Abstract
Background
While some trials suggest benefits of liposomal bupivacaine, data on real-world use and effectiveness is lacking. We aimed to study the impact of liposomal bupivacaine use (regardless of administration route) on inpatient opioid prescription, resource utilization and opioid-related complications among patients undergoing total knee arthroplasties with a peripheral nerve block. We hypothesized that liposomal bupivacaine has limited clinical influence on the studied outcomes.
Methods
We included data on 88,830 total knee arthroplasties performed with a peripheral nerve block (Premier Healthcare Database 2013–2016). Multilevel multivariable regressions measured associations between use of liposomal bupivacaine and (1) inpatient opioid prescription (extracted from billing), length and cost of hospitalization, and (2) opioid-related complications. To reflect the difference between statistical and clinical significance we assumed a relative change of −15% in outcomes to be clinically important.
Results
Overall, liposomal bupivacaine was used in 21.2% (n=18,817) of patients that underwent a total knee arthroplasty with a peripheral nerve block. Liposomal bupivacaine use was not associated with clinically meaningful reduction in inpatient opioid prescription (group median 253 mg oral morphine equivalents, adjusted effect −9.3% CI 11.1%; −7.5% P<0.0001) and length of stay (group median 3 days, adjusted effect −8.8% CI −10.1%; −7.5% P<0.0001) with no effect on cost of hospitalization. Most importantly, liposomal bupivacaine use was not associated with decreased odds for opioid-related complications.
Conclusions
Liposomal bupivacaine was not associated with a clinically relevant improvement in inpatient opioid prescription, resource utilization or opioid-related complications in patients who received modern pain management including a peripheral nerve block.
Introduction
The management of pain after total knee arthroplasty poses a special challenge to perioperative physicians. While opioid based regimens remain the cornerstone of postoperative pain control, a multimodal approach involving a combination of opioids, non-opioid analgesics and regional anesthetic techniques is increasingly being used.1–4 By combining two or more analgesic agents with different mechanisms of action, a multimodal approach should provide adequate pain relief while reducing opioid requirements and opioid-related adverse effects.1,3
In this context, local anesthetics have provided the basis for the performance of peripheral nerve blocks or infiltration techniques, which have been highly effective in reducing pain after joint arthroplasties.5–7 However, their effectiveness has been limited by a relatively short period of action. Catheter approaches have provided the ability to prolong the infusion of local anesthetics but require additional equipment, maintenance and in rare occasions are associated with complications such as infections and in-patient falls.8,9 A recently developed therapy, liposomal bupivacaine, showed promise in addressing these shortfalls. By encapsulating a local anesthetic (bupivacaine) with a lipid-based structure, the local anesthetic is released slowly over time. Thus, liposomal bupivacaine potentially offers the ease of a single shot technique along with the longevity associated with a catheter. However, its utility in clinical practice remains debated.10,11 While liposomal bupivacaine has been approved by the US Food and Drug Administration (FDA) for injection around the surgical site12, as of now its neuraxial, perineural, or intraarticular use is not recommended. Therefore, although off-label use may indeed occur, we expect most use of liposomal bupivacaine to be in line with its FDA approved route.
While several studies in various general patient populations have shown encouraging results, a number of publications have suggested a limited benefit.13–16 Moreover, previous studies have been limited by several factors, including nonequivalence between comparison groups as well as not following best practices with respect to multimodal analgesia techniques. Moreover, these studies have typically been limited to a single institution, so that population-based data representing everyday practice using liposomal bupivacaine and its effectiveness to reduce opioid use and opioid-related adverse effects are lacking.
Therefore, we used a large national database to study the impact of liposomal bupivacaine (regardless of route of administration) in patients undergoing a total knee arthroplasty, with particular focus on those undergoing the procedure with the utilization of a peripheral nerve block. We analyzed the effect of liposomal bupivacaine use on outcomes. Our primary outcome was inpatient opioid prescription (extracted from billing). Secondary outcomes were opioid-related complications, hospitalization cost and length of hospital stay.
We hypothesized that 1) liposomal bupivacaine was used in the minority of cases, 2) that utilization was increasing over time, and 3) that liposomal bupivacaine has limited clinical influence on the studied outcomes in patients who received multimodal analgesia including peripheral nerve blocks.
Methods
Data Source, Study Design, and Study Sample
This retrospective cohort study was considered exempt by the Institutional Review Boards of the Mount Sinai Hospital (# 14-00647) and the Hospital for Special Surgery (# 2012-050) due to the de-identified nature of the data. The data source was the nationwide all-payer Premier Healthcare database (Premier Inc., Charlotte, NC), which contains detailed billing information on 20–25% of US hospitalizations.17,18 The study sample was created using the International Classification of Diseases, Ninth Revision (ICD-9) procedure code for primary knee replacement (81.54). The cohort contained patients undergoing surgery from January 1, 2013 to December 31, 2016. From this sample, we selected patients that received a peripheral nerve block (n=100,795). Patients were excluded in case of non-elective procedures (n=1,852), unknown discharge status (n=9), an outpatient procedure (n=477), treatment at a hospital performing <30 total knee arthroplasties (to ensure sufficient sample size per hospital n=715), 19 absent billing for inpatient opioid prescription (n=4,268), and inpatient opioid prescription >95th percentile (n=4,644). The latter two exclusion criteria were applied in order to account for extremes and potentially unreliable billing for opioids. The Premier data have been used in numerous population-based studies examining health outcomes.20–22 After applying the exclusion criteria there were no missing data for any variable of interest.
We specifically aimed to perform our main analyses in a cohort of patients receiving peripheral nerve blocks to analyze the impact of liposomal bupivacaine in a setting in which state of the art pain management was practiced; this decision was made a priori.
Study Variables
An analysis plan was created a priori which identified all relevant study variables including the main effect of interest and outcomes. The main effect of interest was the use of liposomal bupivacaine; a binary variable was created based on billing for liposomal bupivacaine. Unfortunately, using the Premier dataset, we were not able to differentiate between the various routes of liposomal bupivacaine administration. However, we expect most liposomal bupivacaine to be used in line with its FDA approved administration modality (infiltration at the surgical site). Use of a peripheral nerve block was determined by a combination of billed charges and Current Procedural Terminology codes as previously defined. 23
The primary outcome was inpatient opioid prescription. This was extracted from billing for opioids and converted to oral morphine equivalents using the Lexicomp® “opioid agonist conversion” and the GlobalRPH “opioid analgesic converter”.24,25 We used this as a proxy for opioid administration, recognizing that this may lead to an overestimation as not everything billed for may be actually administered. This bias, however, is minimized as we expect this likely overestimation to be independent of liposomal bupivacaine use and thus affect both groups equally.
Secondary outcomes were length and cost of hospitalization, and opioid-related complications including respiratory, gastrointestinal, central nervous system, genitourinary, and “other” (composite of ICD-9 codes for postoperative bradycardia, rash or itching, drugs causing adverse effects with therapeutic use, and fall from bed) complications.26 Inpatient opioid prescription (in mg oral morphine equivalents, OME) was categorized into prescription totals for the entire hospitalization, and totals for the day of surgery (day 0), the day after (day 1) and the subsequent days (day 1+). Cost of hospitalization was adjusted for inflation and reported in 2016 US dollars. Length of stay was reported in days.
Patient demographics included age, sex and race (White, Black, Other). Healthcare-related variables included insurance type (commercial, Medicaid, Medicare, uninsured, other), hospital location (urban, rural), hospital size (<300, 300–499, ≥ 500 beds), hospital teaching status, and the annual number of total knee arthroplasties performed per hospital. Procedure-related variables included the year of the procedure, the use of neuraxial anesthesia, the use of general anesthesia, patient-controlled analgesia, and use of non-opioid analgesics (intravenous acetaminophen [IV acetaminophen], gabapentin/pregabalin [gabapentinoids], nonsteroidal anti-inflammatory drugs [NSAIDs], cyclooxygenase-2 [cox-2] inhibitors, and ketamine). Overall comorbidity burden was assessed using the Quan adaptation of the Charlson-Deyo Comorbidity Index.27 In addition, we included separate variables indicating substance use/abuse (including smoking), chronic pain conditions, and psychiatric comorbidity variables as they may influence particularly inpatient opioid prescription. The definitions of these variables can be found in Appendix I.
As is typical when using population-based data (i.e. data not specifically collected for any specific study), variables may be under- or over-reported. This holds particularly true for inpatient opioid utilization as the amount billed for may not necessarily reflect the amount administered; thus, a potential overestimation may occur. However, numbers from the current study fall in line with those reported in other studies.28–30 For opioid-related complications we expect an under-reporting as they are defined using ICD-9 codes for which a limited number of fields exist per case. We expect cost and length of stay to be accurate as that is the main focus of the Premier Healthcare dataset (i.e. economic benchmarking for hospitals).18
Statistical Analysis
Univariable associations between liposomal bupivacaine use and study variables were analyzed using the Chi-square test and t-test for categorical and continuous variables, respectively. Multilevel, multivariable regression models were fit to measure associations between liposomal bupivacaine use and outcomes. Multilevel models account for correlation of patients within hospitals (i.e. patients are ‘nested’ within each hospital), and fit one regression line for each hospital, based on all patients within a given hospital.31 This adjustment is necessary as patients within hospitals are correlated as they may experience similar care (e.g. equal chance of liposomal bupivacaine for patients within the same hospital).
Covariates used in the multilevel models were chosen based on clinical importance and/or univariable significance at the P<0.15 level.32 The final covariates in the model are: age, hospital-specific annual volume of total knee arthroplasties, hospital size, Charlson-Deyo Index, sex, race, year, insurance type, hospital teaching status, hospital location, general anesthesia use, neuraxial anesthesia use, liposomal bupivacaine, NSAIDs, cox-2 inhibitors, ketamine, gabapentinoids, patient-controlled analgesia, IV acetaminophen, and the comorbidity variables indicating substance use/abuse, chronic pain conditions, and psychiatric conditions. Full model coefficients can be found in Appendix II. Effect estimates are reported as adjusted odds ratios (OR) with Bonferroni-adjusted p-values and 95% confidence intervals (CI), which will reduce the risk of type I errors while the likelihood of type II errors may be increased.33 Model discrimination was evaluated using the c-statistic (area under the receiver operating curve). A c-statistic value of 0.7 or higher shows good discrimination. 34
All analyses were done using the PROC GLIMMIX procedure in SAS v9.4 statistical software (SAS Institute, Cary, NC). For the continuous outcomes of inpatient opioid prescription, cost of hospitalization, and length of stay the gamma distribution with a log link function were used as these variables are skewed.35,36 Effect estimates for these outcomes are reported as % change and CIs.
The PROC GLIMMIX procedure was additionally used to determine the intraclass correlation coefficient which estimates the percent variation in liposomal bupivacaine use that is accounted for by hospitals included in our study.37 A high percentage would indicate that liposomal bupivacaine use is mainly determined by hospital-level factors.
Sensitivity Analysis
We performed a sensitivity analysis in order to address the possible issue of confounding by indication with patients receiving liposomal bupivacaine because of greater pain and thus more likely to have increased inpatient opioid prescription. In order to address this potential bias, the sensitivity analysis was performed in a cohort restricted to hospitals with ≥50% liposomal bupivacaine use, which may indicate that these hospitals have a perioperative pain protocol that includes liposomal bupivacaine, hence negating the idea of confounding by indication.
Statistical and Clinical Meaningful Significance
As recommended, we judged our inpatient opioid prescription and resource utilization results in respect to their clinically meaningful significance by a priori designating an at least -15% reduction of the reported outcome as clinically meaningful. Authors studying opioid-related complications have suggested that this reduction should be at least 25%38, thus our threshold should be considered less stringent. Statistical significance is reported at the traditional P<0.05 value.
A Priori versus Post Hoc Analyses
Through the course of the peer-review process, we made adjustments to our initial a priori specified analyses. Specifically, we performed the same analyses as mentioned above in two separate cohorts:
Patients undergoing total knee arthroplasty with a peripheral nerve block and use of general anesthesia;
Patients undergoing total knee arthroplasty with a peripheral nerve block and use of neuraxial anesthesia.
The reasoning behind these additional analyses is that we expect a more pronounced effect of liposomal bupivacaine among patients with a higher (general anesthesia patients) compared to a lower (neuraxial anesthesia patients) baseline opioid utilization. Use of general and neuraxial anesthesia is defined through the Premier billing file as previously described.23 An additional analysis was performed to assess if using the individual comorbidities making up the Index in our multivariable models would produce different results compared to the use of the Charlson-Deyo Index itself.
Results
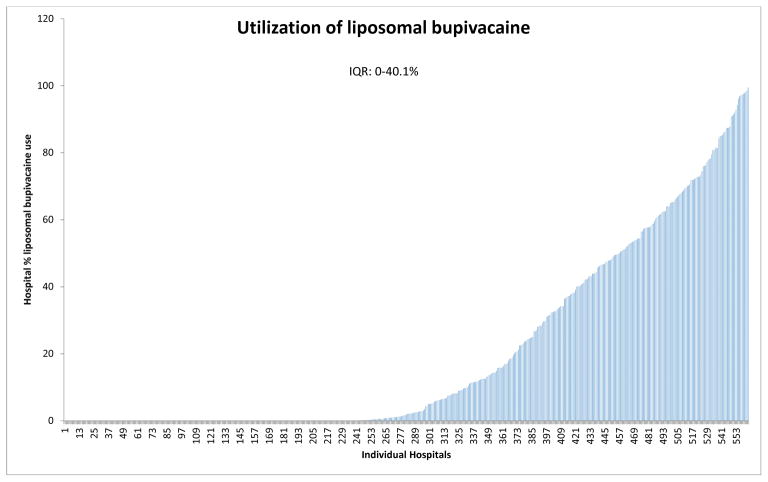
We identified 88,830 hospitalizations from 2013 to 2016 during which a total knee arthroplasty was performed using a peripheral nerve block. Liposomal bupivacaine was used in 21.2% (n=18,817) of patients with substantial inter-hospital variation in liposomal bupivacaine use ranging from 0% to 99.5% (IQR: 0–40.1%) (Figure 1). In addition, use of liposomal bupivacaine increased over time from 7.1% in 2013 to 25.5% in 2016, respectively.
Figure 1.
Interhospital variation (with interquartile range, IQR) in liposomal bupivacaine use.
Table 1 shows patient demographics, healthcare-related and procedure-related variables, and comorbidities by use of liposomal bupivacaine. Differences in patient demographics, comorbidities and insurance type were small between those receiving and not receiving liposomal bupivacaine. Use of liposomal bupivacaine was higher in urban, small and medium-sized hospitals, and non-teaching hospitals. Institutions using liposomal bupivacaine performed a higher number of total knee arthroplasties on an annual basis. Of note, patients who received liposomal bupivacaine also received other non-opioid analgesic medication significantly more often than the control group, except for NSAIDs and Ketamine.
Table 1.
Patient demographics, healthcare related, procedure related and comorbidity related variables (Chi-square and t-test for categorical and continuous variables, respectively) by liposomal bupivacaine use in the peripheral nerve block cohort.
| Use of liposomal bupivacaine | |||
|---|---|---|---|
| Yes (n=18,817) | No (n=70,013) | ||
| n (%) | n (%) | P-value | |
| PATIENT DEMOGRAPHICS | |||
| Age(years) (median (IQR)) | 67 (60–73) | 67 (60–73) | 0.0032 |
| Sex | 0.8101 | ||
| Female | 11,588 (61.6) | 43,183 (61.7) | |
| Male | 7,229 (38.4) | 26,830 (38.3) | |
| Race/Ethnicity | <0.0001 | ||
| White | 15,537 (82.6) | 55,839 (79.8) | |
| Black | 2,184 (11.6) | 5,478 (7.8) | |
| Other | 1,096 (5.8) | 8,696 (12.4) | |
| COMORBIDITY RELATED | |||
| Charlson-Deyo Comorbidity Index (categorized) | <0.0001 | ||
| 0 | 13,843 (73.6) | 50,207 (71.7) | |
| 1 | 3,540 (18.8) | 13,853 (19.8) | |
| 2 | 988 (5.3) | 3,900 (5.6) | |
| 2+ | 446 (2.4) | 2,053 (2.9) | |
| Substance Use/Abuse | 1,362 (7.2) | 5,413 (7.7) | 0.0236 |
| Pain Conditions | 3,191 (17.0) | 12,698 (18.1) | 0.0002 |
| Psychiatric Conditions | 3,762 (20.0) | 15,498 (22.1) | <0.0001 |
| PROCEDURE RELATED | |||
| Year of Procedure | <0.0001 | ||
| 2013 | 1,300 (6.9) | 16,946 (24.2) | |
| 2014 | 4,620 (24.6) | 16,799 (24.0) | |
| 2015 | 6,629 (35.2) | 18,011 (25.7) | |
| 2016 | 6,268 (33.3) | 18,257 (26.1) | |
| General Anesthesia Use | 10,260 (54.5) | 33,021 (47.2) | <0.0001 |
| Neuraxial Anesthesia Use | 1,773 (9.4) | 16,140 (23.1) | <0.0001 |
| NSAIDs Use | 10,748 (57.1) | 39,573 (56.5) | 0.1429 |
| Cox-2 inhibitors Use | 11,095 (59.0) | 32,771 (46.8) | <0.0001 |
| Ketamine Use | 1,127 (6.0) | 5,640 (8.1) | <0.0001 |
| Gabapentinoids Use | 9,011 (47.9) | 22,213 (31.7) | <0.0001 |
| IV Acetaminophen Use | 7,514 (39.9) | 19,163 (27.4) | <0.0001 |
| Patient Controlled Analgesia Use | 237 (1.3) | 6,019 (8.6) | <0.0001 |
| HEALTHCARE RELATED | |||
| Insurance Type | <0.0001 | ||
| Commercial | 6,652 (35.4) | 24,553 (35.1) | |
| Medicaid | 343 (1.8) | 2,319 (3.3) | |
| Medicare | 11,238 (59.7) | 40,704 (58.1) | |
| Uninsured | 33 (0.2) | 163 (0.2) | |
| Unknown | 551 (2.9) | 2,274 (3.2) | |
| Hospital Location | <0.0001 | ||
| Rural | 726 (3.9) | 8,385 (12.0) | |
| Urban | 18,091 (96.1) | 61,628 (88.0) | |
| Hospital Size | <0.0001 | ||
| <300 beds | 8,885 (47.2) | 28,281 (40.4) | |
| 300–499 beds | 6,880 (36.6) | 18,796 (26.8) | |
| ≥500 beds | 3,052 (16.2) | 22,936 (32.8) | |
| Hospital Teaching Status | <0.0001 | ||
| Non-Teaching | 12,572 (66.8) | 37,720 (53.9) | |
| Teaching | 6,245 (33.2) | 32,293 (46.1) | |
| Annual Total Knee Arthroplasties per Hospital (median (IQR)) | 515 (331–770) | 503 (276–774) | <0.0001 |
IQR: Interquartile range
NSAIDs: Nonsteroidal antiinflammatory drugs
Cox-2: Cyclooxygenase-2
Table 2 details the prevalence of complications and provides information on inpatient opioid prescription, cost and length of hospitalization, as well as outcomes from multivariable models.
Table 2.
Univariable (Chi-square and t-test for categorical and continuous variables, respectively) and multivariable results by liposomal bupivacaine use in the peripheral nerve block cohort.
| Univariable results | Multivariable results | ||||
|---|---|---|---|---|---|
| Use of liposomal bupivacaine | Use of liposomal bupivacaine | ||||
| Yes (n=18,817) | No (n=70,013) | Yes (Reference=No) | |||
| n (%) | n (%) | P-value | Odds ratio (95% CI) | P-value | |
| OPIOID-RELATED COMPLICATIONS | |||||
| Respiratory Complications | 199 (1.1) | 1,205 (1.7) | <0.0001 | 1.08 (0.78; 1.50) | >0.999 |
| Genitourinary Complications | 286 (1.5) | 1,233 (1.8) | 0.0235 | 1.01 (0.75; 1.35) | >0.999 |
| Central Nervous System Complications | 117 (0.6) | 491 (0.7) | 0.2402 | 1.09 (0.73; 1.63) | >0.999 |
| Gastrointestinal Complications | 289 (1.5) | 1,671 (2.4) | <0.0001 | 0.97 (0.74; 1.29) | >0.999 |
| “Other” Complications | 137 (0.7) | 897 (1.3) | <0.0001 | 0.83 (0.57; 1.21) | >0.999 |
| Naloxone Use | 107 (0.6) | 620 (0.9) | <0.0001 | 1.01 (0.66; 1.54) | >0.999 |
| Median (IQR) | Median (IQR) | Percent change (95% CI) | |||
| INPATIENT OPIOID PRESCRIPTION | |||||
| Total Opioids (mg OME) | 190 (120–298) | 272 (173–413) | <0.0001 | −9.3% (−11.1%; −7.5%) | <0.0001 |
| Day 0 Opioids (mg OME) | 98 (60–150) | 126 (75–203) | <0.0001 | −7.6% (−9.6%; −5.5%) | <0.0001 |
| Day 1 Opioids (mg OME) | 50 (24–87) | 60 (30–102) | <0.0001 | −7.4% (−10.0%; −4.8%) | <0.0001 |
| Day 1+ Opioids (mg OME) | 30 (0–65) | 55 (23–105) | <0.0001 | −7.6% (−10.8%; −4.3%) | <0.0001 |
| RESOURCE UTILIZATION | |||||
| Cost of Hospitalization (US dollars) | 15,013 (13,113–18,088) | 15,810 (13,503–19,022) | <0.0001 | 2.2% (−0.1%; 4.6%) | 0.0900 |
| Length of Stay (days) | 2 (2–3) | 3 (2–3) | <0.0001 | −8.8% (−10.1%; −7.5%) | <0.0001 |
Multivariable models adjusted for: age, volume of annual total knee arthroplasties per hospital, hospital size, Charlson-Deyo Index, sex, race, year, insurance, teaching status, hospital area, general anesthesia use, neuraxial anesthesia use, liposomal bupivacaine, NSAIDs, Cox-2 inhibitors, ketamine, gabapentinoids, patient-controlled analgesia, IV acetaminophen, and the comorbidities for substance use/abuse, pain conditions, and psychiatric conditions.
OME: Oral morphine equivalents
IQR: Interquartile range
CI: Confidence interval
NSAIDs: Nonsteroidal antiinflammatory drugs
Cox-2: Cyclooxygenase-2
“Other” complications include postoperative bradycardia, rash or itching, drugs causing adverse effects with therapeutic use, and fall from bed
In general, absolute rates of complications in patients who received liposomal bupivacaine were marginally lower compared to those who did not receive liposomal bupivacaine. Univariable results showed that patients in the liposomal bupivacaine group had lower inpatient opioid prescription (190 vs. 272 median mg oral morphine equivalents), length of stay (2 vs. 3 median days) and cost of hospitalization ($15,013 vs. $15,810 median dollars).
However, when controlling for relevant covariates, the use of liposomal bupivacaine was associated with no clinically meaningful reduction in overall inpatient opioid prescription (adjusted effect −9.3% CI −11.1%; −7.5% P<0.0001) and length of stay (−8.8% CI −10.1%; −7.5% P<0.0001), and a non-significant increase in cost of hospitalization (+2.2% CI −0.1%; +4.6% P=0.0900).
Furthermore, liposomal bupivacaine use was not associated with significantly lower odds for opioid-related complications in patients undergoing a total knee arthroplasty with a peripheral nerve block. The c-statistics for the associated models varied between 0.75 and 0.79 indicating sufficient model discrimination.
The intraclass correlation coefficient demonstrated that 79% of the variability in liposomal bupivacaine use can be accounted for by hospital-level factors.
Sensitivity analyses restricting the cohort to hospitals with >50% liposomal bupivacaine use showed similar effect estimates for inpatient opioid prescription and length and cost of hospitalization (Tables 3 and 4). Appendices III–VI depict the same analyses in two subgroups of patients undergoing total knee arthroplasty with a peripheral nerve block and 1) general anesthesia or 2) neuraxial anesthesia. Multivariable results in these cohorts also showed a non-clinically significant reduction in inpatient opioid prescription associated with the use of liposomal bupivacaine when compared to no liposomal bupivacaine use. However, the effect was more pronounced in the general anesthesia subgroup (−12.3% CI −14.8%; −9.7% P<0.0001), whereas in the neuraxial anesthesia subgroup the reduction was not statistically significant (−6.1% CI −14.6%; 3.2%, P>0.999).
Table 3.
Patient demographics, healthcare related, procedure related and comorbidity related variables (Chi-square and t-test for categorical and continuous variables, respectively) by liposomal bupivacaine use for the sensitivity analysis in hospitals with >50% liposomal bupivacaine use.
| Use of liposomal bupivacaine | |||
|---|---|---|---|
| Yes (n=11,606) | No (n=4,428) | ||
| n (%) | n (%) | P-value | |
| PATIENT DEMOGRAPHICS | |||
| Age(years) (median (IQR)) | 67 (61–73) | 66 (59–73) | <0.0001 |
| Sex | 0.9364 | ||
| Female | 7,187 (61.9) | 2,739 (61.9) | |
| Male | 4,419 (38.1) | 1,689 (38.1) | |
| Race/Ethnicity | 0.0008 | ||
| White | 9,691 (83.5) | 3,586 (81.0) | |
| Black | 1,381 (11.9) | 603 (13.6) | |
| Other | 534 (4.6) | 239 (5.4) | |
| COMORBIDITY RELATED | |||
| Charlson-Deyo Comorbidity Index (categorized) | 0.0002 | ||
| 0 | 8,608 (74.2) | 3,150 (71.1) | |
| 1 | 2,193 (18.9) | 915 (20.7) | |
| 2 | 560 (4.8) | 232 (5.2) | |
| 2+ | 245 (2.1) | 131 (3.0) | |
| Substance Use/Abuse | 825 (7.1) | 393 (8.9) | 0.0002 |
| Pain Conditions | 1,937 (16.7) | 696 (15.7) | 0.1377 |
| Psychiatric Conditions | 2,364 (20.4) | 926 (20.9) | 0.4460 |
| PROCEDURE RELATED | |||
| Year of Procedure | <0.0001 | ||
| 2013 | 1,107 (9.5) | 1,754 (39.6) | |
| 2014 | 3,004 (25.9) | 890 (20.1) | |
| 2015 | 3,988 (34.4) | 758 (17.1) | |
| 2016 | 3,507 (30.2) | 1,026 (23.2) | |
| General Anesthesia Use | 7,612 (65.6) | 2,812 (63.5) | 0.0135 |
| Neuraxial Anesthesia Use | 1,247 (10.7) | 507 (11.5) | 0.2007 |
| NSAIDs Use | 6,897 (59.4) | 2,426 (54.8) | <0.0001 |
| Cox-2 Inhibitors Use | 7,195 (62.0) | 1,985 (44.8) | <0.0001 |
| Ketamine Use | 648 (5.6) | 252 (5.7) | 0.7910 |
| Gabapentinoids Use | 6,304 (54.3) | 1,949 (44.0) | <0.0001 |
| IV Acetaminophen Use | 5,729 (49.4) | 1,511 (34.1) | <0.0001 |
| Patient Controlled Analgesia Use | 137 (1.2) | 141 (3.2) | <0.0001 |
| HEALTHCARE RELATED | |||
| Insurance Type | 0.0036 | ||
| Commercial | 3,998 (34.4) | 1,600 (36.1) | |
| Medicaid | 213 (1.8) | 116 (2.6) | |
| Medicare | 7,056 (60.8) | 2,593 (58.6) | |
| Uninsured | 17 (0.1) | 7 (0.2) | |
| Unknown | 322 (2.8) | 112 (2.5) | |
| Hospital Location | 0.3114 | ||
| Rural | 344 (3.0) | 118 (2.7) | |
| Urban | 11,262 (97.0) | 4,310 (97.3) | |
| Hospital Size | <0.0001 | ||
| <300 beds | 6,024 (51.9) | 2,721 (61.5) | |
| 300–499 beds | 5,514 (47.5) | 1,687 (38.1) | |
| ≥500 beds | 68 (0.6) | 20 (0.5) | |
| Hospital Teaching Status | <0.0001 | ||
| Non-Teaching | 8,038 (69.3) | 3,353 (75.7) | |
| Teaching | 3,568 (30.7) | 1,075 (24.3) | |
| Annual Total Knee Arthroplasties per Hospital (median (IQR)) | 485 (331–770) | 435 (331–770) | 0.1328 |
IQR: Interquartile range
NSAIDs: Nonsteroidal antiinflammatory drugs
Cox-2: Cyclooxygenase-2
Table 4.
Univariable (T-test for comparisons) and multivariable results by liposomal bupivacaine use for the sensitivity analysis in hospitals with >50% liposomal bupivacaine use.
| Univariable results | Multivariable results | ||||
|---|---|---|---|---|---|
| Use of liposomal bupivacaine | Use of liposomal bupivacaine | ||||
| Median (IQR) | Median (IQR) | P-value | Percent change (95% CI) | P-value | |
| INPATIENT OPIOID PRESCRIPTION | |||||
| Total Opioids (mg OME) | 170 (110–260) | 235 (140–357) | <0.0001 | −12.6% (−16.0%; −9.0%) | <0.0001 |
| Day 0 Opioids (mg OME) | 95 (55–138) | 113 (60–180) | <0.0001 | −9.4% (−12.9%; −5.7%) | <0.0001 |
| Day 1 Opioids (mg OME) | 45 (20–75) | 56 (30–90) | <0.0001 | −7.7% (−12.5%; −2.6%) | <0.0001 |
| Day 1+ Opioids (mg OME) | 23 (0–60) | 45 (15–90) | <0.0001 | −9.5% (−15.7%; −2.9%) | <0.0001 |
| RESOURCE UTILIZATION | |||||
| Cost of Hospitalization (US dollars) | 14,356 (12,818–16,572) | 14,081 (12,781–16,271) | 0.0002 | −3.1% (−10.6%; 5.0%) | >0.999 |
| Length of Stay (days) | 2 (2–3) | 2 (2–3) | <0.0001 | −13.1% (−15.9%; −10.3%) | <0.0001 |
Multivariable models adjusted for: age, volume of annual total knee arthroplasties per hospital, hospital size, Charlson-Deyo Index, sex, race, year, insurance, teaching status, hospital area, general anesthesia use, neuraxial anesthesia use, liposomal bupivacaine, NSAIDs, Cox-2 inhibitors, ketamine, gabapentinoids, patient-controlled analgesia, IV acetaminophen, and the comorbidities for substance use/abuse, pain conditions, and psychiatric conditions.
OME: Oral morphine equivalents
IQR: Interquartile range
CI: Confidence interval
NSAIDs: Nonsteroidal antiinflammatory drugs
Cox-2: Cyclooxygenase-2
In addition, we performed an analysis to assess if using the individual Charlson-Deyo comorbidities in our multivariable models would produce different results compared to the use of the Charlson-Deyo Index. Liposomal bupivacaine was associated with −9.3% (CI −10.4%; −8.2%) reduction in total inpatient opioid prescription when using a model with individual comorbidities; this was −9.3% (CI −11.1%; −7.5%) when using a model with the Charlson-Deyo Index. Since the results are essentially unaltered, we opted to continue using the Charlson-Deyo Index in our multivariable models.
Discussion
In this observational study of 88,830 total knee arthroplasties in patients who also received a peripheral nerve block, we were unable to determine an association between liposomal bupivacaine use and clinically meaningful reductions in inpatient opioid prescription. Furthermore, no relevant impact of liposomal bupivacaine use on complications or resource utilization were observed. Our sensitivity analyses supported results from our main analysis, demonstrating the robustness of our results. Although only a minority of patients (21.2 %) received liposomal bupivacaine, an initial increase in the utilization of this product was seen over time. Utilization reached a plateau in the latter two years of the study. In addition, we demonstrated substantial inter-hospital variation in the use of liposomal bupivacaine, which was also reflected in the intraclass correlation coefficient.
The reasons for the variability in liposomal bupivacaine use have to remain speculative but are likely to include physician and patient preference. We have previously shown that pain management modalities vary widely among hospitals.39 Factors influencing the choice to use a certain medication may be institution and provider-related. They may include differences in experience and training, the use of protocols and cost considerations.40 While the rapid increase in its utilization in the first three years on the market can be attributed to the fact that liposomal bupivacaine is a new drug, approved by the FDA in 2011, this trend may also reflect the growing awareness for opioid-related adverse effects and the desire to reduce them.41,42 A more recent reduction, however, is noteworthy and while no firm conclusions can be drawn from our data this finding may at least in part be explained by the lack of data supporting its efficacy in this patient population in the context of its relatively high cost.
We did not observe a clinically meaningful reduction in inpatient opioid prescription and resource utilization outcomes. Moreover, liposomal bupivacaine use was not associated with any difference in opioid–related complications. This suggests that, in the context of state-of-the-art pain management including peripheral nerve blocks, liposomal bupivacaine may have a limited role. This is in line with a recent publication by Amundson et al., suggesting that suggesting that liposomal bupivacaine is inferior to the use of femoral catheters plus sciatic blocks in total knee arthroplasties.10 The authors of this study also found that liposomal bupivacaine was not superior to a Ropivacaine mixture.
Bagsby et al. also demonstrated that a periarticular liposomal bupivacaine injection in primary total knee arthroplasty patients had no benefit over Ropivacaine regarding pain and opioid usage.14 The authors concluded that when a multimodal pain protocol is being used, liposomal bupivacaine does not beneficially influence pain control. Similar results have been reported by Schroer et al.13 In their prospective, blinded, randomized study of 111 patients undergoing a total knee arthroplasty and receiving a multimodal pain therapy they found no benefit of liposomal bupivacaine over bupivacaine.
On the other hand, there is a variety of studies that suggest a positive effect of liposomal bupivacaine in total knee arthroplasty. However, some of those studies do not include comparable control groups. In a recent publication Mont et al. concluded that local infiltration of liposomal bupivacaine improves pain scores and opioid use after total knee arthroplasty.43 But as the control group received significantly lower amounts of local anesthetics (100 mg Bupivacaine in the control group vs. 100 mg Bupivacaine plus 266 mg liposomal bupivacaine in the study group) it does not seem surprising that the study group had better outcomes. Next to questionable control groups, currently published studies do not address the fact that potential efficacy of liposomal bupivacaine in highly selective trials may not translate to effectiveness in real-world settings. Indeed, while focusing on its safety profile, liposomal bupivacaine gained FDA acceptance based on trials performed in patients undergoing bunionectomies and hemorrhoidectomies44,45, a far cry from its widespread utilization in much larger surgeries characterized by more pain and opioid utilization.
Interestingly, we found that in those patients within the peripheral nerve block cohort that received general anesthesia liposomal bupivacaine was associated with a more pronounced reduction in inpatient opioid prescription compared to those that received neuraxial anesthesia. While this may be due to the higher baseline opioid utilization (as demonstrated by the higher inpatient opioid prescription) in this cohort, this further questions the value of liposomal bupivacaine in light of real-world practices where state of the art anesthetic and pain management is used including peripheral nerve blocks and neuraxial anesthesia/analgesia.
Our study is subject to a number of limitations. Because of the retrospective design, we can only determine associations and not causal relationships. Therefore, associations have to be interpreted taking into account plausibility. Furthermore, Premier Healthcare data was collected for administrative and not for research purposes thus resulting in a lack of clinical details such as pain scores and clinical conditions only captured if they are billed for. Therefore, as residual confounding may exist, it is prudent to perform sensitivity analyses to assess robustness of results, which we confirmed in the current study. The definition of comorbidities and complications is based on the ICD-9 coding system and may be burdened with coding bias. This can lead to underestimation of adverse events, but again it is likely to affect patients from different groups (those with and without liposomal bupivacaine) equally. Also, administrative data provides information only on billing for medication and not actual administration/consumption. This may include cases where one whole vial was billed, but only a portion administered with the rest discarded. Although it is likely that some level of association between billing and use of opioids exists, the utilization of multimodal analgesia may be associated with a propensity to prescribe less opioids. Thus, prescribers and users of liposomal bupivacaine or other non-opioids may be biased to order/dispense less opioid derivatives than those who do not. Furthermore, it is important to point out the difference between statistical significance and clinical meaningfulness in large database studies. To reflect this, we used a 15% threshold for clinical importance. As the placebo effect is often estimated as a 20–30% clinical effect, our lower threshold would be considered more liberal by many readers. However, as most patients would not be aware of liposomal bupivacaine administration, we expect a limited placebo effect. Moreover, use of a higher threshold could be interpreted as biased towards our hypothesis that liposomal bupivacaine does not improve outcomes, mainly in respect to pain and opioid utilization. These issues will be resolved when more data is available on specifically the minimum reduction in opioid utilization needed to result in reduced risk for adverse effects, a current gap in the literature. Another limitation is that the Premier database does not provide information on preoperative opioid use, which might be a confounder and ideally should be included as a covariate. However, we adjusted for substance use/abuse, chronic pain conditions and psychiatric conditions, which can be associated with preoperative opioid use. Indeed, previous studies have shown that around 20% of patients undergoing total knee arthroplasty use opioids preoperatively which is in line with the prevalence of chronic pain in the current study (for which we adjust).46,47 Finally, using the Premier database, we were not able to differentiate between various routes of administration of liposomal bupivacaine. Although we expect most use to be in line with liposomal bupivacaine’s FDA approval (infiltration at the surgical site), off label use of liposomal bupivacaine (e.g. in a peripheral nerve block) is possible in some cases included in this study. This would entail that the effects demonstrated in our study are an average effect of routes of administration included in our cohort. Under the assumption that most use will be in line with the FDA approval, any other route of administration will need to exert substantial effects in order to affect the mean effect in our cohort. While more detailed studies are needed on differential effectiveness of liposomal bupivacaine by route of administration, this assumption suggests that the effect of liposomal bupivacaine may be limited, especially in light of current state of the art pain management approaches including peripheral nerve and neuraxial blocks.
In conclusion, we failed to show a clinically meaningful reduction in either inpatient opioid prescription, opioid-related complications or resource utilization outcomes among patients receiving liposomal bupivacaine as part of their total knee arthroplasty. Given the number of recent publications that suggest a lack of benefit of the addition of liposomal bupivacaine to a multimodal regimen including a regional analgesic technique, its routine use should be carefully examined, especially given its relatively high cost. Future studies will need to evaluate the effectiveness of liposomal bupivacaine separately for the different routes of administration.
Supplementary Material
Acknowledgments
Funding: Eric C Sun acknowledges funding from the National Institute on Drug Abuse (K08DA032314) and reports consulting fees unrelated to this work from Egalet Inc. Drs. Mazumdar and Poeran are partially funded by the Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai. For all the other authors, support was provided solely from institutional and/or departmental sources.
Footnotes
Disclosure: Dr. Memtsoudis is a director on the boards of the American Society of Regional Anesthesia and Pain Medicine and the Society of Anesthesia and Sleep Medicine. He is a one-time consultant for Sandoz Inc. and the holder of US Patent US-2017-0361063, Multicatheter Infusion System. He is the owner of SGM Consulting, LLC and co-owner of FC Monmouth, LLC. None of the above relations influenced the conduct of the present study.
Clinical trial number: Not applicable
Prior presentations: An abstract of this study was presented as a poster at the 42nd Annual Regional Anesthesiology & Acute Pain Medicine Meeting, April 6–8 2017, San Francisco, CA, USA
Institutional Review Boards that approved the study:
Institutional Review Board of the Hospital for Special Surgery 535 East 71st Street, New York, 10021 NY, USA (# 2012-050)
Icahn School of Medicine at Mount Sinai, One Gustave L. Levy Place, New York, NY 10029-6574, USA (#14-00647)
References
- 1.Fu PL, Xiao J, Zhu YL, Wu HS, Li XH, Wu YL, Qian QR. Efficacy of a multimodal analgesia protocol in total knee arthroplasty: a randomized, controlled trial. J Int Med Res. 2010;38:1404–12. doi: 10.1177/147323001003800422. [DOI] [PubMed] [Google Scholar]
- 2.Grosu I, Lavand’homme P, Thienpont E. Pain after knee arthroplasty: an unresolved issue. Knee Surg Sports Traumatol Arthrosc. 2014;22:1744–58. doi: 10.1007/s00167-013-2750-2. [DOI] [PubMed] [Google Scholar]
- 3.Hebl JR, Dilger JA, Byer DE, Kopp SL, Stevens SR, Pagnano MW, Hanssen AD, Horlocker TT. A pre-emptive multimodal pathway featuring peripheral nerve block improves perioperative outcomes after major orthopedic surgery. Reg Anesth Pain Med. 2008;33:510–7. [PubMed] [Google Scholar]
- 4.Kelley TC, Adams MJ, Mulliken BD, Dalury DF. Efficacy of multimodal perioperative analgesia protocol with periarticular medication injection in total knee arthroplasty: a randomized, double-blinded study. J Arthroplasty. 2013;28:1274–7. doi: 10.1016/j.arth.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Paul JE, Arya A, Hurlburt L, Cheng J, Thabane L, Tidy A, Murthy Y. Femoral nerve block improves analgesia outcomes after total knee arthroplasty: a meta-analysis of randomized controlled trials. Anesthesiology. 2010;113:1144–62. doi: 10.1097/ALN.0b013e3181f4b18. [DOI] [PubMed] [Google Scholar]
- 6.Wiesmann T, Steinfeldt T, Wagner G, Wulf H, Schmitt J, Zoremba M. Supplemental single shot femoral nerve block for total hip arthroplasty: impact on early postoperative care, pain management and lung function. Minerva Anestesiol. 2014;80:48–57. [PubMed] [Google Scholar]
- 7.Marques EM, Jones HE, Elvers KT, Pyke M, Blom AW, Beswick AD. Local anaesthetic infiltration for peri-operative pain control in total hip and knee replacement: systematic review and meta-analyses of short- and long-term effectiveness. BMC Musculoskelet Disord. 2014;15:220. doi: 10.1186/1471-2474-15-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ilfeld BM. Continuous peripheral nerve blocks: a review of the published evidence. Anesth Analg. 2011;113:904–25. doi: 10.1213/ANE.0b013e3182285e01. [DOI] [PubMed] [Google Scholar]
- 9.Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg. 2010;111:1552–4. doi: 10.1213/ANE.0b013e3181fb9507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amundson AW, Johnson RL, Abdel MP, Mantilla CB, Panchamia JK, Taunton MJ, Kralovec ME, Hebl JR, Schroeder DR, Pagnano MW, Kopp SL. A Three-arm Randomized Clinical Trial Comparing Continuous Femoral Plus Single-injection Sciatic Peripheral Nerve Blocks versus Periarticular Injection with Ropivacaine or Liposomal Bupivacaine for Patients Undergoing Total Knee Arthroplasty. Anesthesiology. 2017;126:1139–1150. doi: 10.1097/ALN.0000000000001586. [DOI] [PubMed] [Google Scholar]
- 11.Vandepitte C, Kuroda M, Witvrouw R, Anne L, Bellemans J, Corten K, Vanelderen P, Mesotten D, Leunen I, Heylen M, Van Boxstael S, Golebiewski M, Van de Velde M, Knezevic NN, Hadzic A. Addition of Liposome Bupivacaine to Bupivacaine HCl Versus Bupivacaine HCl Alone for Interscalene Brachial Plexus Block in Patients Having Major Shoulder Surgery. Reg Anesth Pain Med. 2017;42:334–341. doi: 10.1097/AAP.0000000000000560. [DOI] [PubMed] [Google Scholar]
- 12. [Accessed January 22, 2018];FDA information on approval of liposomal bupivacaine. Available at: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=022496.
- 13.Schroer WC, Diesfeld PG, LeMarr AR, Morton DJ, Reedy ME. Does Extended-Release Liposomal Bupivacaine Better Control Pain Than Bupivacaine After Total Knee Arthroplasty (TKA)? A Prospective, Randomized Clinical Trial J Arthroplasty. 2015;30:64–7. doi: 10.1016/j.arth.2015.01.059. [DOI] [PubMed] [Google Scholar]
- 14.Bagsby DT, Ireland PH, Meneghini RM. Liposomal bupivacaine versus traditional periarticular injection for pain control after total knee arthroplasty. J Arthroplasty. 2014;29:1687–90. doi: 10.1016/j.arth.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 15.Sakamoto B, Keiser S, Meldrum R, Harker G, Freese A. Efficacy of Liposomal Bupivacaine Infiltration on the Management of Total Knee Arthroplasty. JAMA Surg. 2017;152:90–95. doi: 10.1001/jamasurg.2016.3474. [DOI] [PubMed] [Google Scholar]
- 16.Webb BT, Spears JR, Smith LS, Malkani AL. Periarticular injection of liposomal bupivacaine in total knee arthroplasty. Arthroplast Today. 2015;1:117–120. doi: 10.1016/j.artd.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makadia R, Ryan PB. Transforming the Premier Perspective Hospital Database into the Observational Medical Outcomes Partnership (OMOP) Common Data Model. EGEMS (Wash DC) 2014;2:1110. doi: 10.13063/2327-9214.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. [Accessed July 21, 2017];Premier Healthcare Database White Paper: Data that Informs and Performs. Available at: https://www.premierinc.com/wpdm-package/research/?wpdmdl=3005&ind=1WPMW7ESmN_SGTnlmo5LNi4O3smwj95mtUScCxFzmgTjO68iqCCmly7wENVgVCavUo7UN3ERg3tk9eJrKPBrpYl3WpNRtwLjT4WtLnmGlKI.
- 19.Moineddin R, Matheson FI, Glazier RH. A simulation study of sample size for multilevel logistic regression models. BMC Med Res Methodol. 2007;7:34. doi: 10.1186/1471-2288-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thacker JK, Mountford WK, Ernst FR, Krukas MR, Mythen MM. Perioperative Fluid Utilization Variability and Association With Outcomes: Considerations for Enhanced Recovery Efforts in Sample US Surgical Populations. Ann Surg. 2016;263:502–10. doi: 10.1097/SLA.0000000000001402. [DOI] [PubMed] [Google Scholar]
- 21.Magee G, Zaloga GP, Turpin RS, Sanon M. A retrospective, observational study of patient outcomes for critically ill patients receiving parenteral nutrition. Value Health. 2014;17:328–33. doi: 10.1016/j.jval.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Cozowicz C, Olson A, Poeran J, Morwald EE, Zubizarreta N, Girardi FP, Hughes AP, Mazumdar M, Memtsoudis SG. Opioid prescription levels and postoperative outcomes in orthopedic surgery. Pain. 2017;158:2422–2430. doi: 10.1097/j.pain.0000000000001047. [DOI] [PubMed] [Google Scholar]
- 23.Memtsoudis SG, Danninger T, Rasul R, Poeran J, Gerner P, Stundner O, Mariano ER, Mazumdar M. Inpatient falls after total knee arthroplasty: the role of anesthesia type and peripheral nerve blocks. Anesthesiology. 2014;120:551–63. doi: 10.1097/ALN.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 24.Wolters Kluwer Clinical Drug Information, Inc. [Accessed September 19, 2016];Opioid Agonist Conversion. Available at: http://online.lexi.com/lco/action/calc/calculator/70050.
- 25.McAuley D., PharmD [Accessed September 19, 2016];GlobalRPH Opioid Analgesic Converter. Available at: http://globalrph.com/narcoticonv.htm.
- 26.Kessler ER, Shah M, Gruschkus SK, Raju A. Cost and quality implications of opioid-based postsurgical pain control using administrative claims data from a large health system: opioid-related adverse events and their impact on clinical and economic outcomes. Pharmacotherapy. 2013;33:383–91. doi: 10.1002/phar.1223. [DOI] [PubMed] [Google Scholar]
- 27.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–9. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 28.Lee S, Rooban N, Vaghadia H, Sawka AN, Tang R. A Randomized Non-Inferiority Trial of Adductor Canal Block for Analgesia After Total Knee Arthroplasty: Single Injection Versus Catheter Technique. J Arthroplasty. 2017 doi: 10.1016/j.arth.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 29.Raiff D, Vaughan C, McGee A. Impact of intraoperative acetaminophen administration on postoperative opioid consumption in patients undergoing hip or knee replacement. Hosp Pharm. 2014;49:1022–32. doi: 10.1310/hpj4911-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sing DC, Barry JJ, Cheah JW, Vail TP, Hansen EN. Long-Acting Opioid Use Independently Predicts Perioperative Complication in Total Joint Arthroplasty. J Arthroplasty. 2016;31:170–174e1. doi: 10.1016/j.arth.2016.02.068. [DOI] [PubMed] [Google Scholar]
- 31.Witte JS, Greenland S, Kim LL, Arab L. Multilevel modeling in epidemiology with GLIMMIX. Epidemiology. 2000;11:684–8. doi: 10.1097/00001648-200011000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Harrell FE. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001. [Google Scholar]
- 33.Perneger TV. What’s wrong with Bonferroni adjustments. Bmj. 1998;316:1236–8. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hosmer DW, Lemesbow S. Goodness of fit tests for the multiple logistic regression model. Communications in Statistics - Theory and Methods. 1980;9:1043–1069. [Google Scholar]
- 35.Moran JL, Solomon PJ. A review of statistical estimators for risk-adjusted length of stay: analysis of the Australian and new Zealand Intensive Care Adult Patient Data-Base, 2008–2009. BMC Med Res Methodol. 2012;12:68. doi: 10.1186/1471-2288-12-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rascati KL, Smith MJ, Neilands T. Dealing with skewed data: an example using asthma-related costs of medicaid clients. Clin Ther. 2001;23:481–98. doi: 10.1016/s0149-2918(01)80052-7. [DOI] [PubMed] [Google Scholar]
- 37.Ene M, Leighton EA, Blue GL, Bell BA. Multilevel Models for Categorical Data using SAS® PROC GLIMMIX: The Basics. [Accessed December 19, 2017];SouthEast SAS Users Group Paper 134–2014. Available at: http://analytics.ncsu.edu/sesug/2014/SD-13.pdf.
- 38.Cherny N, Ripamonti C, Pereira J, Davis C, Fallon M, McQuay H, Mercadante S, Pasternak G, Ventafridda V. Strategies to manage the adverse effects of oral morphine: an evidence-based report. J Clin Oncol. 2001;19:2542–54. doi: 10.1200/JCO.2001.19.9.2542. [DOI] [PubMed] [Google Scholar]
- 39.Memtsoudis SG, Poeran J, Cozowicz C, Zubizarreta N, Ozbek U, Mazumdar M. The impact of peripheral nerve blocks on perioperative outcome in hip and knee arthroplasty-a population-based study. Pain. 2016;157:2341–9. doi: 10.1097/j.pain.0000000000000654. [DOI] [PubMed] [Google Scholar]
- 40.Pomerleau AC, Schrager JD, Morgan BW. Pilot Study of the Importance of Factors Affecting Emergency Department Opioid Analgesic Prescribing Decisions. J Med Toxicol. 2016;12:282–8. doi: 10.1007/s13181-016-0553-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rice DC, Cata JP, Mena GE, Rodriguez-Restrepo A, Correa AM, Mehran RJ. Posterior Intercostal Nerve Block With Liposomal Bupivacaine: An Alternative to Thoracic Epidural Analgesia. Ann Thorac Surg. 2015;99:1953–60. doi: 10.1016/j.athoracsur.2015.02.074. [DOI] [PubMed] [Google Scholar]
- 42.Stone AB, Wick EC, Wu CL, Grant MC. The US Opioid Crisis: A Role for Enhanced Recovery After Surgery. Anesth Analg. 2017 doi: 10.1213/ANE.0000000000002236. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 43.Mont MA, Beaver WB, Dysart SH, Barrington JW, Del Gaizo DJ. Local Infiltration Analgesia With Liposomal Bupivacaine Improves Pain Scores and Reduces Opioid Use After Total Knee Arthroplasty: Results of a Randomized Controlled Trial. J Arthroplasty. 2017 doi: 10.1016/j.arth.2017.07.024. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 44.Golf M, Daniels SE, Onel E. A phase 3, randomized, placebo-controlled trial of DepoFoam(R) bupivacaine (extended-release bupivacaine local analgesic) in bunionectomy. Adv Ther. 2011;28:776–88. doi: 10.1007/s12325-011-0052-y. [DOI] [PubMed] [Google Scholar]
- 45.Gorfine SR, Onel E, Patou G, Krivokapic ZV. Bupivacaine extended-release liposome injection for prolonged postsurgical analgesia in patients undergoing hemorrhoidectomy: a multicenter, randomized, double-blind, placebo-controlled trial. Dis Colon Rectum. 2011;54:1552–9. doi: 10.1097/DCR.0b013e318232d4c1. [DOI] [PubMed] [Google Scholar]
- 46.Kim KY, Anoushiravani AA, Chen KK, Roof M, Long WJ, Schwarzkopf R. Preoperative Chronic Opioid Users in Total Knee Arthroplasty-Which Patients Persistently Abuse Opiates Following Surgery? J Arthroplasty. 2018;33:107–112. doi: 10.1016/j.arth.2017.07.041. [DOI] [PubMed] [Google Scholar]
- 47.Smith SR, Bido J, Collins JE, Yang H, Katz JN, Losina E. Impact of Preoperative Opioid Use on Total Knee Arthroplasty Outcomes. J Bone Joint Surg Am. 2017;99:803–808. doi: 10.2106/JBJS.16.01200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.