Abstract
Hallucinations characterize schizophrenia, with approximately 59% of patients reporting auditory hallucinations and 27% reporting visual hallucinations. Prior neuroimaging studies suggest that hallucinations are linked to disrupted communication across distributed (sensory, salience-monitoring and subcortical) networks. Yet, our understanding of the neurophysiological mechanisms that underlie auditory and visual hallucinations in schizophrenia remains limited.
This study integrates two resting-state functional magnetic resonance imaging (fMRI) analysis methods – amplitudes of low-frequency fluctuations (ALFF) and functional network connectivity (FNC) – to explore the hypotheses that (1) abnormal FNC between salience and sensory (visual/auditory) networks underlies hallucinations in schizophrenia, and (2) disrupted hippocampal oscillations (as measured by hippocampal ALFF) beget changes in FNC linked to hallucinations. Our first hypothesis was supported by the finding that schizophrenia patients reporting hallucinations have higher FNC between the salience network and an associative auditory network relative to healthy controls. Hippocampal ALFF was negatively associated with FNC between primary auditory cortex and the salience network in healthy subjects, but was positively associated with FNC between these networks in patients reporting hallucinations. These findings provide indirect support favoring our second hypothesis. We suggest future studies integrate fMRI with electroencephalogram (EEG) and/or magnetoencephalogram (MEG) methods to directly probe the temporal relation between altered hippocampal oscillations and changes in cross-network functional communication.
Keywords: hallucinations, ALFF, FNC, resting-state, fMRI
1. Introduction
An estimated 59% of patients with schizophrenia (Sz) report auditory hallucinations (AH); nearly half of those reporting AHs also report visual hallucinations (VHs) (Waters et al., 2014). To address the question of how individuals with Sz come to experience hallucinations, researchers have used non-invasive resting-state functional magnetic resonance imaging (rs-fMRI) to compare spontaneous fluctuations in the blood oxygenation level dependent (BOLD) signal in Sz reporting hallucinations relative to control subjects. Resting-state functional connectivity (rs-FC) analyses are commonly employed in hypothesis-driven investigations of Sz symptoms and provide an estimate of how correlated or “in synch” BOLD signal activation is across regions of interest. Both VH and AH are associated with abnormal sensory (Clos et al., 2014; Ford et al., 2015; Gavrilescu et al., 2010; Hoffman, Ralph et al., 2012; Shinn et al., 2013; Sommer et al., 2012), striatal (Amad, A. et al., 2014; Hoffman, Ralph et al., 2012; Rolland et al., 2015), insular (Clos et al., 2014; Rolland et al., 2015), medial frontal (Amad, A. et al., 2014; Clos et al., 2014), and parahippocampal/hippocampal (Amad, A. et al., 2014; Clos et al., 2014; Ford et al., 2015; Rolland et al., 2015; Sommer et al., 2012) rs-FC. Yet, it remains unclear how these widespread disruptions in rs-FC give rise to hallucinations.
The abnormal salience monitoring model proposes that hallucinations may be driven by abnormal functional communication between resting-state networks (e.g. anatomically distributed brain regions that show consistent functional co-activation at rest) (Palaniyappan et al., 2012; Palaniyappan et al., 2011). The salience network (SN) contains hubs in the anterior insula and dorsal anterior cingulate cortex, and activates in response to proximally salient cues — from internal changes in bodily state to demanding tasks that require externally-focused attention (Menon, 2015; Seeley et al., 2007). Dynamic causal modeling and Granger causality analyses suggest the right anterior insula regulates activation/deactivation of the default-mode network (DMN) (Goulden et al., 2014; Sridharan et al., 2008). The DMN is associated with internally-directed attention and self-referential processing (Raichle, 2015); network hubs include medial prefrontal cortex, anterior cingulate, precuneus/posterior cingulate cortex, and bilateral angular gyri. Improper monitoring of salient internal events (e.g. auditory-verbal imagery, visual images) plausibly generates hallucinations. Many studies have explored functional network connectivity (FNC) in Sz (Damaraju et al., 2014; Garrity et al., 2007; Whitfield-Gabrieli et al., 2009), yet no study has tested this hypothesis by examining how primary/associative sensory networks interact with the SN/DMN in the context of hallucinations.
A major advantage of the abnormal salience monitoring model is that it accounts for the distributed changes in functional communication observed in Sz reporting hallucinations. However, this network model fails to incorporate the role of the hippocampus in the generation of hallucinations. Across fMRI investigations of the active AH state (e.g. symptom-capture), the left hippocampus shows the highest likelihood of activation (Jardri et al., 2011). One recent study explored low frequency (<0.1 Hz) power of the BOLD signal across brain voxels during rest. This exploratory analysis of amplitudes of low frequency fluctuations (ALFF) found that Sz patients reporting VH and AH had higher ALFF in the left hippocampus relative to patients that reported AH (but not VH). Variability in left hippocampal ALFF was positively associated with reported VH severity, but was negatively associated with AH severity (Hare et al., 2017).
In a magnetoencephalography (MEG) symptom-capture study of AH, transient decreases in hippocampal theta band power (4–10 Hz) preceded reported AHs (van Lutterveld et al., 2012). Hippocampal theta oscillations are measured in local field potentials of humans (Arnolds et al., 1980), and all other mammals studied to date (Green and Arduini, 1954; Lubenov and Siapas, 2009; Vanderwolf, 1969; Winson, 1972). Medial prefrontal neurons and auditory neurons in the inferior colliculus demonstrate spiking preferences at particular phases of the slow hippocampal theta rhythm (referred to as phase-locking) (Hyman et al., 2011, 2010; Pedemonte et al., 1996; Siapas et al., 2005). Researchers speculate that hippocampal theta waves act like the conductor of an orchestra by synchronizing activation of distributed networks, and temporally ordering information (e.g. sensory percepts, motor representations, and memories) (Buzsaki, 2002; Lisman and Buzsáki, 2008). We propose that disrupted hippocampal oscillations destabilize normal network connections in Sz and might plausibly drive abnormal network connections in Sz patients with hallucinations.
The present study models the relationships between hippocampal ALFF, FNC, and targeted symptomology (AH and VH severity) in the resting-state brain. We first test the hypothesis that altered FNC between salience and sensory networks underlies modality-specific hallucinations, predicting that Sz patients with VH will have higher FNC between visual and salience networks relative to all groups, and patients with AH will have higher FNC between auditory and salience networks relative to nonhallucinating Sz patients and HC.
Next, we explore the hypothesis that disrupted hippocampal oscillations destabilize normal functional network connections in Sz. We predict that (1) hippocampal oscillations (measured indirectly as ALFF within the left hippocampal cluster identified in our previous analysis (Hare et al., 2017) will be associated with FNC in HC; (2) Sz will lack these normal ALFF-FNC relationships, and (3) will have abnormal relationships between hippocampal ALFF and FNC. The poor temporal resolution of fMRI limits our ability to directly test the hypothesis that disrupted hippocampal theta oscillations beget changes in FNC. Nonetheless, we establish links between hippocampal BOLD signal fluctuations and FNC, providing preliminary (indirect) support favoring a novel hippocampal binding model that might explain disrupted auditory network functional communication in Sz.
2. Experimental materials and methods
2.1. Subjects
We analyzed 294 resting-state fMRI scans from the Functional Biomedical Informatics Research Network (FBIRN) dataset (Keator et al., 2016). Schizophrenia patients (n=141) and HC (n=153) were matched for age, reported gender, and handedness (Table 1). Raw imaging data were collected from six sites; written informed consent was obtained from all participants. The consent process was approved by University of California Irvine, University of California San Francisco, Duke University/ University of North Carolina, University of New Mexico, University of Iowa, and University of Minnesota Institutional Review Boards.
Table 1.
Participant Demographic and Clinical Information
| AH (n=42) | VH (n=39) | NH (n=60) | HC (n=153) | |
|---|---|---|---|---|
| Demographic Info | ||||
| Age | 37.8 (11.9) | 37.1 (11.4) | 40.0 (11.8) | 37.8 (11.4) |
| Gender | 32 (m), 10 (f) | 30 (m), 9 (f) | 43 (m), 17 (f) | 108 (m), 45 (f) |
| Handedness (r/l/a) | 36 (r), 5 (l), 1 (a) | 32 (r), 5 (l), 2 (a) | 60 (r), 0 (l), 0 (a) | 83 144 (r), 7 (l), 2 (a) |
| Smoking Status | 19 (s), 23 (n) | 19 (s), 20 (n) | 24 (s), 36 (n) | 14 (s), 139 (n) |
| Socioeconomic Status subject*a | 50.8 (13.1) | 51.2 (13.6) | 50.2 (12.7) | 33.5 (12.7) |
| Socioeconomic Status caregiver*b | 33.8 (14.8) | 35.4 (14.1) | 37.6 (14.6) | 30.4 (14.7) |
| Subject Motion | ||||
| Mean Framewise Displacementc | 0.44 (0.3) | 0.42 (0.3) | 0.35 (0.2) | 0.29 (0.2) |
| Patient Population | ||||
| Duration of Illness | 18.0 (11.0) | 16.9 (12.5) | 17.0 (11.4) | n/a |
| Chlorpromazine equiv.(CPZ Woods)d | 401.1 (443.1) | 335.4 (294.6) | 367.9 (356.2) | n/a |
| Total PANSS*e | 57.7 (12.6) | 63.6 (13.5) | 54.2 (13.1) | n/a |
| PANSS-positive*e | 16.6 (4.5) | 17.8 (4.1) | 13.0 (4.1) | n/a |
| PANSS-negative | 13.7 (5.3) | 15.3 (6.1) | 13.9 (4.8) | n/a |
| Total SAPS*f | 25.1 (13.3) | 40.0 (17.4) | 12.1 (12.3) | n/a |
| Total SAPS adjusted for 2 hallucination items*g | 21.8 (12.8) | 33.9 (16.5) | 12.1 (12.3) | n/a |
Group ANOVA is significant at p=0.05
AH, VH, and NH groups all significantly different than HC (Bonferroni post-hoc, p<0.01)
NH vs. HC significantly different (Bonferroni post-hoc, p<0.01)
AH vs. HC significantly different (Bonferroni post-hoc, p<0.01); VH vs. HC significantly different (Bonferroni post-hoc, p = 0.018).
We only had this information for a subset of patients; percent reporting = 87.2%
VH vs. NH significantly different (Bonferroni post-hoc, p<0.01)
AH vs. NH and VH vs. NH both significantly different (Bonferroni post-hoc, p<0.01)
all post-hoc comparisons are significantly different (Bonferroni post-hoc, p<0.01)
All recruited study participants were between the ages of 18 and 62. All Sz subjects were diagnosed with schizophrenia or schizoaffective disorder by experienced clinicians using the Structural Clinical Interview for DSM-IV-TR Axis I Disorders. Patients were either stable on antipsychotic medication or unmedicated (only 8 out of the 143 Sz subjects were not taking antipsychotic medication at the time of the study). Healthy controls with a first-degree relative with an Axis I disorder or a history of major psychiatric illness were excluded. Exclusion for all participants included history of major medical illness, insufficient eyesight to see with normal acuity with MRI compatible corrective lenses, contraindications for MRI, drug dependence in the last five years or a current substance abuse disorder, an intelligence quotient less than 75.
The present study draws from the FBIRN Phase III study (see (Hare et al., 2017) (Ford et al., 2015) (Damaraju et al., 2014)). Multiple behavioral/symptom assessments were performed as part of the FBIRN Phase III study including the Scale for the Assessment of Positive Symptoms (SAPS) (Andreasen, 1984a) and the Scale for the Assessment of Negative Symptoms (SANS) (Andreasen, 1984b). The protocol required that symptom assessment ratings be completed within one month of scanning. For a detailed description of the multi-phase FBIRN project including subject characteristics, imaging parameters, and behavior assessments see Keator et al., 2016.
2.2. Grouping of Participants
We used the same clinical subgroup sorting strategy used previously in (Hare et al., 2017) and (Ford et al., 2015). Sorting of the 141 Sz into clinical subgroups was achieved by evaluating responses to two SAPS items (Andreasen, 1984a). Item #1 asks if the participant “reports voices, noises, or other sounds that no one else hears,” while Item #6 asks if he/she “sees shapes or people that are not actually present.” Each item is scored using a 1 to 5 rating scale (0 = not present; 1 = questionable; 2 = mild; 3 = moderate; 4 = marked; 5 = severe). The AH (but not VH) group (n = 42) had SAPS Item #1 scores > 1 and SAPS Item #6 scores of zero. The non-hallucinator group (NH, n = 60) scored zero for both items, while the VH group (n = 39) had SAPS Item #6 scores > 1. Due to prevalence of AH in Sz, all but two of the participants in the VH subgroup also reported AH (95%). For a subset of analyses, the VH and AH subgroups were pooled to form a hallucinating (HALL) subgroup reporting AH, VH or both.
2.3. Imaging
Data were acquired using five 3T Siemens TIM Trio scanners and one 3T GE MR750 scanner using an AC-PC aligned echo-planar imaging pulse sequence (TR/TE 2 s/30 ms, flip angle 77°, 32 slices collected sequentially from superior to inferior, 3.4 × 3.4 × 4 mm with mm gap, 162 frames, 5:24 mins) to obtain T2*-weighted images. Subjects were instructed to lie in the scanner with eyes closed.
2.4. Data Processing
Pre-processing was performed using the Data Processing Assistant for Resting-State fMRI (DPARSF) toolbox which runs with the REST software (Song et al., 2011). The first two time frames were removed to allow for signal stabilization. Raw data underwent motion correction to the first image, slice-timing correction to the middle slice, normalization to MNI space, and spatial smoothing with an 8 FWHM Gaussian kernel. Framewise displacement was calculated for each image; framewise displacement differentiates head realignment parameters across frames and generates a 6-dimensional times series that represents instantaneous head motion (Power et al., 2012). To correct for confounding effects of head motion on the fMRI signal, we included mean framewise displacement as a subject-level covariate.
2.5. Group Spatial Independent Component Analysis
We performed spatial group ICA using GIFT software (Calhoun et al., 2001). One hundred independent component networks were obtained from the group principal component analysis matrix using the Infomax algorithm. The ICA algorithm was repeated twenty times in ICASSO and the most central result was used to ensure stability of estimation. Subject-specific spatial maps and time courses were obtained using back reconstruction implemented in GIFT (Erhardt et al., 2011).
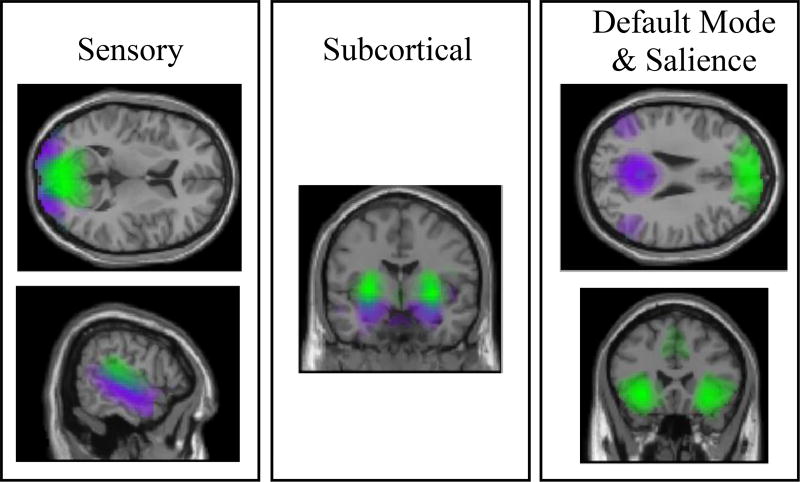
We examined z-transformed spatial maps thresholded at z > 3 to identify artifactual RSNs (e.g. “ringing” motion artifacts, spatial maps with peak signal arising from CSF/white matter). Using the method proposed by (Allen et al., 2011), we discarded components with poor low frequency/high frequency power ratios, and those with stability quotients < 0.85. From the remaining RSNs, nine networks of interest were selected: two visual RSNS, two auditory RSNs, SN, anterior DMN, posterior DMN, bilateral putamen, and bilateral hippocampus (Figure 1, Table 2).
Figure 1. Networks.
Nine networks were selected based on their putative involvement in the generation of auditory and visual hallucinations. Different colors (green/purple) depict distinct resting-state networks. Top left: two visual networks; bottom left: two auditory networks; middle: subcortical networks (hippocampus in purple, putamen in green); top right: default mode network (anterior shown in green and posterior shown in purple); bottom right: salience network. All spatial maps were thresholded at Z > 3.
Table 2.
Nine Networks: Characteristics of Spatial Maps
| Location of Peak Voxel in Group Aggregate Spatial Map |
MNI coordinates of peak voxel |
Other Regions Included in Z- thresholded Aggregate Spatial Map (Z > 3) |
|
|---|---|---|---|
| Network 1 | Right Calcarine/Cuneus BA 17 | [9, −84, 9] | Superior/Middle Occipital (BA 18), Precuneus/PCC (BA 30) |
| Network 2 | Middle Occipital BA 18 | [27, −96, 0] | Precuneus, Calcarine (BA 17) |
| Network 3 | Right Putamen | [30, −3, 0] | Cerebellum, Anterior Lobe/Vermis |
| Network 4 | Left Hippocampus BA 20 | [−30, −9, −18] | Parahippocampal Gyri, Left/Right Amygdala, Anterior Cerebellum (Dentate) |
| Network 5 | Left Superior Temporal BA 41 | [−42, −33, 15] | Opercular/Insular Cortex; Superior Temporal (BA 22) |
| Network 6 | Right Superior Temporal BA 22 | [60, −18, −6] | Middle Temporal (BA 6, 21) |
| Network 7 | Medial Frontal (Interhemispheric) BA 9 | [0, 51, 39] | Superior Frontal (BA 32) |
| Network 8 | Left Precuneus BA 23 | [−6, −54, 27] | Left/Right Angular Gyrus (BA 39); Medial Frontal (BA 10) |
| Network 9 | Left Insula | [−30, 24, −6] | Dorsal Anterior Cingulate, Middle Cingulate (BA 32); Medial Frontal (BA 9) |
Subject timecourses were detrended and despiked, then filtered with a high frequency cutoff of 0.15 Hz prior to computing FNC correlations; FNC correlations are defined as the pairwise correlations between network time courses. For all FNC analyses, FNC correlations were transformed to z-scores using Fisher’s transformation.
2.6. Statistical Analyses
2.6.1. Group Differences
We performed a two-sample t-test (HC vs. Sz) to explore FNC correlations associated with Sz diagnosis. We examined changes in FNC associated with the general trait to experience hallucinations (AH, VH or both) with a three-group level ANCOVA (HALL, NH, HC); FNC associated with modality-specific hallucinations was explored using a four-group level ANCOVA (VH, AH, NH, HC). Age, scanning site, gender, and mean framewise displacement were included as covariates. Statistical significance was a priori specified as p < 0.05 using a false discovery rate (FDR) correction for multiple comparisons.
2.6.2. Symptom Severity & FNC: Regression Analyses
To test the hypothesis that abnormal FNC between salience and sensory networks underlies modality-specific hallucinations, we performed linear regression analyses of FNC. To ensure that observed associations between AH/VH severity and FNC were not driven or influenced by confounding factors, we modeled effects of nuisance covariates (age, gender, and mean framewise displacement; scanning site was dummy coded and modeled as a random effect). Since nicotine use is 2–3 times higher in Sz than in the healthy population (de Leon and Diaz, 2005), and has been shown to significantly impact brain functional connectivity (Jasinska et al., 2014), we examined Spearman correlations between FNC and smoking status (factor with three levels: “never smoker”, “ex-smoker”, “current smoker”) in our sample of 294 subjects. These analyses revealed a significant association between smoking status and SN-STG (BA 22) FNC (rho = −0.244, p < 0.01), so smoking status was included as an additional covariate.
To confirm that observed effects of VH/AH severity on FNC were not driven by confounding effects of antipsychotic medication, we performed post-hoc regression analyses of FNC, including total chlorpromazine equivalents (Woods, 2003) as an additional covariate. We lacked information to derive chlorpromazine equivalents for 18 Sz subjects, so the mean value of total chlorpromazine equivalents was calculated (based on the available data), and interpolated for those subjects with missing data. For all analyses, confidence was specified as p < 0.05.
2.6.3 Hippocampal ALFF & FNC: Regression Analyses
Voxelwise mean ALFF maps were computed for each subject using REST software (Song et al., 2011) as described in (Hare et al., 2017) The left hippocampal cluster that showed significant ALFF variation across VH vs. AH subgroups in Hare et al., 2017, was saved as a binary mask. Subject-specific weighted ALFF averages within this cluster were derived from the 294 ALFF maps using SPM’s MARSBAR utility.
We calculated the relationship between these subject-specific hippocampal ALFF averages and FNC to explore whether the nature and/or strength of ALFF-FNC relationships are different in Sz vs. HC. Only FNC correlations that were significantly different across Sz and HC in the group analysis were examined in these ALFF-FNC regression analyses. First, we examined potential ALFF x diagnosis interactions in a linear regression analysis. Age, gender, mean framewise displacement, and smoking status were included as covariates; scanning site was modeled as a random effect.
To further probe whether the nature and/or strength of ALFF-FNC relationships are different in Sz vs. HC, we explored ALFF-FNC associations in separate analyses of HC and Sz. We modeled effects of hippocampal ALFF on FNC, controlling for confounding influences on FNC (age, gender, mean framewise displacement, smoking status, and random effects of scanning site) in the linear model. Separate regression analyses were performed in HALL and NH to address the question of whether abnormal ALFF-FNC associations are observed in Sz reporting hallucinations exclusively or were also observed in NH patients. We confirmed that observed associations between hippocampal ALFF and FNC were not driven by confounding effects of antipsychotic medication by performing post-hoc regression analyses, including total chlorpromazine equivalents (Woods, 2003) as an additional covariate. Confidence was specified as p < 0.05.
3. Results
3.1. FNC Group Differences
3.1.1. FNC Differences Between Sz Patients and HC
Relative to HC, Sz had higher FNC between STG (BA 22) and hippocampus, and lower FNC between (1) the two STG networks (BA 21, BA 41), (2) STG (BA 22) and visual cortex (BA 17), and (3) STG (BA 41) and SN (see Supplemental Figure 1). For clarity, locations of peak voxels of sensory networks are reported parenthetically.
3.1.2. FNC Differences Between Subgroups of Sz
No significant changes in FNC across hallucination subgroups (NH vs. HALL, NH vs. AH, NH vs. VH, VH vs. AH) survived FDR-correction.
3.1.3. FNC Differences Between NH and HC
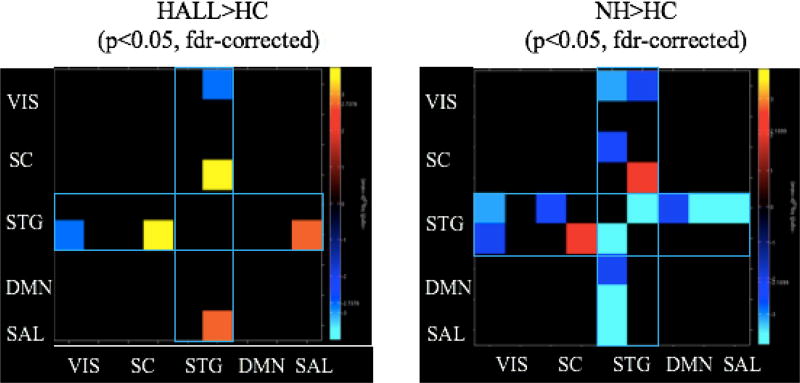
Relative to HC, NH patients showed higher FNC between hippocampus and STG (BA 22), but lower FNC between (1) the two STG networks (BA 22, BA 41), (2) STG (BA 22) and visual cortex (BA 17), (3) STG (BA 41) and visual cortex (BA 17), (4) STG (BA 41) and putamen, (5) STG (BA 41) and SN, and (6) STG (BA 41) and both anterior DMN and posterior DMN (Figure 2).
Figure 2. Altered Superior Temporal Network Connections in Hallucinating and Nonhallucinating Patients.
Warm (yellow/red) colors depict areas of increased network connectivity in patients while cool (blue) colors depict network connectivity that is decreased in patients relative to controls. Relative to healthy subjects, both patient groups show significantly increased connectivity between the STG and hippocampus; hallucinators show elevated connectivity between STG and salience network, while nonhallucinating patients show widespread decreases in STG network connectivity. All significant group differences occur with STG networks (outlined in blue rectangles). VIS: visual networks; SC: subcortical networks; STG: superior temporal gyri; DMN: default mode network; SN: salience network.
3.1.4. FNC Differences Between HALL and HC
Relative to HC, HALL showed higher FNC between STG (BA 22) and hippocampus and between STG (BA 22) and SN, but lower FNC between STG (BA 22) and visual cortex (BA 17) (Figure 2).
3.2. Regression Analyses of FNC
3.2.1. Symptom Severity & FNC
We observed a significant association between AH severity and FNC between STG (BA 22) and SN (t = 2.3, p < 0.05); SN-STG (BA 22) FNC was not associated with VH severity, nor total positive/negative symptoms. This association between AH severity and SN-STG (BA 22) FNC remained significant when we included total chlorpromazine equivalents as an additional regressor in the model (t = 2.0, p < 0.05). There were no other significant associations between FNC correlations and symptom scores.
3.2.2. Hippocampal ALFF & FNC: HC vs. Sz
We observed significant diagnosis x ALFF interactions on (1) FNC between STG networks (BA 41 and BA 22) (t = −2.9, p < 0.01) and (2) SN-STG (BA 41) FNC (t = −3.0, p < 0.01). To ensure that observed effects were not driven by outliers, we re-ran regression analyses after omitting four subjects that had weighted hippocampal ALFF averages exceeding 4 standard deviations from the mean. The diagnosis x ALFF interaction on SN-STG (BA 41) FNC remained significant (t = −3.0, p < 0.01) while the diagnosis x ALFF interaction on FNC between STG networks (BA 41 and BA 22) did not remain significant (t = −1.8, p = 0.08).
In HC, hippocampal ALFF was positively associated with FNC between (1) STG (BA 22) and hippocampus (t = 4.2, p < 0.001), and negatively associated with FNC between (2) the two STG networks (BA 41, BA 22) (t = −3.1, p < 0.01), and (3) STG (BA 41) and SN (t = −2.4, p < 0.05). In Sz, hippocampal ALFF was positively associated with SN-STG (BA 41) FNC (t = 2.2, p < 0.05). This observed association between left hippocampal ALFF and SN-STG (BA 41) connectivity remained significant (t = 2.2, p < 0.05) when total chlorpromazine equivalents were introduced as an additional covariate in the model.
3.2.3. Hippocampal ALFF & FNC: HALL vs. NH
There were no associations between hippocampal ALFF and FNC in NH patients. In HALL patients (n = 81), hippocampal ALFF was associated with FNC between the STG (BA 41) and SN (t = 2.1, p < 0.05). When we included chlorpromazine equivalents as an additional covariate in the regression analysis, the observed association between hippocampal ALFF and SN-STG (BA 41) connectivity remained significant (t = 2.1, p < 0.05).
4. Discussion
This analysis shows higher STG-SN FNC in Sz linked to the trait of experiencing AH. Furthermore, it identifies disrupted patterns of auditory network FNC in Sz and suggests a potential mechanism that may drive these FNC disturbances: hippocampal ALFF. To contextualize these results, we highlight FNC differences in Sz vs. HC before discussing the results of our targeted investigations of AH/VH.
Since convergent evidence from studies examining rs-FC, brain structure, genetics, and neurotransmitters support the hypothesis that Sz is a disorder of brain dysconnectivity (Friston et al., 2016), we anticipated that Sz would show widespread differences in cross-network communication. Significant increases and decreases in FNC were observed in Sz patients (Supplemental Figure 1), consistent with results from a prior analysis using this dataset (Damaraju et al., 2014). In both studies, Sz had lower FNC between sensory networks, and higher FNC between subcortical and sensory networks. In the present analysis, we observed STG-hippocampal hyperconnectivity in patients (Supplemental Figure 1); Damaraju et al. did not include a hippocampal network and observed sensory-thalamic hyperconnectivity in Sz patients. While Damaraju et al. investigated FNC linked to Sz diagnosis, a central aim of this study was to identify targeted markers of hallucinations in Sz.
Prior findings support the hypothesis that abnormal salience monitoring underlies AH (Palaniyappan et al., 2012; Palaniyappan et al., 2011). Reported AH severity correlates negatively with FC within the SN (between SN hubs and intrinsic FC of the right anterior insula) (Manoliu et al., 2014; Pu et al., 2012). In addition, SN hubs showed increased FC with dorsomedial prefrontal cortex in patients with AH relative to NH patients (Alonso-Solís et al., 2015). While these studies delineate links between SN dysfunction and AH, changes in SN functional communication are also linked to diverse behaviors and clinical outcomes (see Menon, 2015, Figure 14; Palaniyappan et al., 2013, Supplemental Figure S3).
In this study, we find that hallucinating patients (98% reporting AH, 48% reporting VH), but not NH patients, had higher FNC between STG (BA 22) and SN relative to HC. Regression analysis revealed that SN-STG (BA 22) FNC was associated with AH severity (and not VH severity nor global assessments of positive/negative symptoms). This targeted association between AH severity and SN-STG (BA 22) FNC provides support favoring the hypothesis that disrupted FNC between SN and associative-auditory cortex underlies AH in Sz.
We predicted that patients with VH would have higher FNC between visual and salience networks relative to all groups. Our failure to detect this anticipated effect could be driven by low statistical power (i.e. only 39 Sz patients reported VH), but might also be interpreted as evidence favoring rejection of the hypothesis that abnormal SN-visual FNC underlies VH in Sz.
Our analyses exploring the relation between hippocampal ALFF and FNC were motivated by theoretical and methodological shortcomings of prior analyses. First, although numerous studies report links between abnormal hippocampal function and hallucinations, the hippocampus remains absent from dominant models of hallucinations including abnormal salience monitoring theories (Menon, 2015; Palaniyappan and Liddle, 2012), and abnormal self-monitoring (forward modeling) theories (Frith, 1996). Second, while many fMRI studies have examined the neural basis of hallucinations in Sz, fewer studies have used MEG/EEG to examine neurophysiological changes that occur on a millisecond scale.
A rare MEG symptom-capture study found that transient decreases in hippocampal theta band power (4–10 Hz) preceded reported AHs (van Lutterveld et al., 2012). Slow theta oscillations are thought to play a key role in temporally coordinating local network oscillations in the faster gamma range (> 30 Hz) (Lisman and Buzsáki, 2008). Fast gamma cycles in local networks can couple to the same theta phase, providing a means for cross-network functional communication. The precise phase and timing information provided by slow theta rhythms may be essential for coordinating and synchronizing activity across distributed networks (Buzsaki, 2002; Lisman and Buzsáki, 2008). In line with this view, we hypothesized that abnormal hippocampal theta oscillations in Sz disrupt normal brain FNC.
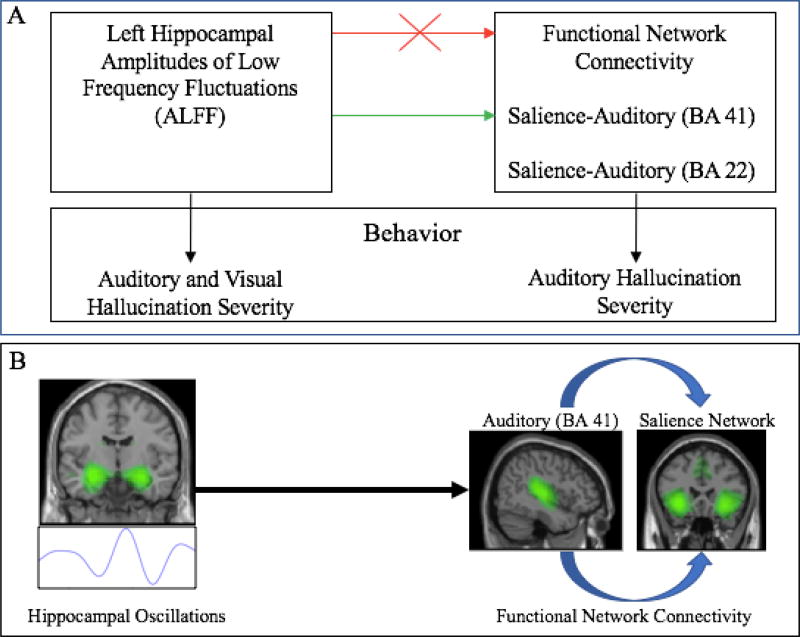
Due to fMRI’s poor temporal resolution, we were unable to directly test the hypothesis that abnormal hippocampal theta oscillations beget changes in brain FNC. Our finding that hippocampal ALFF was associated with different FNC correlations in Sz and HC provides preliminary, indirect support favoring this hypothesis. In HC, hippocampal ALFF was positively associated with FNC between (1) hippocampus and STG (BA 22), and negatively associated with FNC between (2) BA 41 and BA 22 auditory networks, and (3) STG (BA 41) and SN. These findings suggest that the hippocampus may regulate auditory FNC in healthy subjects. In Sz, we observed an abnormal positive association between hippocampal ALFF and SN-STG (BA 41) FNC; this association was observed only in Sz reporting AH and/or VH (no significant association was observed in NH). Our findings (summarized in Figure 3a) support a hippocampal binding model of FNC in which abnormal hippocampal oscillations in Sz disrupt normal auditory FNC and beget abnormal functional communication between salience and primary-auditory networks (Figure 3b).
Figure 3. Abnormal Hippocampal Activity and Functional Communication Between Salience and Auditory Networks in Schizophrenia.
(A) Reported VH and AH severity are associated with left hippocampal ALFF (Hare et al. 2017), while AH severity is associated with salience-auditory (BA 22) FNC. In schizophrenia, there is a loss of normal relationships between hippocampal ALFF and FNC found in healthy subjects (red arrow). In hallucinating (HALL) patients, there is an abnormal positive association between hippocampal ALFF and FNC between salience and auditory (BA 41) networks (green arrow). (B) These results favor an abnormal hippocampal binding model in which disrupted hippocampal oscillations beget a loss of normal FNC in schizophrenia patients, and may drive abnormal FNC between salience and auditory networks. ALFF: amplitudes of low frequency fluctuations; AH = auditory hallucination; FNC = functional network connectivity; VH = visual hallucination
A recent dynamic causal modeling study examined interactions between the left hippocampus, DMN, SN and an executive network in Sz actively experiencing AH (Lefebvre et al., 2016). Hallucination transition periods (e.g. periods of transition from no reported AH to reported AH) were associated with disruptions to all network connections, while active AH periods were associated with left hippocampal input to the SN. The authors speculate that AH are the result of misattributing salience to auditory memory fragments that are brought into consciousness (Copolov et al., 2003).
Our findings are consistent with this hypothesis, but allow us to glean further insight into the mechanisms that drive salience misattribution. Proper functional communication between hippocampal, salience and auditory networks facilitates our ability to recall auditory memories, tag them as salient, and bring them into consciousness at will. In the case of volitional recall, one anticipates bringing an auditory memory into consciousness, and recognizes it as self-generated. We would expect the phenomenology associated with this type of event to be different from the phenomenology associated with SN-auditory (BA 22) hyperconnectivity that drives abnormal attribution of salience to auditory images, which are brought into consciousness at random. The Sz patient would not anticipate the auditory image(s) being brought into consciousness, and might conclude that the conscious percept was generated by an alien source. In this respect, our SN-auditory hyperconnectivity theory of AH may provide an account of why AHs feel alien.
Finally, our findings link up with neurochemical hypotheses of Sz. One model proposes that hyperactive phasic midbrain dopaminergic responses stem from a loss of inhibitory regulation of hippocampal pyramidal neurons (Grace and Gomes, 2018). Phasic dopaminergic signaling plays an essential role in encoding motivational/behavioral salience (Bromberg-Martin et al., 2010). The SN contains network hubs in dopamine-rich midbrain regions (e.g. ventral tegmental area, substantia nigra) (Seeley et al., 2007), and may rely on these phasic signals to orient our attention to threats, rewards, and other salient cues. This neurochemical hypothesis predicts that abnormal hippocampal activity may lead to abnormal tracking and monitoring of salient stimuli in Sz, which is consistent with our findings.
There are several limitations of this cross-sectional analysis. We scanned subjects at one point in time, and don’t know how neural function changed in patients over the course of the disorder, and whether observed FNC effects reflect chronic dispositions. In this study, we were interested in identifying trait markers of AH/VH, but, we expect that symptoms fluctuate over the course of the illness; those in the NH group reported neither VH nor AH at the time of the scan, but they might have reported VH and/or AH at earlier time(s). These realities should be considered when developing inferences from these data. Second, patients had chronic schizophrenia; all but eight patients were taking antipsychotic medication at the time of the study. This precluded our ability to control for extraneous effects of antipsychotic medication on FNC by performing separate analyses of patients on medication and those not taking medication. Post-hoc analyses of FNC showed that observed associations with FNC (e.g. symptom-FNC, ALFF-FNC) remained significant after modeling effects of total chlorpromazine equivalents. Particular antipsychotic treatments such as clozapine have been shown to influence brain areas related to default mode (Gillespie et al., 2017; Mouchlianitis et al., 2016). We lacked detailed drug information to explore these targeted effects, so this limitation must be acknowledged.
Due to AH prevalence in Sz, we were unable to study VH independent of AH (95% of patients reporting VH also reported AH). However, our results allow us to glean insight into why AHs are roughly twice as prevalent as VH in Sz. Patients reporting neither AH nor VH show widespread decreases in STG network connectivity relative to HC (Figure 2), suggesting that STG network connectivity is especially vulnerable to disruption in all Sz patients (including those that do not report hallucinations). Future studies should explore the mechanisms that underlie normal STG functional network communication in healthy subjects to better understand how functional communication with STG networks becomes disrupted in Sz.
Finally, low frequency BOLD signal fluctuations (<0.1 Hz) are associated with changes in local field potentials (van Lutterveld et al., 2012), which are driven by voltage-dependent neural oscillations, but also by summed synaptic activities of local networks, fast action potentials, and neuron-glial interactions (Buzsáki et al., 2012). Thus, our findings suggest important links between altered hippocampal activity and abnormal FNC in Sz. We speculate that disrupted hippocampal theta oscillations may disrupt functional communication between auditory and salience networks in Sz patients reporting hallucinations, but alternative hypotheses of AH could be proposed. In line with the dynamic causal modeling analysis findings (Lefebvre et al., 2016), abnormal coupling between hippocampal oscillations and SN oscillations may give rise to the active AH state. Our findings suggest that disturbed oscillatory coupling between salience and auditory networks may play a role in the generation of AHs.
To date, these hypotheses have not been tested. In general, very little is known regarding SN oscillations and their functional/behavioral significance. One study found that reduced insular thickness in Sz was associated with inefficient resetting of frontal theta oscillations (Palaniyappan et al., 2012), while another study reported that Sz patients had abnormally high beta oscillations in the insula in response to task-irrelevant stimuli (Liddle et al., 2016). Future studies of SN oscillations need to be performed to refine our understanding of how the SN communicates with other functional networks in healthy subjects, and how disrupted SN oscillations may give rise to various symptoms such as hallucinations.
In sum, our findings raise a number of interesting hypotheses and provide indirect support favoring our proposed hippocampal binding hypothesis of AH. Innovative fMRI methods are currently being developed that explore FNC dependence on different spectral frequency modes of the BOLD signal (Yaesoubi et al., 2017). Future studies should use a combination of methodological approaches (including combined EEG/MEG + fMRI approaches) to explore frequency-dependent coupling between salience, hippocampal and sensory networks, and directly test the hypothesis that disrupted hippocampal theta oscillations beget changes in functional network communication in Sz.
Supplementary Material
Acknowledgments
FBIRN was supported by a grant from NCRR to S. Potkin (NIH 1 U24 RR021992). This work was supported in part through a 2nd Century Initiative fellowship to S. Hare, and by NIMH to J. Turner & V. Calhoun (1R01MH094524-01A1).
Role of the Funding Source(s)
These funding sources had no role in the design of this study nor any role during its execution, analyses, interpretation of the data, or decision to submit results. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health nor Georgia State University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Ms. Hare designed and performed the FNC/ALFF analyses, and took primary responsibility for drafting and revising the manuscript. Ms. Law assisted with the FNC analyses, outlining, and writing a first draft of the manuscript. Drs. Ford, Mathalon, and Turner provided guidance with data interpretation, while Dr. Calhoun provided guidance on the group ICA methods. Drs. Ford, Mathalon, Bustillo, Mueller, van Erp, Calhoun, and Turner provided suggestions for revisions at various stages of the manuscript. Ms. Ahmadi, Mr. Damaraju and Ms. Hare assisted with data preprocessing and curation. Dr. Hyo Jong Lee collected and quality checked data. Drs. Bustillo, Belger, Mueller, Lim, Brown, Preda, van Erp, Calhoun, Turner and Potkin contributed to the FBIRN study design and implementation, data collection, and data sharing. All authors reviewed and approved the final manuscript.
Disclosures
Dr. Mathalon has served as a consultant for Boehringer Ingelheim, Alkermes, Takeda, and Upsher-Smith. All other authors declare no conflicts of interest.
References
- Allen EA, Erhardt EB, Damaraju E, Gruner W, Segall JM, Silva RF, Havlicek M, Rachakonda S, Fries J, Kalyanam R, Michael AM, Caprihan A, Turner JA, Eichele T, Adelsheim S, Bryan AD, Bustillo J, Clark VP, Feldstein Ewing SW, Filbey F, Ford CC, Hutchison K, Jung RE, Kiehl KA, Kodituwakku P, Komesu YM, Mayer AR, Pearlson GD, Phillips JP, Sadek JR, Stevens M, Teuscher U, Thoma RJ, Calhoun VD. A baseline for the multivariate comparison of resting-state networks. Front. Syst. Neurosci. 2011;4(5):2. doi: 10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Solís A, Vives-Gilabert Y, Grasa E, Portella MJ, Rabella M, Sauras RB, Roldán A, Núñez-Marín F, Gómez-Ansón B, Pérez V, Alvarez E, Corripio I. Resting-state functional connectivity alterations in the default network of schizophrenia patients with persistent auditory verbal hallucinations. Schizophr. Res. 2015;161:261–268. doi: 10.1016/j.schres.2014.10.047. [DOI] [PubMed] [Google Scholar]
- Amad A, Cachia A, Gorwood P, Pins D, Delmaire C, Rolland B, Mondino M, Thomas P, Jardri R. The multimodal connectivity of the hippocampal complex in auditory and visual hallucinations. Mol. Psychiatry. 2014;19:184–191. doi: 10.1038/mp.2012. [DOI] [PubMed] [Google Scholar]
- Andreasen N. Scale for the Assessment of Positive Symptoms (SAPS) Iowa City IA Univ; Iowa: 1984a. [Google Scholar]
- Andreasen N. Scale for the Assessment of Negative Symptoms (SANS) Iowa City IA Univ; Iowa: 1984b. [Google Scholar]
- Arnolds DE, Lopes da Silva FH, Aitink JW, Kamp A, Boeijinga P. The spectral properties of hippocampal EEG related to behaviour in man. Electroencephalogr. Clin. Neurophysiol. 1980;50:324–328. doi: 10.1016/0013-4694(80)90160-1. [DOI] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/S0896-6273(02)00586-X. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents — EEG, ECoG, LFP and spikes. Nat. Rev. Neurosci. 2012;13:407–420. doi: 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum. Brain Mapp. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clos M, Diederen KMJ, Meijering AL, Sommer IE, Eickhoff SB. Aberrant connectivity of areas for decoding degraded speech in patients with auditory verbal hallucinations. Brain Struct. Funct. 2014;219:581–594. doi: 10.1007/s00429-013-0519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copolov DL, Seal ML, Maruff P, Ulusoy R, Wong MTH, Tochon-Danguy HJ, Egan GF. Cortical activation associated with the experience of auditory hallucinations and perception of human speech in schizophrenia: a PET correlation study. Psychiatry Res. 2003;122:139–152. doi: 10.1016/s0925-4927(02)00121-x. [DOI] [PubMed] [Google Scholar]
- Damaraju E, Allen EA, Belger A, Ford JM, McEwen S, Mathalon DH, Mueller BA, Pearlson GD, Potkin SG, Preda A, Turner JA, Vaidya JG, van Erp TG, Calhoun VD. Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. NeuroImage Clin. 2014;5:298–308. doi: 10.1016/j.nicl.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr. Res. 2005;76:135–157. doi: 10.1016/j.schres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Erhardt EB, Rachakonda S, Bedrick EJ, Allen EA, Adali T, Calhoun VD. Comparison of multi-subject ICA methods for analysis of fMRI data. Hum. Brain Mapp. 2011;32:2075–2095. doi: 10.1002/hbm.21170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Palzes VA, Roach BJ, Potkin SG, Van Erp TGM, Turner JA, Mueller BA, Calhoun VD, Voyvodic J, Belger A, Bustillo J, Vaidya JG, Preda A, McEwen SC, Mathalon DH. Visual hallucinations are associated with hyperconnectivity between the amygdala and visual cortex in people with a diagnosis of schizophrenia. Schizophr. Bull. 2015;41:223–232. doi: 10.1093/schbul/sbu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, Brown HR, Siemerkus J, Stephan KE. The dysconnection hypothesis (2016) Schizophr. Res. 2016;176:83–94. doi: 10.1016/j.schres.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith C. The role of the prefrontal cortex in self-consciousness: the case of auditory hallucinations. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1996;351:1505–1512. doi: 10.1098/rstb.1996.0136. [DOI] [PubMed] [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am. J. Psychiatry. 2007;164:450–457. doi: 10.1176/appi.ajp.164.3.450. [DOI] [PubMed] [Google Scholar]
- Gavrilescu M, Rossell S, Stuart GW, Shea TL, Innes-Brown H, Henshall K, McKay C, Sergejew AA, Copolov D, Egan GF. Reduced connectivity of the auditory cortex in patients with auditory hallucinations: a resting state functional magnetic resonance imaging study. Psychol. Med. 2010;40:1149–1158. doi: 10.1017/S0033291709991632. [DOI] [PubMed] [Google Scholar]
- Gillespie AL, Samanaite R, Mill J, Egerton A, MacCabe JH. Is treatment-resistant schizophrenia categorically distinct from treatment-responsive schizophrenia? a systematic review. BMC Psychiatry. 2017:17. doi: 10.1186/s12888-016-1177-y. [DOI] [PMC free article] [PubMed]
- Goulden N, Khusnulina A, Davis NJ, Bracewell RM, Bokde AL, McNulty JP, Mullins PG. The salience network is responsible for switching between the default mode network and the central executive network: Replication from DCM. NeuroImage. 2014;99:180–190. doi: 10.1016/j.neuroimage.2014.05.052. [DOI] [PubMed] [Google Scholar]
- Grace AA, Gomes FV. The Circuitry of Dopamine System Regulation and its Disruption in Schizophrenia: Insights Into Treatment and Prevention. Schizophr. Bull. 2018 doi: 10.1093/schbul/sbx199. [DOI] [PMC free article] [PubMed]
- Green JD, Arduini AA. Hippocampal electrical activity in arousal. J. Neurophysiol. 1954;17:533–557. doi: 10.1152/jn.1954.17.6.533. [DOI] [PubMed] [Google Scholar]
- Hare SM, Ford JM, Ahmadi A, Damaraju E, Belger A, Bustillo J, Lee HJ, Mathalon DH, Mueller BA, Preda A, Erp V, M TG, Potkin SG, Calhoun VD, Turner JA. Modality-Dependent Impact of Hallucinations on Low-Frequency Fluctuations in Schizophrenia. Schizophr. Bull. 2017;43:389–396. doi: 10.1093/schbul/sbw093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman R, Fernandez T, Pittman B, Hampson M. Elevated Functional Connectivity Along the Corticostriatal Loop and the Mechanism of Auditory/Verbal Hallucinations in Patients with Schizophrenia. Biol. Psychiatry. 2012;69:407–414. doi: 10.1016/j.biopsych.2010.09.050.ELEVATED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman JM, Hasselmo ME, Seamans JK. What is the Functional Relevance of Prefrontal Cortex Entrainment to Hippocampal Theta Rhythms? Front. Neurosci. 2011;5:24. doi: 10.3389/fnins.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Working Memory Performance Correlates with Prefrontal-Hippocampal Theta Interactions but not with Prefrontal Neuron Firing Rates. Front. Integr. Neurosci. 2010;4:2. doi: 10.3389/neuro.07.002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardri R, Pouchet A, Pins D, Ph D, Thomas P. Cortical Activations During Auditory Verbal Hallucinations in Schizophrenia : A Coordinate-Based Meta-Analysis. Am. J. Psychiatry. 2011;168:73–81. doi: 10.1176/appi.ajp.2010.09101522. [DOI] [PubMed] [Google Scholar]
- Jasinska AJ, Zorick T, Brody AL, Stein EA. Dual role of nicotine in addiction and cognition: A review of neuroimaging studies in humans. Neuropharmacology. 2014;0:111–122. doi: 10.1016/j.neuropharm.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keator DB, van Erp TGM, Turner JA, Glover GH, Mueller BA, Liu TT, Voyvodic JT, Rasmussen J, Calhoun VD, Lee HJ, Toga AW, McEwen S, Ford JM, Mathalon DH, Diaz M, O’Leary DS, Jeremy Bockholt H, Gadde S, Preda A, Wible CG, Stern HS, Belger A, McCarthy G, Ozyurt B, Potkin SGFBIRN. The Function Biomedical Informatics Research Network Data Repository. NeuroImage. 2016;124:1074–1079. doi: 10.1016/j.neuroimage.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre S, Demeulemeester M, Leroy A, Delmaire C, Lopes R, Pins D, Thomas P, Jardri R. Network dynamics during the different stages of hallucinations in schizophrenia. Hum. Brain Mapp. 2016;37:2571–2586. doi: 10.1002/hbm.23197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle EB, Price D, Palaniyappan L, Brookes MJ, Robson SE, Hall EL, Morris PG, Liddle PF. Abnormal salience signaling in schizophrenia: The role of integrative beta oscillations. Hum. Brain Mapp. 2016;37:1361–1374. doi: 10.1002/hbm.23107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Buzsáki G. A neural coding scheme formed by the combined function of gamma and theta oscillations. Schizophr. Bull. 2008;34:974–980. doi: 10.1093/schbul/sbn060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubenov EV, Siapas AG. Hippocampal theta oscillations are travelling waves. Nature. 2009;459:534–539. doi: 10.1038/nature08010. [DOI] [PubMed] [Google Scholar]
- Manoliu A, Riedl V, Zherdin A, Mühlau M, Schwerthöffer D, Scherr M, Peters H, Zimmer C, Förstl H, Bäuml J, Wohlschläger AM, Sorg C. Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophr. Bull. 2014;40:428–437. doi: 10.1093/schbul/sbt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Salience Network, Brain Mapping: An Encyclopedic Reference. Vol. 2. Elsevier Inc; 2015. pp. 597–611. [Google Scholar]
- Mouchlianitis E, McCutcheon R, Howes OD. Brain-imaging studies of treatment-resistant schizophrenia: a systematic review. Lancet Psychiatry. 2016;3:451–463. doi: 10.1016/S2215-0366(15)00540-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniyappan L, Balain V, Radua J, Liddle PF. Structural correlates of auditory hallucinations in schizophrenia: A meta-analysis. Schizophr. Res. 2012;137:169–173. doi: 10.1016/j.schres.2012.01.038. [DOI] [PubMed] [Google Scholar]
- Palaniyappan L, Doege K, Mallikarjun P, Liddle E, Francis-Liddle P. Cortical thickness and oscillatory phase resetting: a proposed mechanism of salience network dysfunction in schizophrenia. Psychiatr. Psychiatr. 2012;23:117–129. [PubMed] [Google Scholar]
- Palaniyappan L, Liddle PF. Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. J. Psychiatry Neurosci. 2012;37:17–27. doi: 10.1503/jpn.100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniyappan L, Mallikarjun P, Joseph V, White TP, Liddle PF. Reality distortion is related to the structure of the salience network in schizophrenia. Psychol. Med. 2011;41:1701–1708. doi: 10.1017/S0033291710002205. [DOI] [PubMed] [Google Scholar]
- Palaniyappan L, Simmonite M, White TP, Liddle EB, Liddle PF. Neural Primacy of the Salience Processing System in Schizophrenia. Neuron. 2013;79:814–828. doi: 10.1016/j.neuron.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedemonte M, Peña JL, Velluti RA. Firing of inferior colliculus auditory neurons is phase-locked to the hippocampus theta rhythm during paradoxical sleep and waking. Exp. Brain Res. 1996;112:41–46. doi: 10.1007/BF00227176. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes Ka, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu W, Li L, Zhang H, Ouyang X, Liu H, Zhao J, Li L. Morphological and functional abnormalities of salience network in the early-stage of paranoid schizophrenia. Schizophr. Res. 2012;141:15–21. doi: 10.1016/j.schres.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Raichle ME. The Brain’s Default Mode Network. Annu. Rev. Neurosci. 2015:413–427. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed]
- Rolland B, Amad A, Poulet E, Bordet R, Vignaud A, Bation R, Delmaire C, Thomas P, Cottencin O, Jardri R. Resting-state functional connectivity of the nucleus accumbens in auditory and visual hallucinations in schizophrenia. Schizophr. Bull. 2015;41:291–299. doi: 10.1093/schbul/sbu097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinn AK, Baker JT, Cohen BM, Ongur D. Functional connectivity of left Heschl’s gyrus in vulnerability to auditory hallucinations in schizophrenia. Schizophr. Res. 2013;143:260–268. doi: 10.1016/j.schres.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46:141–151. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Sommer IE, Clos M, Meijering AL, Diederen KMJ, Eickhoff SB. Resting State Functional Connectivity in Patients with Chronic Hallucinations. PLoS ONE. 2012;7:3–10. doi: 10.1371/journal.pone.0043516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, He Y, Yan CG, Zang YF. REST: A Toolkit for Resting-State Functional Magnetic Resonance Imaging Data Processing. PLoS ONE. 2011;6:e25031–e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. U. S. A. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lutterveld R, Hillebrand A, Diederen KMJ, Daalman K, Kahn RS, Stam CJ, Sommer IEC. Oscillatory cortical network involved in auditory verbal hallucinations in Schizophrenia. PLoS ONE. 2012:7. doi: 10.1371/journal.pone.0041149. [DOI] [PMC free article] [PubMed]
- Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr. Clin. Neurophysiol. 1969;26:407–418. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- Waters F, Collerton D, Ffytche DH, Jardri R, Pins D, Dudley R, Blom JD, Mosimann UP, Eperjesi F, Ford S, Larøi F. Visual hallucinations in the psychosis spectrum and comparative information from neurodegenerative disorders and eye disease. Schizophr. Bull. 2014;(40 Suppl. 4):S233–45. doi: 10.1093/schbul/sbu036. [DOI] [PMC free article] [PubMed]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, LaViolette P, Wojcik J, Gabrieli JDE, Seidman LJ. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2009;106:1279–84. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winson J. Interspecies differences in the occurrence of theta. Behav. Biol. 1972;7:479–487. doi: 10.1016/s0091-6773(72)80210-4. [DOI] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J. Clin. Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Yaesoubi M, Miller RL, Calhoun VD. Time-varying spectral power of resting-state fMRI networks reveal cross-frequency dependence in dynamic connectivity. PloS ONE. 2017;12:e0171647. doi: 10.1371/journal.pone.0171647. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.