdavas et al., (2011) found more left-directed eye movements after terminal than after concurrent exposure. Importantly, they found that the magnitude of shift in eye movements was positively related to the amount of rapid online error correction during prism adaptation. Rapid online error correction during prism adaptation is thought to reflect strategic compensation for errors rather than an adaptation of the visual and motor systems. However, reducing detection of, and strategic compensation for, errors during prism exposure increases the size of the observed aftereffects, the hallmark of an implicit adaptation of the visual and motor systems (Fernández-Ruiz, Hall, Vergara, & Díaz, 2000; Michel, Pisella, Prablanc, Rode, & Rossetti, 2007).
These differences between terminal and concurrent exposure conditions may be one reason why a recent study comparing prism adaptation with visual scanning training and limb activation therapy found no difference among the ability of the therapies to rehabilitate neglect symptoms (Priftis et al., 2013; see also Ten Brink et al., 2017). Employing a terminal exposure procedure for prism adaptation may have necessitated strategic error correction and thus, the results from that procedure were similar to other treatments that rely on implementation of top-down strategies.
Functional Assessment of Spatial Neglect
Importantly, our study results indicate that the effects of frontal brain lesions are relevant to how functional performance of daily life behaviors improves after spatial neglect treatment. The study results do not extend to paper-and-pencil measures of spatial neglect in the Behavioural Inattention Test. This replicates what we observed in a prior study (Goedert et al., 2014). It is possible that we did not observe differences in the BIT because the CBS, which is an observational evaluation of asymmetry in performing daily life activities, has more established external validity—it is extremely sensitive to variance in functional disability (Goedert et al., 2012) as well as spatial neglect impairment tasks (Azouvi et al., 2006). Consistent with our own prior work (Goedert et al., 2012), Pitteri et al. (2017) recently demonstrated that the CBS, performed via the KF-NAP procedure, was more strongly associated with motor FIM and Barthel Index scores than the BIT-conventional. Furthermore, the CBS detected higher rates of spatial neglect than did individual neuropsychological tests targeting personal, peripersonal, or extrapersonal space. They suggested that the CBS may be a more sensitive test of neglect because it assesses behavior in all three spaces under the attentionally complex and demanding context of daily activity.
In contrast, the BIT-conventional may be more sensitive to perceptual-attentional deficits. Previously we demonstrated an association between the BIT and visual extinction (Goedert et al., 2012). Others have demonstrated baseline BIT scores to be positively associated with tests of visual, but not proprioceptive, straight ahead (Rode et al., 2015). Thus, the BIT may be more likely to detect improvements in spatial neglect after prism adaptation employing a terminal exposure procedure, which is more likely to induce perceptual-attentional changes, than a concurrent exposure procedure, which is more likely to induce motor-intentional changes. Indeed, studies employing a terminal exposure prism adaptation procedure have observed significant improvements on the BIT (e.g., Frassinetti, Angeli, Meneghello, Avanzi, & Ladavas, 2002; Làdavas et al., 2011; Mizuno et al., 2011), while those employing a concurrent exposure procedure have not (e.g., Goedert et al., 2014; Nys, Haan, Kunneman, Kort, & Dijkerman, 2008; Rode et al., 2015). The BIT may also be less sensitive than the CBS to spatial-motor deficits associated with frontal lesions that are strongly linked to functional recovery. Nonetheless, other assessments that mimic real-life motor demands, such as virtual-reality based navigation, may also be effective and sensitive at detecting neglect (Buxbaum, Dawson, & Linsley, 2012), as well as at detecting spatial-motor improvements associated with concurrent exposure prism adaptation procedures in neglect treatment (Glize et al., 2017). Future studies establishing the external validity of virtual reality and other similar assessment procedures, in large, representative patient groups with spatial neglect, are needed.
Limitations of the current study
Although the current study results are exciting because they open a new path in the treatment of spatial neglect, several questions still need to be addressed. First, as we note above, we did not examine both impairment-level changes in directional hypokinesia and functional performance. We suggest that frontal lesions are associated with prism adaptation treatment response from spatial-motor Aiming errors (Garza, Eslinger, & Barrett, 2008) to spatial motor-intentional neglect (Heilman, 2004), to functional movements during performance of daily life tasks (Fortis, Chen, et al., 2011; Goedert et al., 2014). Future studies are needed in which patients with well-characterized brain lesions undergo prism adaptation treatment and functional performance assessment that includes assessment of directional hypokinesia during, immediately after, and in the months following the treatment.
Our results, using the mixed effects linear modeling approach, are highly internally valid, and generate important information about patients receiving prism adaptation treatment during the critical, first 30-day window of stroke recovery (Cramer et al., 2012). However, further research is needed to confirm that these results are applicable to other groups that differ in demographic characteristics, treatment settings, time post-stroke, and exposure to other treatments. A large-scale, multi-site study confirming the feasibility and cost-efficacy of implementing prism adaptation treatment, and tracking the influence of lesion location on recovery, could address this research objective. It would be important for future studies to report the specifics of the prism adaptation procedure, and to systematically investigate the relationship between the terminal and concurrent exposure procedures and potential neuroanatomical moderators of prism response under these different conditions.
In the current study, we elected to employ a standard care control group as opposed to a placebo control using neutral glasses (e.g., Nys et al., 2008; Rode et al., 2015) or pointing movements without glasses (e.g., Mizuno et al., 2011). Rossetti et al. (1998) observed significant neglect improvement specific to left-shifting prism lenses (and not right-shifting prism lenses) using a concurrent exposure prism adaptation procedure similar to that which we employed in Chen et al., 2014 and the present study. Given these findings, we suspect that functional improvements that we observed in our prism-treated group with frontal lesions were indeed prism-specific rather than attributable to repetitive pointing. We suggest that to determine whether to administer 1) usual and standard care, or 2) concurrent prism adaptation, within rehabilitative clinical practice, researchers should compare those two conditions. Neutral glasses may be useful to investigate the mechanism of improvement, for example when a placebo response is suspected. However, future studies to develop consensus about when to use usual and standard care, versus neutral glasses, as a control in prism adaptation trials, are needed.
Our failure to replicate our previous post-hoc finding of intact medial temporal cortex mediating improvements associated with prism adaptation and frontal lesions emphasizes the hypothesis-generating nature of voxel-based lesion-symptom mapping and the need for additional, confirmatory research as we determine the critical neural substrates associated with treatment effects. It is still possible that differences between the individuals in the present study, and those in our previous study (e.g., presence or absence of other lesions that may interact with the medial temporal complex) may account for the difference in effects.
Furthermore, while lesion-behavior mapping, as used in this study, is a potent method to study brain–behavior relationships, this method may overlook structural disconnections to seemingly spared but functionally related brain regions. Future large-sample studies should use a predictive multivariate approach to investigate the relationship between deficits in right hemisphere damaged patients with chronic neglect and the distribution of structural brain damage associated with response to prism adaptation. Connectome-based analysis should be used in combination with lesion-based approaches to fully elucidate whether structurally damaged or structurally disconnected regions relate to neglect deficits and its recovery.
Conclusion
In this study, we produce evidence supporting our suggestion for translational treatment of spatial neglect, by matching mechanisms of treatment to impaired spatial cognitive mechanisms (Barrett et al., 2012). Because impairments in spatial-motor exploratory systems are related to functional competence in activities of daily living (Goedert et al., 2012), prism adaptation treatment with a concurrent exposure procedure, and other treatments improving spatial-motor function may be particularly well-suited for broad application (Barrett & Muzaffar, 2014). Indeed, a number of characteristics make this type of prism adaptation a potentially desirable treatment. First, it does not rely on top-down strategies by the patient. This is ideal for individuals with spatial neglect, who frequently experience a lack of awareness of their deficit (Vossel, Weiss, Eschenbeck, & Fink, 2013; Vossel et al., 2012). Second, while prism adaptation treatment requires repeated administrations (e.g., the tacitly accepted dosage for prism treatment is a minimum of one session a day for ten days; Goedert, Zhang, & Barrett, 2015), it does not require extensive clinician training. Further, the treatment is neither invasive, nor does it require an expensive apparatus. Thus, prism adaptation treatment is highly feasible, even for home use (Fortis et al., 2010). Accumulating evidence shows it facilitates functional improvement (Champod et al., 2016), and its beneficial effects have the potential to last for months (Fortis et al., 2010; Mizuno et al., 2011; Serino, Barbiani, Rinaldesi, & Ladavas, 2009).
Our work confirms our previous report that patients with frontal cortical lesions may be excellent candidates to receive prism adaptation treatment for spatial neglect. However, because spatial neglect is not documented and potentially undiagnosed in as many as 50–80% of stroke patients (Chen et al., 2013), it is critical that we consider whether patients with frontal brain lesions and hemiparesis are being identified during routine care. Now that a set of prospective cognitive research studies are available demonstrating that these patients benefit from spatial neglect treatment, we urge spatial neglect researchers to join us in educating our clinical colleagues that “typical” stroke patients with hemiparesis, who may not have obvious visual-perceptual deficits, may still have spatial neglect manifest during movements, causing functional disability (Barrett & Muzzafar, 2014).
Given the tremendous cost and burden of spatial neglect after stroke and brain injury, targeting prism adaptation treatment could increase treatment efficiency and improve public health. As above, a large-sample implementation study across multiple sites, to confirm cost-efficacy and functional improvement as well as confirm mechanistic predictors of clinical response, is needed.
Acknowledgements
The authors thank Dr. James Danckert for prompting the analysis of the insula lesions. We also thank the participants for donating their time to take part in the study, Jenny Masmela for assistance with institutional reporting, data management, and staff supervision, and Meghan Caulfield for assistance with lesion mapping.
Funding details
Funded by The National Institute for Disability, Independent Living and Rehabilitation Research (NIDILRR, 901F0037 PI: Barrett), National Institutes of Health (R01 NS 055808 and K24 HD062647; PI:Barrett), the Wallerstein Foundation for Geriatric Improvement (PI: Barrett) and the Kessler Foundation (Chen and Barrett). The contents of this report do not necessarily represent the policy of NIDILRR, the US Administration for Community Living, or the US Department of Health and Human Services, and you should not assume endorsement by the Federal Government.
Appendix A
Randomization Procedure
At the start of the study, one of the PIs created a list of potential subject identification numbers for study participants. From the master list of subject numbers, this PI created four distinct randomization lists. In all lists, the randomization of subjects to the prism treatment and standard care controls was a simple randomization with a 1:1 ratio. The four randomization lists were given to an administrative staff member of the research facility who was not involved in carrying out the project. This staff member was directed to randomly select one randomization list to keep and to discard the other three lists. Research assistants carrying out the study identified potential study participants from the inpatient rehabilitation hospital system. Once research participants completed the screening and were determined to meet the inclusion and exclusion criteria of the protocol, the research assistant asked the administrative staff member keeping the randomization list for the condition assignment corresponding to the participant’s identification number.
Approximately half-way through data collection, the PIs evaluated the success of the simple random assignment procedure to produce relatively equal numbers of participants in the prism-treatment and control groups. By chance, more participants had been enrolled into the control than the treatment group. At this point, four new distinct randomization lists were created with a randomization ratio of 3:1 (prism: control). The administrative staff member was again directed to select one list and discard the other three. The randomization assignment continued to be revealed to research assistants after successful screening, as described above.
Appendix B
Table B1.
Slopes (b) on the recovery trajectories for each cell of the exploratory, post-hoc insula X frontal X condition MLM analysis of the CBS.
| Insula Damage | Frontal Damage | Condition | n | b | p | 95% confidence interval |
|---|---|---|---|---|---|---|
| no | yes | control | 0 | |||
| no | no | control | 2 | 0.40 | .644 | −1.31, 2.12 |
| yes | yes | control | 3 | −0.30 | .675 | −1.68, 1.08 |
| yes | no | control | 3 | −0.89 | .205 | −2.26, 0.49 |
| yes | no | prisms | 1 | 1.37 | .136 | −0.43, 3.17 |
| no | no | prisms | 3 | −0.60 | .275 | −1.67, 0.47 |
| yes | yes | prisms | 3 | −1.36 | .014 | −2.44, −0.28 |
| no | yes | prisms | 1 | −1.87 | .042 | −3.67, −0.07 |
Note: Because lower scores on the CBS indicate less neglect, greater negative slopes indicate greater improvement over time.
Footnotes
Disclosure of Interest
Authors Goedert, and Foundas report no conflicts of interest. Drs. Chen and Barrett report the scientific funding disclosed above, and are both employees of the Kessler Foundation.
Contributor Information
Kelly M. Goedert, Department of Psychology, Seton Hall University, 400 South Orange Ave., South Orange, NJ 07079, phone: 1-973-275-2703; Kelly.goedert@shu.edu
Peii Chen, Stroke Rehabilitation Research, Kessler Foundation, Department of Physical Medicine and Rehabilitation, Rutgers- New Jersey Medical School, 1199 Pleasant Valley Way, West Orange, NJ 07052, phone: 1-973-324-2574; pchen@kesslerfoundation.org.
Anne L. Foundas, Department of Psychology, Tulane University, 2007 Percival Stern Hall, New Orleans, Louisiana 70118, phone: (504) 865-5331, anne.foundas@gmail.com
A.M. Barrett, Stroke Rehabilitation Research, Kessler Foundation, Department of Physical Medicine and Rehabilitation, Rutgers-New Jersey Medical School, Kessler Institute for Rehabilitation, 1199 Pleasant Valley Way, West Orange, NJ 07052, phone: 1-973-324-3569; abarrett@kesslerfoundation.org
References
- Adair JC, & Barrett AM (2008). Spatial neglect: clinical and neuroscience review: a wealth of information on the poverty of spatial attention. Annals of the New York Academy of Sciences, 1142, 21–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azouvi P, Bartolomeo P, Beis JM, Perennou D, Pradat-Diehl P, & Rousseaux M (2006). A battery of tests for the quantitative assessment of unilateral neglect. Restorative Neurology and Neuroscience, 24(4–6), 273–285. [PubMed] [Google Scholar]
- Azouvi P, Marchal F, Samuel C, Morin L, Renard C, Louis-Drefus A, … Bergego C. (1996). Functional consequences and awareness of unilateral neglect: Study of an evaluation scale. Neuropsychological Rehabilitation, 6(2), 133–150. [Google Scholar]
- Barrett AM, & Burkholder S (2006). Monocular patching in subjects with right-hemisphere stroke affects perceptual-attentional bias. Journal of Rehabilitation Research and Development, 43(3), 337–346. [DOI] [PubMed] [Google Scholar]
- Barrett AM, & Muzaffar T (2014). Spatial cognitive rehabilitation and motor recovery after stroke. Current Opinion in Neurology, 27(6), 653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett AM, Goedert KM, & Basso JC (2012). Prism adaptation for spatial neglect after stroke: translational practice gaps. Nature Reviews.Neurology, 8(10), 567–577. 10.1038/nrneurol.2012.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomeo P, Schotten M. T. de, & Doricchi F (2007). Left unilateral neglect as a disconnection syndrome. Cerebral Cortex, 17(11), 2479–2490. [DOI] [PubMed] [Google Scholar]
- Bartolomeo Paolo, Schotten M. T. de, & Chica AB (2012). Brain networks of visuospatial attention and their disruption in visual neglect. Frontiers in Human Neuroscience, 6. doi: 10.3389/fnhum.2012.00110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisiach E, Ricci R, Lualdi M, & Colombo MR (1998). Perceptual and response bias in unilateral neglect: two modified versions of the Milner landmark task. Brain and Cognition, 37(3), 369–386. [DOI] [PubMed] [Google Scholar]
- Bowen A, Hazelton C, Pollock A, & Lincoln NB (2013). Cognitive rehabilitation for spatial neglect following stroke. The Cochrane Database of Systematic Reviews, (7):CD003586. doi(7), CD003586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum LJ, Dawson AM, & Linsley D (2012). Reliability and validity of the Virtual Reality Lateralized Attention Test in assessing hemispatial neglect in right-hemisphere stroke. Neuropsychology, 26(4), 430–441. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Ferraro MK, Veramonti T, Farne A, Whyte J, Ladavas E, … Coslett HB (2004). Hemispatial neglect: Subtypes, neuroanatomy, and disability. Neurology, 62(5), 749–756. [DOI] [PubMed] [Google Scholar]
- Champod AS, Frank RC, Taylor K, & Eskes GA (2016, epub). The effects of prism adaptation on daily life activities in patients with visuospatial neglect: A systematic review. Neuropsychological Rehabilitation, 1–24. doi: 10.1080/09602011.2016.1182032 [DOI] [PubMed] [Google Scholar]
- Chen P, Goedert KM, Murray E, Kelly K, Ahmeti S, & Barrett AM (2011). Spatial bias and right hemisphere function: Sex-specific changes with aging. Journal of the International Neuropsychological Society, 17(3), 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Goedert KM, Shah P, Foundas AL, & Barrett AM (2014). Integrity of medial temporal structures may predict better improvement of spatial neglect with prism adaptation treatment. Brain Imaging and Behavior, 8(3), 346–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, McKenna C, Kutlik AM, & Frisina PG (2013). Interdisciplinary communication in inpatient rehabilitation facility: evidence of under-documentation of spatial neglect after stroke. Disability and Rehabilitation, 35(12), 1033–1038. 10.3109/09638288.2012.717585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Peii, Chen CC, Hreha K, Goedert KM, & Barrett AM (2015). Kessler Foundation Neglect Assessment Process uniquely measures spatial neglect during activities of daily living. Archives of Physical Medicine and Rehabilitation, 96(5), 869–876.e1. 10.1016/j.apmr.2014.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Peii, Hreha K, Kong Y, & Barrett AM (2015). Impact of spatial neglect on stroke rehabilitation: evidence from the setting of an inpatient rehabilitation facility. Archives of Physical Medicine and Rehabilitation, 96(8), 1458–1466. 10.1016/j.apmr.2015.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clower DM, Hoffman JM, Votaw JR, Faber TL, Woods RP, & Alexander GE (1996). Role of posterior parietal cortex in the recalibration of visually guided reaching. Nature, 383(6601), 618–621. [DOI] [PubMed] [Google Scholar]
- Cramer S, Duncan P, Barrett A. (2012). Report of the NIH Stroke Progress Review Group (SPRG): Recovery and Rehabilitation. [Accessed May 14, 2017] https://www.ninds.nih.gov/About-NINDS/Strategic-Plans-Evaluations/Strategic-Plans/Final-Report-Stroke-Progress-Review-Group-1#recoverypriority
- Crottaz-Herbette S, Fornari E, Notter MP, Bindschaedler C, Manzoni L, & Clarke S (2017). Reshaping the brain after stroke: The effect of prismatic adaptation in patients with right brain damage. Neuropsychologia, 104(Supplement C), 54–63. 10.1016/j.neuropsychologia.2017.08.005 [DOI] [PubMed] [Google Scholar]
- Danckert J, Ferber S, & Goodale MA (2008). Direct effects of prismatic lenses on visuomotor control: an event-related functional MRI study. The European Journal of Neuroscience, 28(8), 1696–1704. 10.1111/j.1460-9568.2008.06460.x [DOI] [PubMed] [Google Scholar]
- Danckert J, Stöttinger E, Quehl N, & Anderson B (2012). Right hemisphere brain damage impairs strategy updating. Cerebral Cortex, 22(12), 2745–2760. 10.1093/cercor/bhr351 [DOI] [PubMed] [Google Scholar]
- Doricchi F, & Tomaiuolo F (2003). The anatomy of neglect without hemianopia: a key role for parietal-frontal disconnection? Neuroreport, 14(17), 2239–2243. [DOI] [PubMed] [Google Scholar]
- Doricchi F, Schotten M. T. de, Tomaiuolo F, & Bartolomeo P (2008). White matter (dis)connections and gray matter (dys)functions in visual neglect: gaining insights into the brain networks of spatial awareness. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 44(8), 983–995. [DOI] [PubMed] [Google Scholar]
- Eskes GA, & Barrett AM (2009). Neuropsychological rehabilitation. In Lazar RM (Ed.), Neurovascular neuropsychology. (pp. 281–305). New York, NY US: Springer Science + Business Media. [Google Scholar]
- Farne A, Buxbaum L, Ferraro M, Frassinetti F, Whyte J, Veramonti T, … Ladavas E (2004). Patterns of spontaneous recovery of neglect and associated disorders in acute right brain-damaged patients. Journal of Neurology, Neurosurgery, and Psychiatry, 75(10), 1401–1410. 10.1136/jnnp.2002.003095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferber S, Danckert J, Joanisse M, Goltz HC, & Goodale MA (2003). Eye movements tell only half the story. Neurology, 60(11), 1826–1829. [DOI] [PubMed] [Google Scholar]
- Fernández-Ruiz J, Hall C, Vergara P, & Díaz R (2000). Prism adaptation in normal aging: Slower adaptation rate and larger aftereffect. Cognitive Brain Research, 9(3), 223–226. [DOI] [PubMed] [Google Scholar]
- Fink JN, Selim MH, Kumar S, Voetsch B, Fong WC, & Caplan LR (2005). Insular cortex infarction in acute middle cerebral artery territory stroke: predictor of stroke severity and vascular lesion. Archives of Neurology, 62(7), 1081–1085. 10.1001/archneur.62.7.1081 [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. [DOI] [PubMed] [Google Scholar]
- Fortis P, Chen P, Goedert KM, & Barrett AM (2011). Effects of prism adaptation on motor-intentional spatial bias in neglect. Neuroreport, 22(14), 700–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortis P, Chen P, Goedert KM, & Barrett AM (2011). Effects of prism adaptation on motor-intentional spatial bias in neglect. Neuroreport, 22(14), 700–705. 10.1097/WNR.0b013e32834a3e20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortis P, Maravita A, Gallucci M, Ronchi R, Grassi E, Senna I, … Vallar G (2010). Rehabilitating patients with left spatial neglect by prism exposure during a visuomotor activity. Neuropsychology, 24(6), 681–697. 10.1037/a0019476 [DOI] [PubMed] [Google Scholar]
- Fortis P, Goedert KM, & Barrett AM (2011). Prism adaptation differently affects motor-intentional and perceptual-attentional biases in healthy individuals. Neuropsychologia, 49(9), 2718–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frassinetti F, Angeli V, Meneghello F, Avanzi S, & Ladavas E (2002). Long-lasting amelioration of visuospatial neglect by prism adaptation. Brain : A Journal of Neurology, 125(Pt 3), 608–623. [DOI] [PubMed] [Google Scholar]
- Garza JP, Eslinger PJ, & Barrett AM (2008). Perceptual-attentional and motor-intentional bias in near and far space. Brain and Cognition, 68(1), 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghacibeh GA, Shenker JI, Winter KH, Triggs WJ, & Heilman KM (2007). Dissociation of neglect subtypes with transcranial magnetic stimulation. Neurology, 69(11), 1122–1127. [DOI] [PubMed] [Google Scholar]
- Gillen R, Tennen H, & McKee T (2005). Unilateral spatial neglect: relation to rehabilitation outcomes in patients with right hemisphere stroke. Archives of Physical Medicine and Rehabilitation, 86(4), 763–767. [DOI] [PubMed] [Google Scholar]
- Glize B, Lunven M, Rossetti Y, Revol P, Jacquin-Courtois S, Klinger E, … Rode G (2017). Improvement of navigation and representation in virtual reality after prism adaptation in neglect patients. Frontiers in Psychology, 8(November). 10.3389/fpsyg.2017.02019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert KM, Boston RC, & Barrett AM (2013). Advancing the science of spatial neglect rehabilitation: an improved statistical approach with mixed linear modeling. Frontiers in Human Neuroscience, 7, 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert KM, Chen P, Boston RC, Foundas AL, & Barrett AM (2014). Presence of Motor-Intentional Aiming Deficit Predicts Functional Improvement of Spatial Neglect With Prism Adaptation. Neurorehabilitation and Neural Repair, 28(5), 483–493. 10.1177/1545968313516872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert KM, Chen P, Botticello A, Masmela JR, Adler U, & Barrett AM (2012). Psychometric evaluation of neglect assessment in an acute post-stroke sample reveals novel predictor of functional outcomes. Archives of Physical Medicine and Rehabilitation, 93(1), 137–142. 10.1016/j.apmr.2011.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert KM, Zhang JY, & Barrett AM (2015). Prism adaptation and spatial neglect: the need for dose-finding studies. Frontiers in Human Neuroscience, 9, 243 10.3389/fnhum.2015.00243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hair JF, Black WC, Babin BJ, & Anderson RE (2009). Advanced Diagnostics for Multiple Regression: A Supplement to Multivariate Data Analysis (7th ed). Upper Saddle River, NJ: Prentice Hall. [Google Scholar]
- Halligan PW, & Marshall JC (1989). Perceptual cueing and perceptuo-motor compatibility in visuo-spatial neglect: A single case study. Cognitive Neuropsychology, 6(4), 423–435. 10.1080/02643298908253291 [DOI] [Google Scholar]
- Halligan PW, Cockburn J, & Wilson BA (1991). The behavioral assessment of visual neglect. Neuropsychological Rehabilitation, 1(1), 5–32. [Google Scholar]
- Heilman KM (2004). Intentional neglect. Frontiers in Bioscience : A Journal and Virtual Library, 9, 694–705. [DOI] [PubMed] [Google Scholar]
- Heilman KM, & Valenstein E (1972). Frontal lobe neglect in man. Neurology, 22(6), 660–664. [DOI] [PubMed] [Google Scholar]
- Heilman Kenneth M., Watson RT, & Valenstein E (2011). Neglect and related disorders. In Heilman Kenneth M. & Valenstein E (Eds.) (5th ed, pp. 296–348). New York: Oxford University Press. [Google Scholar]
- Herlihey TA, Black SE, & Ferber S (2012). Terminal, but not concurrent prism exposure produces perceptual aftereffects in healthy young adults. Neuropsychologia, 50(12), 2789–2795. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Chang S, Heidler-Gary J, Newhart M, Kleinman JT, Davis C, … Ken L (2006). Neural correlates of modality-specific spatial extinction. Journal of Cognitive Neuroscience, 18(11), 1889–1898. [DOI] [PubMed] [Google Scholar]
- Kalra L, Perez I, Gupta S, & Wittink M (1997). The influence of visual neglect on stroke rehabilitation. Stroke; a Journal of Cerebral Circulation, 28(7), 1386–1391. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Zopf R, Johannsen L, Berger MF, Nagele T, & Klose U (2005). Normalized perfusion MRI to identify common areas of dysfunction: patients with basal ganglia neglect. Brain : A Journal of Neurology, 128(Pt 10), 2462–2469. https://doi.org/awh629 [pii] [DOI] [PubMed] [Google Scholar]
- Kerkhoff G, & Schenk T (2012). Rehabilitation of neglect: An update. Neuropsychologia, 50(6), 1072–1079. [DOI] [PubMed] [Google Scholar]
- Kitazawa S, Kimura T, & Uka T (1997). Prism adaptation of reaching movements: specificity for the velocity of reaching. The Journal Of Neuroscience: The Official Journal Of The Society For Neuroscience, 17(4), 1481–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC (2016). Messages for Clinicians: Moderators and Mediators of Treatment Outcome in Randomized Clinical Trials. American Journal of Psychiatry, 173(7), 672–679. 10.1176/appi.ajp.2016.15101333 [DOI] [PubMed] [Google Scholar]
- Làdavas E, Bonifazi S, Catena L, & Serino A (2011). Neglect rehabilitation by prism adaptation: Different procedures have different impacts. Neuropsychologia, 49(5), 1136–1145. 10.1016/j.neuropsychologia.2011.01.044 [DOI] [PubMed] [Google Scholar]
- Luaute J, Michel C, Rode G, Pisella L, Jacquin-Courtois S, Costes N, … Rossetti Y (2006). Functional anatomy of the therapeutic effects of prism adaptation on left neglect. Neurology, 66(12), 1859–1867. [DOI] [PubMed] [Google Scholar]
- Mancuso M, Pacini M, Gemignani P, Bartalini B, Agostini B, Ferroni L, … Cantagallo A (2012). Clinical application of prismatic lenses in the rehabilitation of neglect patients. A randomized controlled trial. European Journal of Physical and Rehabilitation Medicine, 48(2), 197–208. [PubMed] [Google Scholar]
- Michel C, Pisella L, Prablanc C, Rode G, & Rossetti Y (2007). Enhancing visuomotor adaptation by reducing error signals: single-step (aware) versus multiple-step (unaware) exposure to wedge prisms. Journal of Cognitive Neuroscience, 19(2), 341–350. 10.1162/jocn.2007.19.2.341 [DOI] [PubMed] [Google Scholar]
- Mizuno K, Tsuji T, Takebayashi T, Fujiwara T, Hase K, & Liu M (2011). Prism adaptation therapy enhances rehabilitation of stroke patients with unilateral spatial neglect: a randomized, controlled trial. Neurorehabilitation and Neural Repair, 25(8), 711–720. [DOI] [PubMed] [Google Scholar]
- Morris AP, Kritikos A, Berberovic N, Pisella L, Chambers CD, & Mattingley JB (2004). Prism adaptation and spatial attention: A study of visual search in normals and patients with unilateral neglect. Cortex: A Journal Devoted to the Study of the Nervous System and Behavior, 40(4–5), 703–721. [DOI] [PubMed] [Google Scholar]
- Na DL, Adair JC, Williamson DJ, Schwartz RL, Haws B, & Heilman KM (1998). Dissociation of sensory-attentional from motor-intentional neglect. Journal of Neurology, Neurosurgery, and Psychiatry, 64(3), 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijboer TC, Kollen BJ, & Kwakkel G (2014). The impact of recovery of visuo-spatial neglect on motor recovery of the upper paretic limb after stroke. PloS One, 9(6), e100584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijboer TC, Olthoff L, Van der Stigchel S, & Visser-Meily JM (2014). Prism adaptation improves postural imbalance in neglect patients. Neuroreport, 25(5), 307–311. 10.1097/WNR.0000000000000088 [DOI] [PubMed] [Google Scholar]
- Nys GM, Haan E. H. de, Kunneman A, Kort P. L. de, & Dijkerman HC (2008). Acute neglect rehabilitation using repetitive prism adaptation: a randomized placebo-controlled trial. Restorative Neurology and Neuroscience, 26(1), 1–12. [PubMed] [Google Scholar]
- Priftis K, Passarini L, Pilosio C, Meneghello F, & Pitteri M (2013). Visual Scanning Training, Limb Activation Treatment, and Prism Adaptation for Rehabilitating Left Neglect: Who is the Winner? Frontiers in Human Neuroscience, 7, 360 10.3389/fnhum.2013.00360 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redding GM, & Wallace B (1990). Effects on prism adaptation of duration and timing of visual feedback during pointing. Journal of Motor Behavior, 22(2), 209–224. [DOI] [PubMed] [Google Scholar]
- Redding GM, & Wallace B (2006). Prism adaptation and unilateral neglect: review and analysis. Neuropsychologia, 44(1), 1–20. 10.1016/j.neuropsychologia.2005.04.009 [DOI] [PubMed] [Google Scholar]
- Riestra AR, & Barrett AM (2013). Rehabilitation of spatial neglect. Handbook of Clinical Neurology, 110, 347–355. 10.1016/B978-0-444-52901-5.00029-0 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson IH, & North N (1992). Spatio-motor cueing in unilateral left neglect: the role of hemispace, hand and motor activation. Neuropsychologia, 30(6), 553–563. [DOI] [PubMed] [Google Scholar]
- Robertson IH, & North N (1993). Active and passive activation of left limbs: Influence on visual and sensory neglect. Neuropsychologia, 31(3), 293–300. 10.1016/0028-3932(93)90093-F [DOI] [PubMed] [Google Scholar]
- Robertson I, & Halligan PW (1999). Clinical presentation of spatial neglect. In Halligan PW and Robertson I (Eds.) Spatial Neglect: A Clinical Handbook for Diagnosis and Treatment. (pp. 21–24). Hove, East Sussex, UK: Psychology Press. [Google Scholar]
- Rode G, Lacour S, Jacquin-Courtois S, Pisella L, Michel C, Revol P, … Rossetti Y (2015). Long-term sensorimotor and therapeutical effects of a mild regime of prism adaptation in spatial neglect. A double-blind RCT essay. Annals of Physical and Rehabilitation Medicine, 58(2), 40–53. 10.1016/j.rehab.2014.10.004 [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath H-O, & Bonilha L (2007). Improving lesion-symptom mapping. Journal of Cognitive Neuroscience, 19(7), 1081–1088. 10.1162/jocn.2007.19.7.1081 [DOI] [PubMed] [Google Scholar]
- Rossetti Y, Rode G, Pisella L, Farne A, Li L, Boisson D, & Perenin MT (1998). Prism adaptation to a rightward optical deviation rehabilitates left hemispatial neglect. Nature, 395(6698), 166–169. 10.1038/25988 [DOI] [PubMed] [Google Scholar]
- Rousseaux M, Bernati T, Saj A, & Kozlowski O (2006). Ineffectiveness of prism adaptation on spatial neglect signs. Stroke; a Journal of Cerebral Circulation, 37(2), 542–543. 10.1161/01.STR.0000198877.09270.e8 [DOI] [PubMed] [Google Scholar]
- Saj A, Cojan Y, Vocat R, Luaute J, & Vuilleumier P (2013). Prism adaptation enhances activity of intact fronto-parietal areas in both hemispheres in neglect patients. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 49(1), 107–119. 10.1016/j.cortex.2011.10.009 [DOI] [PubMed] [Google Scholar]
- Schotten M. T. de, Dell’Acqua F, Forkel SJ, Simmons A, Vergani F, Murphy DGM, & Catani M (2011). A lateralized brain network for visuospatial attention. Nature Neuroscience, 14(10), 1245–1246. [DOI] [PubMed] [Google Scholar]
- Schotten M. T. de, Tomaiuolo F, Aiello M, Merola S, Silvetti M, Lecce F, … Doricchi F (2014). Damage to white matter pathways in subacute and chronic spatial neglect: A group study and 2 single-case studies with complete virtual “in vivo” tractography dissection. Cerebral Cortex, 24(3), 691–706. [DOI] [PubMed] [Google Scholar]
- Shiraishi H, Muraki T, Itou YSA, & Hirayama K (2010). Prism intervention helped sustainability of effects and ADL performances in chronic hemispatial neglect: a follow-up study. NeuroRehabilitation, 27(2), 165–172. https://doi.org/10.3233/NRE-2010-0593 ; https://doi.org/10.3233/NRE-2010-059310.3233/NRE-2010-0593; 10.3233/NRE-2010-0593 [DOI] [PubMed] [Google Scholar]
- Stone SP, Halligan PW, & Greenwood RJ (1993). The incidence of neglect phenomena and related disorders in patients with an acute right or left hemisphere stroke. Age and Ageing, 22(1), 46–52. [DOI] [PubMed] [Google Scholar]
- Striemer CL, & Danckert J (2010a). Dissociating perceptual and motor effects of prism adaptation in neglect. Neuroreport, 21(6), 436–441. 10.1097/WNR.0b013e328338592f [DOI] [PubMed] [Google Scholar]
- Striemer CL, & Danckert JA (2010b). Through a prism darkly: re-evaluating prisms and neglect. Trends in Cognitive Sciences, 14(7), 308–316. 10.1016/j.tics.2010.04.001 [DOI] [PubMed] [Google Scholar]
- Taniguchi H, Hiyamizu M, Tominaga T, & Morioka S (2012). Brain Activity Stimulated by Prism Adaptation Tasks Utilized for the Treatment of Unilateral Spatial Neglect: A Study with fNIRS. Rehabilitation Research and Practice, 2012. 10.1155/2012/312781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Brink AF, Visser-Meily JMA, Schut MJ, Kouwenhoven M, Eijsackers ALH, & Nijboer TCW (2017, epub ahead of print). Prism Adaptation in Rehabilitation? No Additional Effects of Prism Adaptation on Neglect Recovery in the Subacute Phase Poststroke: A Randomized Controlled Trial. Neurorehabilitation and Neural Repair. 10.1177/1545968317744277 [DOI] [PubMed] [Google Scholar]
- Triggs WJ, Gold M, Gerstle G, Adair J, & Heilman KM (1994). Motor neglect associated with a discrete parietal lesion. Neurology, 44(6), 1164–1166. [DOI] [PubMed] [Google Scholar]
- Turton AJ, O’Leary K, Gabb J, Woodward R, & Gilchrist ID (2010). A single blinded randomised controlled pilot trial of prism adaptation for improving self-care in stroke patients with neglect. Neuropsychological Rehabilitation, 20(2), 180–196. 10.1080/09602010903040683 [DOI] [PubMed] [Google Scholar]
- Valenstein E, & Heilman KM (1981). Unilateral hypokinesia and motor extinction. Neurology, 31(4), 445–448. [DOI] [PubMed] [Google Scholar]
- Verdon V, Schwartz S, Lovblad K-O, Hauert C-A, & Vuilleumier P (2010). Neuroanatomy of hemispatial neglect and its functional components: A study using voxel-based lesion-symptom mapping. Brain, 133(3), 880–894. 10.1093/brain/awp305 [DOI] [PubMed] [Google Scholar]
- Vossel S, Weiss ES, Eschenbeck P, & Fink GR (2013). Anosognosia, neglect, extinction and lesion site predict impairment of daily living after right-hemispheric stroke. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 49(7), 1782–1789. 10.1016/j.cortex.2012.12.011 [DOI] [PubMed] [Google Scholar]
- Vossel S, Weiss PH, Eschenbeck P, Saliger J, Karbe H, & Fink GR (2012). The neural basis of anosognosia for spatial neglect after stroke. Stroke, 43(7), 1954–1956. 10.1161/STROKEAHA.112.657288 [DOI] [PubMed] [Google Scholar]
- Watson RT, Miller BD, & Heilman KM (1978). Nonsensory neglect. Annals of Neurology, 3(6), 505–508. 10.1002/ana.410030609 [DOI] [PubMed] [Google Scholar]
- Yang NYH, Zhou D, Chung RCK, Li-Tsang CWP, & Fong KNK (2013). Rehabilitation interventions for unilateral neglect after stroke: A systematic review from 1997 through 2012. Frontiers in Human Neuroscience, 7, 187 10.3389/fnhum.2013.00187 [DOI] [PMC free article] [PubMed] [Google Scholar]