Abstract
AIM
To investigate the anti-fibrotic effects of the traditional oriental herbal medicine Daikenchuto (DKT) associated with transient receptor potential ankyrin 1 (TRPA1) channels in intestinal myofibroblasts.
METHODS
Inflammatory and fibrotic changes were detected in a 2,4,6-trinitrobenzenesulfonic acid (TNBS) chronic colitis model of wild-type and TRPA1-knockout (TRPA1-KO) mice via pathological staining and immunoblotting analysis. Ca2+ imaging experiments examined the effects of DKT and its components/ingredients on intestinal myofibroblast (InMyoFib) cell TRPA1 channel function. Pro-fibrotic factors and transforming growth factor (TGF)-β1-associated signaling were tested in an InMyoFib cell line by qPCR and immunoblotting experiments. Samples from non-stenotic and stenotic regions of the intestines of patients with Crohn’s disease (CD) were used for pathological analysis.
RESULTS
Chronic treatment with TNBS caused more severe inflammation and fibrotic changes in TRPA1-KO than in wild-type mice. A one-week enema administration of DKT reduced fibrotic lesions in wild-type but not in TRPA1-KO mice. The active ingredients of DKT, i.e., hydroxy α-sanshool and 6-shogaol, induced Ca2+ influxes in InMyoFib, and this was antagonized by co-treatment with a selective TRPA1 channel blocker, HC-030031. DKT counteracted TGF-β1-induced expression of Type I collagen and α-smooth muscle actin (α-SMA), which were accompanied by a reduction in the phosphorylation of Smad-2 and p38-mitogen-activated protein kinase (p38-MAPK) and the expression of myocardin. Importantly, 24-h incubation with a DKT active component Japanese Pepper increased the mRNA and protein expression levels of TRPA1 in InMyoFibs, which in turn negatively regulated collagen synthesis. In the stenotic regions of the intestines of CD patients, TRPA1 expression was significantly enhanced.
CONCLUSION
The effects of DKT on the expression and activation of the TRPA1 channel could be advantageous for suppressing intestinal fibrosis, and benefit inflammatory bowel disease treatment.
Keywords: Intestinal fibrosis, Myofibroblast, Transient receptor potential ankyrin 1, Crohn’s disease, Collagen, α-smooth muscle actin
Core tip: Active ingredients of the famous Chinese medicine Da-Jian-Zhong-Tang (Daikenchuto; DKT), i.e., hydroxy α-sanshool and 6-shogaol, induced Ca2+ influxes in intestinal myofibroblast (InMyoFib), which were antagonized by co-treatment with a selective transient receptor potential ankyrin 1 (TRPA1) channel blocker HC-030031. DKT counteracted the transforming growth factor (TGF)-β1-induced expression of Type I collagen, α-smooth muscle actin (α-SMA), and this was accompanied by a reduction in fibrosis signaling downstream of the TGF-β1 receptor. Importantly, a 24-h incubation with another DKT active ingredient of Japanese Pepper increased mRNA and protein expression in TRPA1, which in turn negatively regulated collagen synthesis in InMyoFibs. In stenotic regions of the intestines of patients with Crohn’s disease, TRPA1 expression was significantly increased.
INTRODUCTION
Daikenchuto (DKT; Da-Jian-Zhong-Tang in Chinese) is a traditional herbal medicine, originally described in Jin Gui Yao Lue (“Essential Prescriptions from the Golden Cabinet”), a classic book of Chinese medicine published more than 1800 years ago. DKT is often used for postoperative ileus and constipation, and is composed of several crude components, Zingiberis rhizoma (Ginger), Panax ginseng (Ginseng; Ginseng radix), Zanthoxyli fructus (Japanese pepper), and malt sugar. DKT generally accelerates gastrointestinal motility; it increases intestinal blood flow and gastrointestinal hormone secretion[1-3]. Numerous basic studies have demonstrated the effects of DKT on vasodilation, inflammation, and bacterial translocation[4-9]. Ginger contains several active ingredients, such as gingerols and shogaols (6-, 8-, and 10- isomers), which have anti-inflammatory and vasoprotective effects via modulating the activities of mitogen-activated protein kinase (MAPK), protein kinase B (Akt), and NF-κB[10-12]. Japanese pepper contains hydroxy-α and hydroxy-β-sanshools that alter intestinal blood flow, motility, and barrier function by inducing the synthesis/secretion of adrenomedullin and calcitonin gene-related peptides[1,13]. DKT accelerates the recovery of gastrointestinal function in patients undergoing an open colectomy for colon cancer[14,15]. However, the essential pharmacological mechanism(s) underlying these effects remains largely unclear.
Myofibroblasts are crucial for the pathogenesis of tissue fibrosis. The formation of stress fibers in activated myofibroblasts results in the release of a myocardin-related transcription factor, a transcriptional coactivator of serum response factor. The major pro-fibrotic factor, transforming growth factor (TGF)-β, secreted from many types of cells, induces or augments myofibroblast functions, i.e., transformation, proliferation, invasion, migration, stress fiber formation, and collagen synthesis[16,17]. Previously, we used an intestinal myofibroblast (InMyoFib) cell line, stimulated by TGF-β1, to produce a pathological fibrosis model[18]. TGF-β1 treatment induced dramatic morphological changes in myofibroblasts, including an enlarged shape and a filamentous microstructure characteristic in transformed cells[19]. The activated TGF-β receptor 1 (TGFR) complex phosphorylates the transcription factors Smad-2 and Smad-3, which in turn promote collagen synthesis[20]. TGF-β can also signal through non-canonical pathways involving extracellular signal-regulated kinases (ERKs), c-Jun N-terminal kinases, and p38-MAPK. Both pathways are implicated in myofibroblast-mediated cytokine production and fibrosis in the gut[21,22]. Levels of TGF-β were found to be elevated in the inflamed intestines of patients with Crohn’s disease (CD) and ulcerative colitis. TGF-β1 is also essential for anti-inflammatory responses. Thus, there is a caveat against the use of a TGF-β1-neutralizing strategy for anti-fibrotic treatment in clinical practice, as TGF-β1-neutralizing antibodies might exacerbate the progression of CD by attenuating the anti-inflammatory actions of TGF-β1. Further, abnormal TGF-β signaling was reported to impair intestinal immune tolerance and tissue repair[23].
There is mounting evidence for the role of transient receptor potential (TRP) channels in a variety of cellular remodeling processes[24-27]. For instance, TRPA1 is expressed not only in peripheral sensory neurons, but also in intestinal epithelial cells[7,28]. The TRPA1 agonist allyl isothiocyanate (AITC) exhibits anti-fibrogenic effects in hepatic stellate cells, and another TRPA1 agonist allicin prevents fibrotic changes in the oral submucosa and heart[29,30]. Our recent study also demonstrated that TRPA1 mediates the anti-fibrotic effects of steroids and pirfenidone in the mouse TNBS-colitis model, and TRPA1 expression is significantly increased in the intestinal stenotic regions of CD patients[18]. Moreover, TRPA1 activity has been ascribed to anti-inflammatory responses in dextran sulfate sodium-induced chronic colitis, where TRPA1 regulates the inflammatory potential of T cells[31]. A later study based on interleukin knockout and T-cell-adoptive transfer colitis models suggested that TRPA1 likely counteracted the transient receptor potential vanilloid type 1 (TRPV1)-mediated differentiation of CD4+-T cells into Th1-effector cells[32]. Finally, TRPA1 activation by cannabichromene was found to reduce the severity of dinitrobenzene sulfonic acid (DNBS)-induced colitis[33].
Hydroxy-α-sanshool and 6-shogaol are the active ingredients of DKT components ginger and Japanese pepper. Both are capable of activating TRPA1 and TRPV1 channels[34,35]. Activation by DKT of the TRPA1 channel endogenously expressed in intestinal epithelial cells improves gastrointestinal microcirculation via adrenomedullin release[28], and facilitates intestinal transit in a murine model of postoperative ileus[36].
Currently, surgical operation is the only available option that prevents the fibrotic complications of CD, but it often only has temporary benefits. It is, therefore, crucial to develop new alternative treatments for fibrotic stenosis. Recently, DKT has often been used to mitigate inflammatory bowel disease (IBD)-associated fibrosis and the resulting stenosis and strictures, common and severe complications in CD patients. However, the pathogenesis of CD-related fibrosis and how DKT exerts its therapeutic effects are poorly understood[37].
In this study, we tested whether DKT can activate myofibroblast TRPA1 channels to reduce TGF-β1-induced fibrotic events. To this end, we examined the effects of DKT and its ingredients on TRPA1 channel activity and the expression of pro-fibrotic factors downstream of the TGF-β1 signaling pathway. Moreover, surgical samples from stenotic and non-stenotic regions of the intestines of patients with CD were used to confirm the validity of the observations in cell experiments for human pathogenesis.
MATERIALS AND METHODS
Materials
DKT is an aqueous extract containing processed ginger, ginseng (ginseng radix), and Japanese pepper at a ratio of 5:3:2. The dried powdered extract forms of DKT (TU-100: without maltose syrup), ginger, ginseng, and Japanese pepper were obtained from Tsumura Co. (Tokyo, Japan). These extracted powders were dissolved in ethanol by sonication and purified by filtering. Ginsenoside (Wako, Japan), 6-shogaol (Wako, Japan), hydroxy-α-sanshool (Adipogen Life Science), AITC, and HC-030031 (Sigma-Aldrich, St Louis, MO, United States) were also used in this study. Recombinant human TGF-β1 (Wako) and Type I collagen (IFP, Higashine, Japan) were added to cultured cells, as indicated by the manufacturer’s instructions. Human stealth siRNAs for TRPA1: TRPA1HSS113276 (5′-GGAGCAAUUGCUGUUUACUUCUAUU-3′ and 5′-AAUAGAAGUAAACAGCAAUUGCUCC-3′) were obtained from Invitrogen (Carlsbad, CA, United States) and used for gene silencing, according to the manufacturer’s instructions. Other TRPA1-siRNAs, TRPA1HSS113277 and TRPA1HSS189723, were also evaluated, but the silencing efficacy was inferior to that of TRPA1HSS113276 for InMyoFibs. Antibodies against TRPA1 (mouse; Sigma-Aldrich), α-SMA (Abcam, Cambridge, MA, United States), β-actin (Abcam, Cambridge, MA, United States), Collagen I (Abcam, Cambridge, MA, United States), Smad-2/3, phospho-Smad-2, and p38-MAPK (Cell Signaling Technology, Beverly, MA, United States) were used for immunoblotting and immunostaining experiments. An ELISA Kit was used to measure the Type I collagen levels in the conditioned medium.
Trinitrobenzenesulfonic acid chronic colitis model
Chronic TNBS-associated colitis was induced as previously reported[18]. TNBS intracolonic administration for mice (8-9 wk old, n = 8) was achieved after 24-h fasting by inserting a polyethylene catheter 4 cm into the rectum under pentobarbital (40 mg/kg injected i.p. with saline) anesthesia. TNBS solution in 30% ethanol/phosphate-buffered saline (PBS) (10 mg/mL; 50 μL) and sonicated was weekly injected for six weeks, as illustrated in Figure 1A. The vehicle control group received 30% ethanol/PBS (50 μL). DKT was administered daily (5 mg/kg/d; anesthetized by isoflurane) by enema for one week after the last TNBS treatment. Colonic tissues were excised from the anus to the caecum at week six after cervical dislocation. All animal experiments were conducted in accordance with the guidelines of the Animal Center of Fukuoka University.
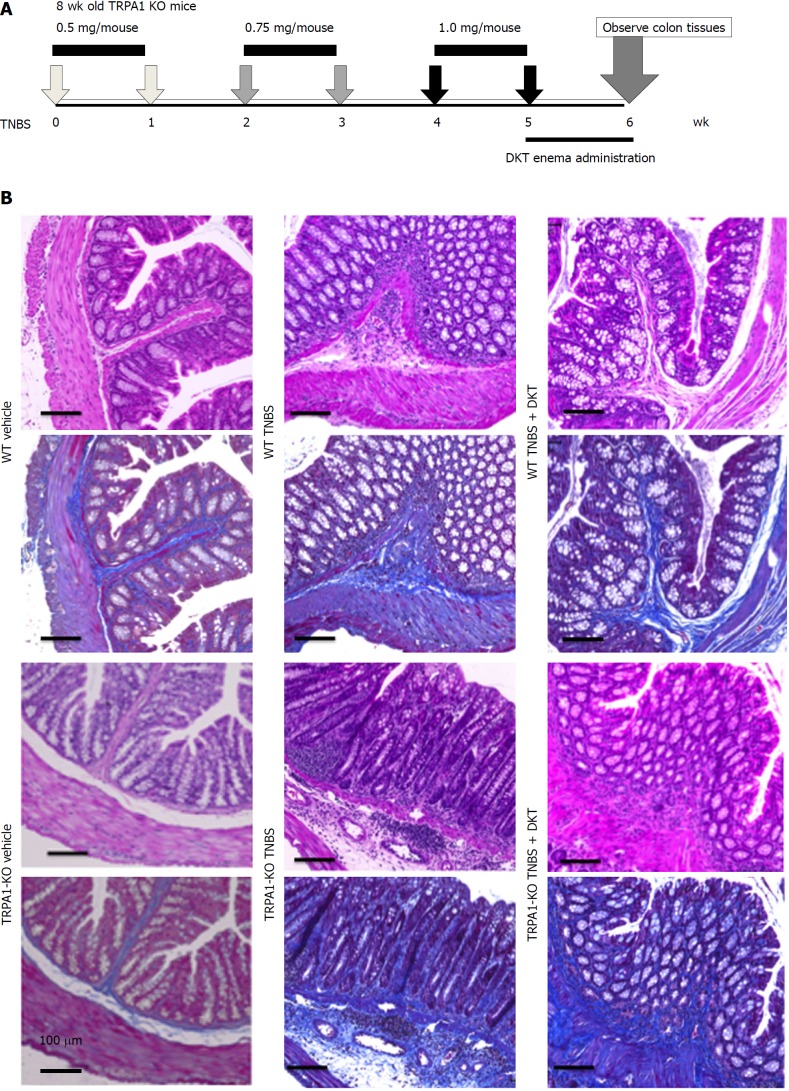
Figure 1.
Chronic 2, 4, 6-trinitrobenzenesulfonic acid-induced colitis models of wild-type and transient receptor potential ankyrin 1-knockout mice. Chronic colitis was induced by weekly intrarectal injection of TNBS for six weeks. Immediately after the final TNBS treatment, a DKT enema was administered daily for one week. A: schematic presentation of TNBS injection and DKT enema; B: Hematoxylin–eosin (HE)- and Masson's trichrome (MT)-stained tissues from the vehicle control, TNBS and TNBS + DKT groups of WT and TRPA1-/- mice. TNBS: 2, 4, 6-trinitrobenzenesulfonic acid; WT: Wild-type; TRPA1: Transient receptor potential ankyrin 1; TRPA1-/-: Transient receptor potential ankyrin 1-knockout; DKT: Daikenchuto; HE: Hematoxylin-eosin; MT: Masson's trichrome.
Histological evaluation
The mouse distal colon and patient samples were fixed in 10% buffered formalin, embedded in paraffin, and cut into 4-μm-thick sections for routine hematoxylin-eosin (HE) and Masson’s trichrome (MT) staining. Stained colon tissues were micro-photographed at 200 × magnification on six randomly chosen locations of each tissue, and analyzed by a pathologist (Koga K) in a blinded manner. For all mice, the fibrosis score was measured at the tissue located 1 cm from the anus, where the most obvious fibrosis was observed. The optical density of the blue-stained collagen fibers was measured and expressed as a fibrosis score on a scale of 0-3: (0) No fibrosis wild-type vehicle control mouse; (1) mild fibrosis; (2) moderate fibrosis; and (3) severe fibrosis as described previously[18].
Cell culture
Normal human intestinal myofibroblasts (InMyoFibs) were purchased from Lonza (CC-2902; Basel, Switzerland) and grown in smooth muscle basal medium (SmBM), supplemented with 5% fetal bovine serum (FBS), antibiotics (gentamicin/amphotericin-B), and growth factors (insulin, human epidermal growth factor-β, and human fibroblastic growth factor). InMyoFibs were passaged 10-19 times. TGF-β1 (5 ng/mL) was used under low serum (1% FBS) conditions.
Electrophysiological study
Whole-cell voltage clamp recording was implemented by an EPC-10 patch-clamp amplifier (HEKA Electronics, Lambrecht, Pfalz, Germany). The recording electrodes (patch pipettes), with resistances of 2-4 MΩ, were fabricated from borosilicate glass capillaries. An Ag-AgCl wire was used as a grounding electrode. Capacitive currents were electronically compensated, and the linear leak and residual capacitance were subtracted. More than 70% of series resistance was compensated to minimize voltage errors.
Data analysis and illustration were performed offline using the versatile analysis software programs Clampfit v.9.2 (Axon Instruments, Foster City, CA, United States) and KaleidaGraph v.4.0 (Hulinks, Tokyo, Japan). Long-term recordings were performed at a sampling rate of 100 Hz in conjunction with an A/D, D/A-converter PowerLab/400 (ADInstruments, Australia) and analyzed by the accessory software LabChart v.8.1.5. A solenoid valve-driven fast solution change device similar to the “Y-tube” system was used to rapidly apply drugs onto cells as previously described[38]. The density of the membrane current (pA/pF) was calculated by normalizing its amplitude by the cell capacitance to minimize cell size-dependent variations. To prevent the quick Ca2+-dependent inactivation of TRPA1 currents, a Ca2+ chelator BAPTA was added to both the bath and pipette solutions[39].
The bath solution contained 140 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L MgCl2, 5 mmol/L EGTA, 10 mmol/L HEPES, and 10 mmol/L glucose (pH = 7.4, adjusted with Tris base). K+ currents were eliminated by including Cs+ and TEA in the patch pipette solution. Patch pipettes were filled with 130 mmol/L Cs-aspartate, 10 mmol/L TEA-Cl, 5 mmol/L BAPTA, 1.374 mmol/L Ca-gluconate, 1 mmol/L MgCl2, 2 mmol/L MgSO4, 2 mmol/L Na2ATP, and 10 mmol/L HEPES (pH = 7.2, adjusted with Tris base).
Measurement of [Ca2+]i
The intracellular Ca2+ concentration ([Ca2+]i) of myofibroblasts was monitored using a digital fluorescence imaging technique with Fura-2. Briefly, InMyoFibs were enzymatically dispersed and lodged on a poly-L-lysine-coated (Sigma-Aldrich, United States) glass chamber placed on the stage of an inverted fluorescent microscope (DMI600B; Leica, Germany). InMyoFibs cells were then loaded with 5 μmol/L Fura-2/AM in the dark at 20-25 °C for 30 min. The intensities of Fura-2 emissions at 510 nm (± 10 nm) resulting from excitation at 340 or 380 nm were measured through a fluorescent microscope (DMI600B) and a Cascade EMCCD camera (Nippon Roper, Tokyo, Japan). Data acquisition and analysis were performed using SlideBook v.4.2 software (Intelligent Imaging Innovation Inc., Denver, Colorado, United States). Fluorescence intensities were corrected for background fluorescence, and changes in [Ca2+]i were defined as the ratio of corrected fluorescence intensities at 340 and 380 nm excitations (F340/F380). The normal external solution for Ca2+-imaging experiments contained 140 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L CaCl2, 1.2 mmol/L MgCl2, 10 mmol/L HEPES, and 10 mmol/L glucose (pH = 7.4, adjusted with Tris base).
Immunostaining
InMyoFibs cells were fixed in 4% formaldehyde for 15 min and permeabilized with 0.3% Triton X-100 in 5% normal goat serum (Wako). Cells were then incubated overnight with primary antibodies (1:200 dilution) against α-SMA at 4 °C, washed with PBS, and incubated with an Alexa Fluor-conjugated secondary antibody (Life Technologies, Carlsbad, California, United States, 1:200 dilution) for an additional 1 h. Immunostained cells were analyzed using a Zeiss LSM 710 Confocal Microscope (Oberkochen, Germany). Images were taken through a 40 × objective lens using the default settings for pinhole width, laser intensity, and detector gain.
Real-time RT-PCR
Total RNA was extracted from InMyoFibs using the RNeasy RNA Extraction Kit (Qiagen, Venlo, Netherlands). To quantify mRNA expression levels, real-time PCR was performed using a BioMark™ HD System (Fluidigm, South San Francisco, CA, United States). Thermocycling was performed using an initial denaturation at a hot start phase of 60 s at 95 °C. This was followed by 35 cycles of 5 s at 96 °C and 20 s at 60 °C. The following TaqMan® Gene Expression Assay kits from Life Technologies were used for real-time PCR reactions: acta2 (α-SMA; Hs00426835_g1), myocd (myocardin; Hs00538071_m1), and col1a1 (Hs00164004_m1).
Immunoblotting analysis
Immunoblotting experiments were performed to examine the protein levels of Type I collagen, α-SMA, β-actin, Smad-2/3, phospho-Smad-2, and p38-MAPK in InMyoFibs, as described previously[19]. Total cell lysates were homogenized in radioimmunoprecipitation assay buffer with protease inhibitors, and prepared in a sample buffer and diluted in 5% (v/v) 2-mercaptoethanol and 1% (w/v) bromophenol blue prior to electrophoresis. Proteins were resolved using 10% (w/v) sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to polyvinyldifluoridine (PVDF) membranes. The membranes were blocked with Blocking One (Nacalai Tesque, Kyoto, Japan) and incubated overnight (at 4 °C) with the appropriate primary antibodies. The protein levels were detected by incubating the PVDF membranes with appropriate species-specific, horseradish peroxidase-conjugated secondary antibodies (20-25 °C, 45 min), and visualized using the ECL Western Blotting Detection System (GE Healthcare, Little Chalfont, United Kingdom).
Patient samples
We obtained surgical samples from three male CD patients with colonic lesions with a median age of 47 years (range: 40-51 years) and a median history of CD 22 years (range: 15-26 years). All patients had suffered, in addition to coexisting stenosis, an intra-abdominal or perianal fistula and/or abscess, during the course of the disease. All patients received an anti-TNF agent. The Fukuoka University Hospital Ethics Committee approved the protocol, and written informed consent was obtained from all patients.
Statistical analysis
Results are expressed as means ± SEM. Experimental protocols were repeated with at least four different batches of cells under each condition, and pooled data were averaged and subjected to statistical analyses. Statistically significant differences among groups were evaluated by analysis of variance (ANOVA), and Dunnett’s test was employed for multiple comparisons. P-values < 0.01 or 0.05 were considered statistically significant.
RESULTS
Chronic TNBS fibrosis model of TRPA1-KO mice
To better visualize the time course of chronic colitis, we adopted the TNBS-induced colitis model, as it is known to allow a more moderate and slower progression of gut inflammation and fibrosis in mice than the other colitis models.
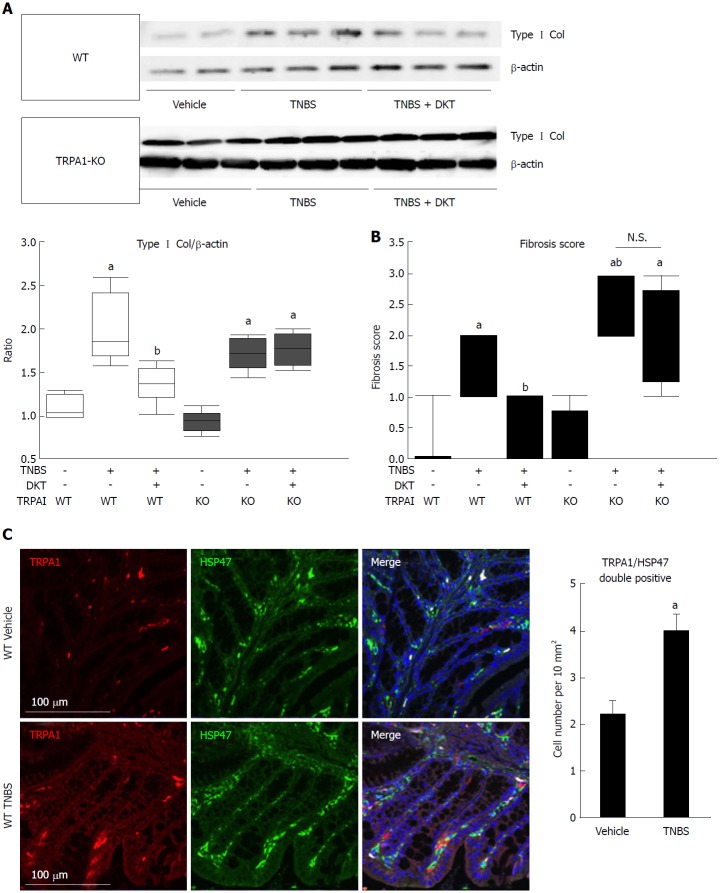
At the sixth week of TNBS treatment, TRPA1-KO mice showed more severe signs of inflammation/fibrosis than did wild-type mice, where prominent cell infiltration occurred in some mucosal and submucosal layers. The co-administration of DKT reduced these pathological changes in wild-type (WT), but not in TRPA1-KO mice (Figure 1B). Concomitantly, the Type I collagen protein expression and fibrosis score, which were remarkably enhanced and aggravated by TNBS treatment, respectively, were significantly reduced in WT but not in TRPA1-KO mice (Figures 1B, 2A and B). Double immunostaining experiments indicated a high co-incidence of immunoreactivities against TRPA1 and heat shock protein (HSP) 47, an endoplasmic reticulum-resident molecule essential for correct procollagen folding[18]. The number of TRPA1/HSP47-double-positive cells per unit area greatly increased in the TNBS-treated groups compared to in the vehicle control group (Figure 2C).
Figure 2.
Fibrotic changes in wild-type and transient receptor potential ankyrin 1-knockout CRISPR mice treated with 2, 4, 6-trinitrobenzenesulfonic acid and/or Daikenchuto. A: Type I collagen expression was examined by western blotting. The ratio of changes in each experimental condition is shown in the middle lower-left graph. aP < 0.01 vs vehicle control, bP < 0.01 vs TNBS (n = 6); B: Fibrosis scores based on histological examination ranging from 0 (no fibrosis) to 3 (severe fibrosis) are shown as the dot-plot graph. The leftmost circles (n = 8) show absolutely zero values. Values are the means ± SD; C: Co-localization of TRPA1 and HSP47 proteins in the mouse intestine. WT vehicle control, WT TNBS: Immunostaining images of mouse intestine stained with anti-TRPA1 (red) antibody, anti-HSP47 (green), and DAPI (blue). Cell number per 10 mm2 is summarized in right graph, aP < 0.05 vs vehicle control (n = 6). TNBS: 2, 4, 6-trinitrobenzenesulfonic acid; WT: Wild-type; HSP47: Heat shock protein 47; TRPA1: Transient receptor potential ankyrin 1; DAPI: 4',6-diamidino-2-phenylindole.
DKT activates TRPA1 channel and antagonizes fibrotic changes in InMyoFibs
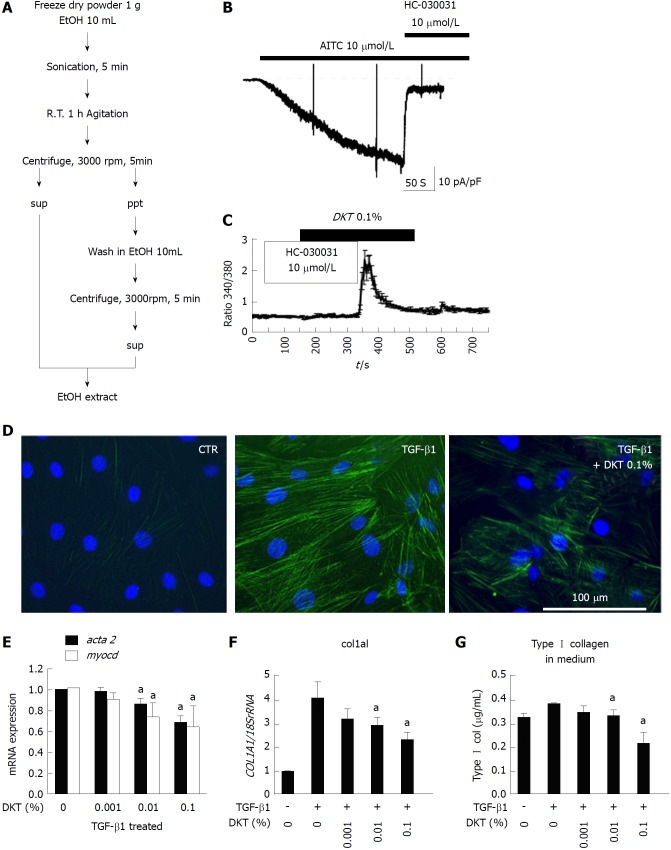
We next examined the ability of DKT to induce TRPA1 activity in InMyoFib. The workflow for ethanol extraction of crude components from DKT, i.e., ginger, ginseng radix, and Japanese pepper, are shown in Figure 3A. In the whole-cell mode of patch-clamp recording (holding potential: -60 mV), a potent TRPA1 agonist, AITC (10 μmol/L), induced a robust non-selective cation current. This current was strongly inhibited by a TRPA1-selective antagonist, HC-030031 (10 μmol/L) (Figure 3B). The same concentration of AITC also evoked a prominent rise in [Ca2+]i, which was completely inhibited by the application of HC-030031 (10 μmol/L)[18]. A similar-magnitude rise in HC-030031-sensitive [Ca2+]i was also induced by DKT (0.1% EtOH extract) (Figure 3C). These results suggest that InMyoFibs express functional TRPA1 channels with Ca2+ permeability, which can be activated by DKT.
Figure 3.
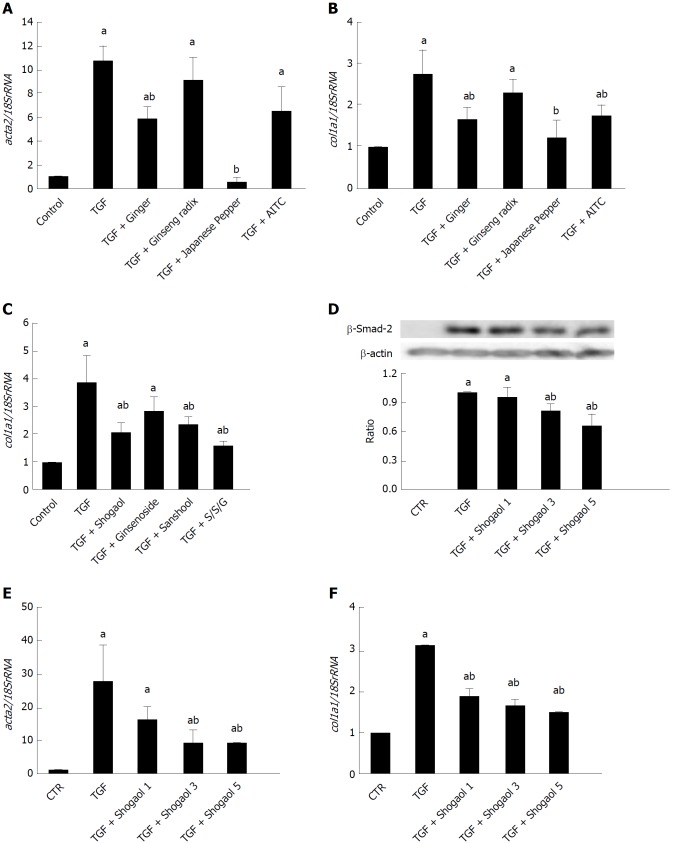
Daikenchuto-induced Ca2+ influx via transient receptor potential ankyrin 1 and anti-fibrosis effects in intestinal myofibroblasts. A: Method for ethanol-extraction of DKT and its components, i.e., ginger, Japanese pepper, and ginseng; B: Representative traces of whole-cell currents recorded from InMyoFibs cells at a holding potential of -60 mV. TRPA1 nonselective cation currents are activated by AITC (10 μmol/L) and inhibited by HC-030031 (10 μmol/L); C: Representative [Ca2+]i response in InMyoFibs cells. Cells were exposed to DKT extracts (0.1%), which evoked prominent increases in [Ca2+]i. The TRPA1 antagonist HC-030031 (10 μmol/L) was added to examine whether the [Ca2+]i response involved the activation of TRPA1 channels. Respective data points represent means ± SEM from > 30 cells; D: Immunostaining images of InMyoFibs with anti-α-SMA (green) antibody and DAPI (blue) in untreated controls, 24 h TGF-β1 (5 ng/mL) treatment, or 24 h TGF-β1 (5 ng/mL) + DKT extracts (0.1%) treatment; E and F: DKT extracts (0.001%, 0.01%, and 0.1%) were co-administered with TGF-β1, and acta2 (α-SMA), myocd (myocardin), and col1a1 mRNA expression levels were quantified by real-time PCR; G: Concentrations of Type I collagen in the culture medium were measured by ELISA from the control and TGF-β1-treated cells. aP < 0.05 vs TGF-β1-treated cells (n = 4). DKT extracts (0.001%, 0.01%, and 0.1%) were co-administered with TGF-β1. DKT: Daikenchuto; InMyoFibs: Intestinal myofibroblasts; TRPA1: Transient receptor potential ankyrin 1; AITC: Allyl isothiocyanate; α-SMA: α-Smooth muscle actin; DAPI: 4',6-diamidino-2-phenylindole; TGF-β1: Transforming growth factor-β1.
We then examined how DKT affects the fibrotic changes induced by TGF-β1 treatment. As shown in Figure 3D, the 24-h treatment of InMyoFibs with 5 ng/mL TGF-β1 facilitated stress fiber formation and protein expression of α-SMA, which was effectively suppressed by co-treatment with 0.1% EtOH extract of DKT. Concomitantly, the 24-h treatment of InMyoFibs with 5 ng/mL TGF-β1 enhanced the mRNA expression levels of the master transcription regulator MYOCD, α-SMA (ACTA2), collagen Type I alpha 1 (COL1A1), and Type I collagen released in the culture medium. DKT (0.01%, 0.1% EtOH extract) effectively suppressed these mRNA upregulations in a dose-dependent manner (Figure 3E-G). In addition, DKT (0.001%, 0.01%, 0.1% EtOH extract) did not affect the mRNA levels of COL1A1 in the absence of TGF-β1 stimulation, but 100 μg/mL DKT suppressed Type III collagen gene (COL3A1) expression, irrespective of TGF-β1 treatment (data not shown).
Active components and ingredients of DKT activate TRPA1 and show anti-fibrotic effects
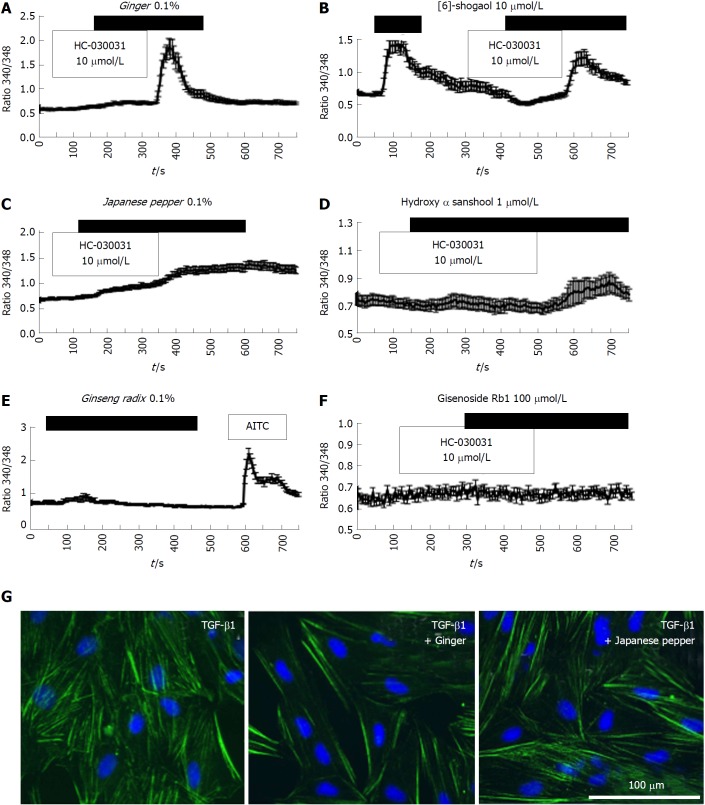
We next examined the efficacies of the active components and ingredients contained in DKT. Among the three active components of DKT (ginger, ginseng, Japanese pepper), ginger was the most effective, showing a similar efficacy to DKT (Figure 4A). In contrast, Japanese pepper was less efficacious at activating Ca2+ influx, and ginseng had almost no effect (Figure 4C and 4E, respectively). The Ca2+ mobilizing effects of ginger and Japanese pepper were almost completely antagonized by the prior application of a selective TRPA1 channel blocker HC-030031, suggesting the involvement of TRPA1 activation (Figure 4A and C). The active ingredients of the respective DKT components were further examined by the same Ca2+-imaging protocol as used above. The active ingredient of ginger, 6-shogaol (100 μmol/L), induced a comparable Ca2+ influx to that of AITC (10 μmol/L), while that of Japanese pepper, hydroxy α-sanshool (1 μmol/L), induced a much milder influx. The active ingredient of ginseng radix, ginsenoside Rb1, was virtually ineffective (Figure 4B, D and F).
Figure 4.
Crude components/ingredient of Daikenchuto induce Ca2+ responses via the transient receptor potential ankyrin 1 channel with anti-fibrotic effects in intestinal myofibroblasts. A-D: Representative [Ca2+]i responses in InMyoFibs cells. Cells were exposed to ginger (0.1%), Japanese pepper (0.1%), 6-shogaol (10 μmol/L), or hydroxy-α-sanshool (1 μmol/L), which evoked measurable increases in [Ca2+]i; E and F: Cells were exposed to ginseng radix (0.1%), ginsenoside Rb1 (100 μmol/L), or AITC (10 μmol/L). Only AITC evoked a measurable increase in [Ca2+]i in InMyoFib. The vertical axis indicates the 340/380 fluorescence ratio. Respective data points represent means ± SEM from > 30 cells; G: Ginger (0.1%) or Japanese pepper (0.1%) suppressed TGF-β1-induced α-SMA expression in InMyoFibs. Immunostaining images of InMyoFibs with anti-α-SMA (green) antibody and DAPI (blue), and no treatment or 24 h TGF-β1 (5 ng/mL) treatment. AITC: Allyl isothiocyanate; InMyoFib: Intestinal myofibroblast; α-SMA: α-Smooth muscle actin; TGF-β1: Transforming growth factor-β1.
We then explored the inhibitory effects of ginger and Japanese pepper on the fibrotic changes induced by 24-h treatment with 5 ng/mL TGF-β1. Co-treatment with ginger or Japanese pepper significantly suppressed TGF-β1-induced stress (α-SMA) fiber formation in InMyoFibs (Figure 4G). In accord with these immunostaining results, ginger, Japanese pepper, and AITC all significantly inhibited the TGF-β1-induced mRNA transcriptions of α-SMA (ACTA2) and Type I collagen (COL1A1) (Figure 5A and 5B).
Figure 5.
Daikenchuto components/ingredients suppress increased expression of α-smooth muscle actin and collagen by transforming growth factor-β1. ACTA2 and COL1A1 mRNAs were quantified by real-time PCR in control and TGF-β1-treated (5 ng/mL, 24 h) InMyoFibs. A-C: DKT components Ginger (0.1%), Japanese pepper (0.1%) and Ginseng radix (0.1%) (A, B), and DKT ingredients 6-shogaol (10 μmol/L), hydroxy-α-sanshool (1 μmol/L) and ginsenoside Rb1 (100 μmol/L) (C) were co-administered with TGF-β1. AITC (10 μmol/L) was used as the reference of full TRPA1 activation; D: Phosphorylation of Smad-2 was measured by western blot analysis. 1, 3, or 5 μmol/L 6-shogaol was co-administered with TGF-β1. Relative expression of Smad-2 protein (normalized to β-actin) in TGF-β1-treated cells is shown as the ratio of the two in the histogram; E and F: COL1A1 and ACTA2 mRNAs were quantified by RT-PCR in TGF-β1 treated InMyoFibs and normalized to 18S rRNA. 1, 3 or 5 μmol/L 6-shogaol was co-administered with TGF-β1. aP < 0.05 vs control cells; bP < 0.05 vs TGF-β1-treated cells (n = 4). TGF-β1: Transforming growth factor-β1; InMyoFibs: Intestinal myofibroblasts; DKT: Daikenchuto; AITC: Allyl isothiocyanate; TRPA1: Transient receptor potential ankyrin 1.
The active ingredient of ginger, 6-shogaol, and that of Japanese pepper, hydroxy α-sanshool, also significantly suppressed the TGF-β1-induced increase in COL1A1 mRNA expression, with a similar efficacy to that of AITC (Figure 5B, C and F). The 6-shogaol dose-dependently suppressed the Smad-2 phosphorylation and α-SMA mRNA expression (ACTA2) induced by TGF-β1 (Figure 5D and F). Although ineffective at evoking a rise in TRPA1-mediated [Ca2+]i, ginsenoside inhibited the TGF-β1-induced increase in COL1A1 mRNA expression.
DKT upregulates TRPA1 channel expression
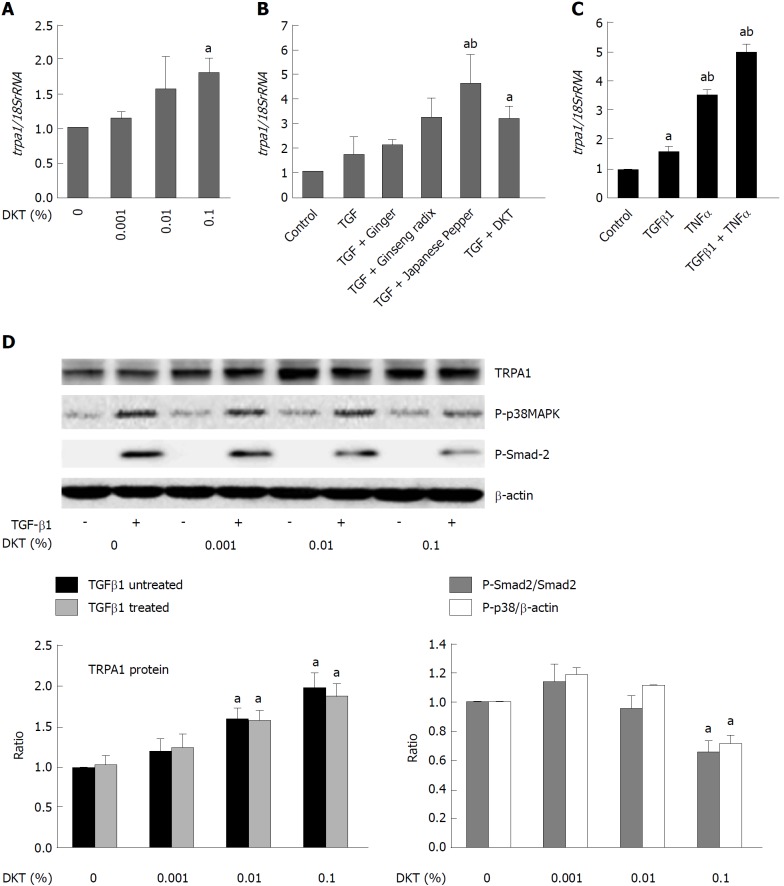
In another series of experiments, we found that in addition to activating TRPA1 channels, DKT also enhanced TRPA1 expression in InMyoFibs. As shown in Figure 6A, 24-h incubation with DKT dose-dependently increased TRPA1 mRNA expression in InMyoFibs. The TRPA1-upregulating effects were also observed for the DKT components Japanese pepper and ginseng radix, but not ginger, and this was particularly prominent when TGF-β1 coexisted (Figure 6B). Notably, treatment with TNFα or TGF-β1 themselves enhanced TRPA1 expression in InMyoFibs: when treated with both, they seemingly acted additively or synergistically (Figure 6C). In parallel to the upregulation of TRPA1 expression, the key downstream events of TGF-β1 signaling, phosphorylations of Smad-2 and p38-MAPK, were also dose-dependently inhibited by the presence of DKT (Figure 6D).
Figure 6.
Daikenchuto upregulates transient receptor potential ankyrin 1 expression and suppresses phosphorylation events downstream of transforming growth factor-β1 signaling. A: trpa1 mRNA expression quantified by real-time PCR in control and TGF-β1-treated (5 ng/mL, 24 h) InMyoFibs (normalized to 18S rRNA). DKT extracts (0.001%, 0.01%, 0.1%) were applied for 24 h; B: Relative mRNA expression of trpa1 with ginger (0.1%), ginseng radix (0.1%), Japanese pepper (0.1%), or DKT (0.1%) plus co-administered TGF-β1; C: trpa1 mRNA expression was upregulated by TGF-β1, TNF-α, or both; D: TRPA1 protein expression and phosphorylation of Smad-2 or p38-MAPK with and without TGF-β1 treatment at various concentrations of DKT extracts (0.001%, 0.01%, and 0.1%). The upper panel shows actual immunoblots. Graphs show relative expression level of TRPA1 (normalized to β-actin) and the relative ratios of phosphorylated/unphosphorylated Smad-2 and p38-MAPK (normalized to β-actin) after 1 h TGF-β1 treatment at various DKT concentrations. aP < 0.05 vs control cells, bP < 0.05 vs TGF-β1-treated cells (n = 4). TGF-β1: Transforming growth factor-β1; InMyoFibs: Intestinal myofibroblasts; DKT: Daikenchuto; TRPA1: Transient receptor potential ankyrin 1; p38-MAPK: p38-mitogen-activated protein kinase.
TRPA1 is a negative feedback regulator of collagen synthesis/secretion
We previously found that extracellular applied collagen downregulated many pro-fibrotic gene transcripts involved in TGF-β signaling in InMyoFibs, including CDH2, ACTA2, and COL1A1[19]. We therefore examined whether a major extracellular matrix collagen also regulated TRPA1 expression. When Type I collagen was added into the culture medium, the expression of TRPA1 was enhanced more than two-fold, resulting in the decreased endogenous production of collagen (Figure 7A and B). Concomitantly, the expression levels of COL1A1, ACTA2, and CDH2 mRNAs in InMyoFibs were downregulated by extracellular applied Type I collagen, regardless of the presence of TGF-β1. Moreover, extracellular applied collagen caused a significant decrease in TGF-β1-induced MYOCD expression (Figure 7B-D). Strikingly, following siRNA knockdown of TRPA1 expression, which exacerbated the fibrogenic effects of TGF-β1[18], extracellular applied collagen was no longer effective in suppressing the mRNA expression of fibrotic factors COL1A1, MYOCD, and ACTA2 (Figure 7E). Taken together, these results indicate that TRPA1 activity may be commonly involved in anti-fibrotic processes in InMyoFibs, which are activated by DKT and extracellular applied collagen.
Figure 7.
Extracellular collagen upregulates transient receptor potential ankyrin 1 expression to exert anti-fibrotic effects. A: Type I collagen solution (0, 0.1, or 1 μg/mL) was added into the culture medium, and immunoblotting was performed to detect TRPA1 and Type I collagen expression in InMyoFibs. The rightmost graph shows the summary (n = 4) B-E: Relative expression levels (shown as the post/pre ratio) of col1a1, trpa1, myocd, and acta2 mRNAs quantified by RT-PCR in Type Icollagen- or TGF-β1-treated InMyoFibs. In E, InMyoFibs were pre-treated with TRPA1 siRNA. aP < 0.05 vs control cells; bP < 0.05 vs TGF-β1-treated cells (n = 4). TRPA1: Transient receptor potential ankyrin 1; InMyoFibs: Intestinal myofibroblasts; TGF-β1: Transforming growth factor-β1.
Enhanced TRPA1 expression in stenotic tissues of CD patients
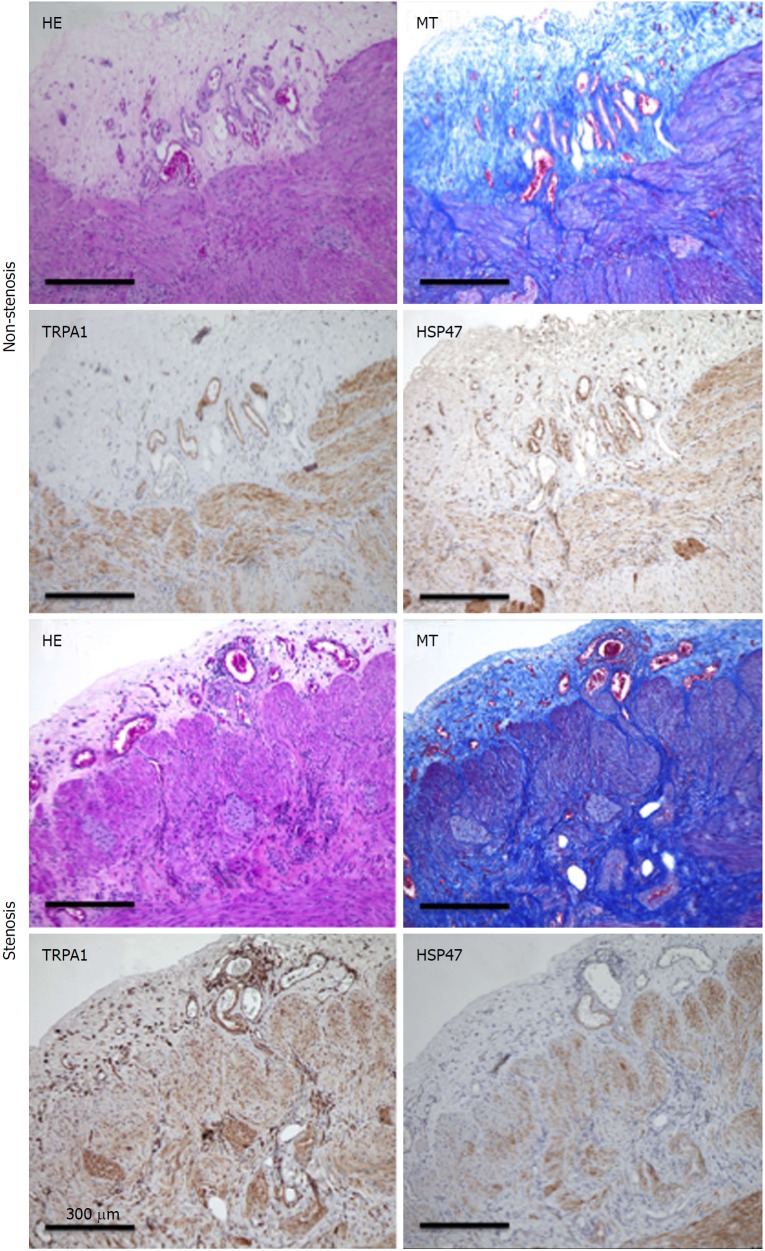
Because intestinal stricture formation in CD is driven by a local excessive accumulation of myofibroblasts, we examined whether TRPA1 is upregulated in highly fibrotic areas of human CD patients. Surgical samples obtained from the patient’s intestines were subjected to histological examination and immunostaining. HE and MT staining revealed a much denser deposition of collagen fibers in the mucosal layer of the fibrotic stenotic areas than those of the non-stenotic areas. Moreover, TRPA1 immunoreactivity detected by diaminobenzidine (DAB) staining was more diffusely distributed in the mucosal and submucosal layers of the stenotic regions in the intestines of CD patients (Figure 8).
Figure 8.
Hematoxylin-eosin, Masson’s trichrome staining. TRPA1-DAB, and HSP47-DAB-stained non-stenosis/stenosis surgical tissue from the transverse colon of a CD patient. TRPA1: Transient receptor potential ankyrin 1; DAB: Diaminobenzidine; HSP47: Heat shock protein 47; CD: Crohn’s disease.
DISCUSSION
Daikenchuto (DKT), a traditional oriental herbal medicine, is widely used for the treatment of gastrointestinal disorders. DKT is known to improve post-operative complications and is frequently prescribed for ileus, abdominal bloating, and cold sensation[40]. Several clinical meta-analyses have shown, for instance, that the perioperative administration of DKT effectively relieves the symptoms of post-operative ileus in patients undergoing gastrointestinal cancer surgery[41]. These beneficial effects of DKT are in part ascribed to its multiple actions through differential pharmacokinetics of respective DKT compounds on a number of ion channels (TRPA1, TRPV1, two-pore-domain KCNK channels) distributed throughout the enteric/myenteric and sensory nerves and intestinal epithelial cells. The activation or inhibition of these channels are thought to cause the release of two vasoactive/anti-inflammatory peptides, CGRP and adrenomeduline, or enhance the excitability of smooth muscle and neurons in the intestine, which improve intestinal microcirculation and motility, and induce anti-inflammatory responses[40]. However, although a few studies using chronic inflammation models have already reported the anti-fibrotic effects of DKT[3,42,43], the critical involvement of intestinal myoblasts in fibrogenesis and the therapeutic potential of the myofibroblast TRPA1 channel against it have not yet been addressed[18].
In this respect, the present study has provided several novel findings. First, our TNBS colitis model with the comparative use of wild-type and TRPA1-KO mice successfully demonstrated the anti-fibrotic actions of DKT via TRPA1 channel activation (Figure 1).
Specifically, the fibrosis score exacerbated by TNBS treatment was significantly improved by enema administration of DKT during the chronic phase of inflammation (i.e., at five weeks after TNBS), which was abrogated by the deletion of the trpa1 gene (Figure 2)[18]. Second, our in vitro experiments with a human myofibroblast cell line clearly indicated that myofibroblast TRPA1 channel activation is a requisite for the downregulation of fibrotic factors/activities by DKT. Additionally, among its components, ginger and Japanese pepper, both being able to activate the TRPA1 channel in the expression system, showed comparable anti-fibrotic effects. Further investigation of active DKT ingredients suggested that 6-shogaol is likely the most effective ingredient, and that hydroxy α-sanshool may also mediate some part of DKT’s anti-fibrotic actions (Figures 3-5). Intriguingly, DKT has an additional action of enhancing TRPA1 expression per se, which appears to be exerted by its Japanese pepper component. Finally, in support of these findings, histological and immunohistochemical examinations of human intestinal samples showed that myofibroblasts expressing TRPA1, as well as the fibrosis biomarker HSP47, were coincidentally accumulated in the stenosis areas of CD patients (Figure 8)[18], as observed in the mice TNBS-colitis model (Figure 2C).
Taken together, these findings can be interpreted to indicate that in chronically inflamed intestines, TRPA1 channel may be antagonistically upregulated in both expression and activity against the growing fibrogenic drive, which could further be potentiated by TRPA1-stimulating actions of DKT. As we discussed in a recent paper[18], this view could be extended to various tissues and organs undergoing chronic inflammation where therapeutic activation TRPA1 channels in situ may work beneficially to decelerate locally enhanced fibrogenic processes. In fact, there are several lines of evidence that TRPA1 channels in both neuronal and non-neuronal cells modulate local tissue inflammation accompanying fibrotic changes, as exemplified by the animal models of acute pulmonary inflammation induced by acrolein and cigarette smoke, experimental colitis, or carrageenan-induced paw edema[7,44,45].
In addition to the above findings, we also found that extracellular applied collagen can induce the expression of TRPA1, with downregulation of the mRNAs of fibrotic factors MYOCD, COL1A1, and ACTA2 (Figure 9). It has been reported that cell contact with collagen reduces cadherin expression, which in turn eliminates the mechanotransduction between fibroblasts[46]; therefore, extracellular collagen might modulate the expression of cadherin and the dynamics of cytoskeleton stress fiber formation via an as-yet-unknown membrane sensor(s), which is crucial for the modulation of fibrogenesis signaling. In our study, extracellular collagen application downregulated the expression levels of intrinsic collagen, α-SMA, and myocardin, but these changes were not observed in TRPA1-siRNA-treated InMyoFibs. These data strongly support the idea that TRPA1 channel activity is negatively correlated with the collagen synthesis of intestinal myofibroblasts. In the context of the present study, this would again represent another type of TRPA1-mediated negative feedback regulation that resists against a heightening fibrogenic potential.
Figure 9.

Graphical abstract.
In a few clinical studies, TGF-β1 mRNA levels were found elevated in the affected intestinal mucosa from the patients with CD and ulcerative colitis, particularly in the regions confined to the lamina propria where immune cells, as well as myofibroblasts, preferentially reside[23]. Combining this fact with our own finding from human surgical samples (i.e., the co-accumulation of TRPA1-/HSP47-double positive myofibroblasts in the stenotic regions), it seems plausible to assume that in an interwoven network of TGF-β-mediated signaling, TRPA1 in myofibroblasts may operate as a crucial anti-fibrotic negative feedback to lower the pathologically increased fibrogenic potential. Thus, targeting the myofibroblast TRPA1 signaling axis could be a tenable therapeutic strategy to reinforce the anti-fibrotic potential in chronic fibrotic diseases such as CD.
The higher prevalence of fibrosis in CD is thought to be the complex consequences of transmural bowel inflammation that exposes all mesenchymal cells producing extracellular matrix to fibrotic mediators[47]. Therefore, anti-TNF (i.e., anti-inflammatory) treatment is generally recommended as the first-line therapy for CD patients with poor prognoses who have severe complications or bowel damages[48]. However, anti-TNF treatment has an increased risk of worsening stenotic fibrosis[49], and stenotic lesions can be present in variable pathological states, such as inflammatory, fibrogenic, and neoplastic, or a combination of these states. Therefore, a therapeutic strategy that can distinguish between these different states might be more desirable than the currently available anti-inflammatory approaches. In this respect, the present finding, that the activation of myofibroblast TRPA1 attenuates intestinal fibrosis, may provide great therapeutic benefits, and could presumably be generalized to other fibrotic lesions such as those in the skin, lungs, and liver, where several TRP channels, including TRPA1, are highly expressed.
Finally, we should point out several limitations of this study. First, the method adopted in this study (i.e., TNBS treatment) could not create a severe fibrotic stenosis model, which only allowed us to see rather mild fibrotic changes. Second, all biopsy samples were obtained from IBD patients but not from healthy donors. If this had been done, the results of this study might clinically be more attractive. Third, other Chinese medicines that are potentially capable of activating TRPA1 channel were not tested in this study. This would somewhat compromise the first priority of DKT for anti-fibrotic treatment. In addition, DKT used in this study was obtained from a single source (i.e., Tsumura Co.); therefore, the possibility cannot be entirely excluded that the observed effects in this study might differ in details from those obtained with the same drug of a different origin.
In summary, the present study revealed a new role for myofibroblast TRPA1 channels in intestinal fibrosis, i.e., its negative regulation, and showed that DKT upregulates this channel protein in both activity and expression to exert its anti-fibrotic actions. These findings not only provide a novel molecular target for the anti-fibrotic therapy of IBDs in the future, but also a framework to reinterpret the unique theories of traditional Oriental medicine.
ARTICLE HIGHLIGHTS
Research background
Daikenchuto (DKT) is a traditional oriental herbal medicine, widely used to mitigate post-operative ileus and constipation. The mechanism of its actions remains less understood, in particular regarding its molecular target(s). However, an important hint to addressing this issue has come from our previous findings that the transient receptor potential ankyrin 1 (TRPA1) is highly expressed in intestinal myofibroblasts and mediates the antifibrotic effects of steroid and pirfenidone.
Research motivation
We hypothesized that DKT may activate TRPA1 channels in myofibroblasts to directly counteract the fibrotic changes of inflamed intestines. Although DKT has been shown to be beneficial for gut motility, intestinal blood flow and gastrointestinal hormone secretion, its anti-fibrotic actions through TRPA1 channel activation will be an entirely new finding and of considerable clinical significance.
Research objectives
We aimed at investigating at both in vitro and in vivo levels whether DKT targets TRPA1 channels expressed in intestinal myofibroblasts to exert its anti-fibrotic actions. The results would not only disclose a novel molecular target of anti-fibrotic therapy for inflammatory bowel diseases such as Crohn’s disease (CD) and ulcerative colitis, but also promote our general understanding about the complex actions of herbal medicines.
Research methods
A trinitrobenzene sulfonic acid (TNBS) chronic colitis model was established in both wild-type and TRPA1 knock out mice, in which the impact of DKT administration were evaluated by pathological and biochemical analyses. An intestinal myofibroblast cell line (InMyoFibs) stimulated by transforming growth factor-β1 (TGF-β1) was used as a cellular model of intestinal fibrosis, where expression of TRPA1 and pro-fibrotic factors and related intracellular signaling (in particular TGF-β-mediated one) were examined in detail. The counteracting effects of DKT on these changes were also evaluated. Biopsy samples from non-stenotic and stenotic regions of CD patient’s intestines were subjected to histological examination and immunoblot analysis with particular respect to the altered expression pattern of TRPA1 channel and fibrotic factors. As compared with preceding works on a similar topic, the methodological approach applied to this study is more comprehensive covering a broad range of DKT’s actions that include cellular and animal models and human patients.
Research results
In TNBS chronic colitis model mice, the extents of inflammation and fibrotic changes were more prominent in TRPA1-/- knockout than in wild-type mice. One-week enema administration of DKT suppressed fibrotic lesions in wild-type mice, but not in TRPA1 knockout mice. Active ingredients of DKT, induced Ca2+ influxes in InMyoFib, which were antagonized by TRPA1 channel blocker HC-030031. DKT counteracted TGF-β1-induced expression of Type 1 collagen, α-SMA, and this was accompanied by attenuated fibrosis-associated signaling. A DKT’s active ingredient Japanese Pepper increased the mRNA and protein expressions of TRPA1, which in turn negatively regulated collagen synthesis in InMyoFibs. In stenotic regions of CD patient’s intestines, TRPA1 expression was significantly increased. However, several limitations remain in this study that may compromise the value and relevance of this study, which include the inability of TNBS to create a severe phenotype of chronic colitis, the lack of control data from human healthy donors, and no comparative data from other Chinese (herbal) medicines that may have similar actions.
Research conclusions
DKT-induced expression and activation of TRPA1 could be important mechanisms for suppressing intestinal fibrosis, which would in part account for the reported beneficial actions of DKT on inflamed intestines. Thus, targeting myofibroblast TRPA1 may serve as a new and promising therapeutic strategy for fibrotic stenotic changes occurring in incurable chronic inflammatory bowel diseases such as CD and ulcerative colitis.
Research perspectives
Our findings not only provide a novel molecular target for the anti-fibrotic therapy of inflammatory bowel diseases in the future, but also a framework to reinterpret the unique theories of traditional Oriental medicine.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the Clinical Research Ethics Committee of Fukuoka University, No. 15-10-04.
Institutional animal care and use committee statement: All procedures involving animals were reviewed and approved by the Animal experiment ethics committee of Fukuoka University, No. 1709099; and the Genetic Modification Experiment Safety Commission of Fukuoka University approved experiments on genetically modified animals, No. 167.
Conflict-of-interest statement: The authors declare that they have no competing interests.
Data sharing statement: Readers can request the data of this paper by contacting us via hailin@fukuoka-u.ac.jp.
ARRIVE guidelines statement: The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
Peer-review started: May 16, 2018
First decision: June 13, 2018
Article in press: July 22, 2018
P- Reviewer: Masia DO, Naser SA, Vasudevan A S- Editor: Gong ZM L- Editor: A E- Editor: Yin SY
Contributor Information
Keizo Hiraishi, Department of Physiology, Graduate School of Medical Sciences, Fukuoka University, Fukuoka 8140180, Japan.
Lin-Hai Kurahara, Department of Physiology, Graduate School of Medical Sciences, Fukuoka University, Fukuoka 8140180, Japan. hailin@fukuoka-u.ac.jp.
Miho Sumiyoshi, Department of Physiology, Graduate School of Medical Sciences, Fukuoka University, Fukuoka 8140180, Japan.
Yao-Peng Hu, Department of Physiology, Graduate School of Medical Sciences, Fukuoka University, Fukuoka 8140180, Japan.
Kaori Koga, Department of Pathology, Faculty of Medicine, Fukuoka University, Fukuoka 8140180, Japan.
Miki Onitsuka, Department of Pathology, Faculty of Medicine, Fukuoka University, Fukuoka 8140180, Japan.
Daibo Kojima, Department of Gastroenterological Surgery, Faculty of Medicine, Fukuoka University, Fukuoka 8140180, Japan.
Lixia Yue, Department of Cell Biology, University of Connecticut Health Center, Farmington, CT 06030, United States.
Hidetoshi Takedatsu, Department of Gastroenterology and Medicine, Faculty of Medicine, Fukuoka University, Fukuoka 8140180, Japan.
Yu-Wen Jian, College of Letters and Science, University of California, Davis, CA 95616, United States.
Ryuji Inoue, Department of Physiology, Graduate School of Medical Sciences, Fukuoka University, Fukuoka 8140180, Japan.
References
- 1.Kono T, Omiya Y, Hira Y, Kaneko A, Chiba S, Suzuki T, Noguchi M, Watanabe T. Daikenchuto (TU-100) ameliorates colon microvascular dysfunction via endogenous adrenomedullin in Crohn’s disease rat model. J Gastroenterol. 2011;46:1187–1196. doi: 10.1007/s00535-011-0438-2. [DOI] [PubMed] [Google Scholar]
- 2.Endo M, Hori M, Ozaki H, Oikawa T, Hanawa T. Daikenchuto, a traditional Japanese herbal medicine, ameliorates postoperative ileus by anti-inflammatory action through nicotinic acetylcholine receptors. J Gastroenterol. 2014;49:1026–1039. doi: 10.1007/s00535-013-0854-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kitamura M, Nishino T, Obata Y, Oka S, Abe S, Muta K, Ozono Y, Koji T, Kohno S. The kampo medicine Daikenchuto inhibits peritoneal fibrosis in mice. Biol Pharm Bull. 2015;38:193–200. doi: 10.1248/bpb.b14-00469. [DOI] [PubMed] [Google Scholar]
- 4.Ogasawara T, Morine Y, Ikemoto T, Imura S, Fujii M, Soejima Y, Shimada M. Influence of Dai-kenchu-to (DKT) on human portal blood flow. Hepatogastroenterology. 2008;55:574–577. [PubMed] [Google Scholar]
- 5.Pan MH, Hsieh MC, Hsu PC, Ho SY, Lai CS, Wu H, Sang S, Ho CT. 6-Shogaol suppressed lipopolysaccharide-induced up-expression of iNOS and COX-2 in murine macrophages. Mol Nutr Food Res. 2008;52:1467–1477. doi: 10.1002/mnfr.200700515. [DOI] [PubMed] [Google Scholar]
- 6.Penuelas A, Tashima K, Tsuchiya S, Matsumoto K, Nakamura T, Horie S, Yano S. Contractile effect of TRPA1 receptor agonists in the isolated mouse intestine. Eur J Pharmacol. 2007;576:143–150. doi: 10.1016/j.ejphar.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 7.Poole DP, Pelayo JC, Cattaruzza F, Kuo YM, Gai G, Chiu JV, Bron R, Furness JB, Grady EF, Bunnett NW. Transient receptor potential ankyrin 1 is expressed by inhibitory motoneurons of the mouse intestine. Gastroenterology. 2011;141:565–575, 575.e1-575.e4. doi: 10.1053/j.gastro.2011.04.049. [DOI] [PubMed] [Google Scholar]
- 8.Satoh K, Hayakawa T, Kase Y, Ishige A, Sasaki H, Nishikawa S, Kurosawa S, Yakabi K, Nakamura T. Mechanisms for contractile effect of Dai-kenchu-to in isolated guinea pig ileum. Dig Dis Sci. 2001;46:250–256. doi: 10.1023/a:1005636412287. [DOI] [PubMed] [Google Scholar]
- 9.Tokita Y, Yamamoto M, Satoh K, Nishiyama M, Iizuka S, Imamura S, Kase Y. Possible involvement of the transient receptor potential vanilloid type 1 channel in postoperative adhesive obstruction and its prevention by a kampo (traditional Japanese) medicine, daikenchuto. J Pharmacol Sci. 2011;115:75–83. doi: 10.1254/jphs.10226fp. [DOI] [PubMed] [Google Scholar]
- 10.Kim MO, Lee MH, Oi N, Kim SH, Bae KB, Huang Z, Kim DJ, Reddy K, Lee SY, Park SJ, et al. [6]-shogaol inhibits growth and induces apoptosis of non-small cell lung cancer cells by directly regulating Akt1/2. Carcinogenesis. 2014;35:683–691. doi: 10.1093/carcin/bgt365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radhakrishnan EK, Bava SV, Narayanan SS, Nath LR, Thulasidasan AK, Soniya EV, Anto RJ. [6]-Gingerol induces caspase-dependent apoptosis and prevents PMA-induced proliferation in colon cancer cells by inhibiting MAPK/AP-1 signaling. PLoS One. 2014;9:e104401. doi: 10.1371/journal.pone.0104401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubowski KM, Luke JL. Measurement of carboxyhemoglobin and carbon monoxide in blood. Ann Clin Lab Sci. 1973;3:53–65. [PubMed] [Google Scholar]
- 13.Kono T, Kaneko A, Hira Y, Suzuki T, Chisato N, Ohtake N, Miura N, Watanabe T. Anti-colitis and -adhesion effects of daikenchuto via endogenous adrenomedullin enhancement in Crohn’s disease mouse model. J Crohns Colitis. 2010;4:161–170. doi: 10.1016/j.crohns.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Katsuno H, Maeda K, Kaiho T, Kunieda K, Funahashi K, Sakamoto J, Kono T, Hasegawa H, Furukawa Y, Imazu Y, et al. Clinical efficacy of Daikenchuto for gastrointestinal dysfunction following colon surgery: a randomized, double-blind, multicenter, placebo-controlled study (JFMC39-0902) Jpn J Clin Oncol. 2015;45:650–656. doi: 10.1093/jjco/hyv056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katsuno H, Maeda K, Ohya M, Yoshioka K, Tsunoda A, Koda K, Matsuoka H, Ohge H, Morita S, Saji S, et al. Clinical pharmacology of daikenchuto assessed by transit analysis using radiopaque markers in patients with colon cancer undergoing open surgery: a multicenter double-blind randomized placebo-controlled study (JFMC39-0902 additional study) J Gastroenterol. 2016;51:222–229. doi: 10.1007/s00535-015-1100-1. [DOI] [PubMed] [Google Scholar]
- 16.Rieder F, Brenmoehl J, Leeb S, Schölmerich J, Rogler G. Wound healing and fibrosis in intestinal disease. Gut. 2007;56:130–139. doi: 10.1136/gut.2006.090456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 18.Kurahara LH, Hiraishi K, Hu Y, Koga K, Onitsuka M, Doi M, Aoyagi K, Takedatsu H, Kojima D, Fujihara Y, et al. Activation of Myofibroblast TRPA1 by Steroids and Pirfenidone Ameliorates Fibrosis in Experimental Crohn’s Disease. Cell Mol Gastroenterol Hepatol. 2017;5:299–318. doi: 10.1016/j.jcmgh.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurahara LH, Sumiyoshi M, Aoyagi K, Hiraishi K, Nakajima K, Nakagawa M, Hu Y, Inoue R. Intestinal myofibroblast TRPC6 channel may contribute to stenotic fibrosis in Crohn’s disease. Inflamm Bowel Dis. 2015;21:496–506. doi: 10.1097/MIB.0000000000000295. [DOI] [PubMed] [Google Scholar]
- 20.Medina C, Santos-Martinez MJ, Santana A, Paz-Cabrera MC, Johnston MJ, Mourelle M, Salas A, Guarner F. Transforming growth factor-beta type 1 receptor (ALK5) and Smad proteins mediate TIMP-1 and collagen synthesis in experimental intestinal fibrosis. J Pathol. 2011;224:461–472. doi: 10.1002/path.2870. [DOI] [PubMed] [Google Scholar]
- 21.Biancheri P, Giuffrida P, Docena GH, MacDonald TT, Corazza GR, Di Sabatino A. The role of transforming growth factor (TGF)-β in modulating the immune response and fibrogenesis in the gut. Cytokine Growth Factor Rev. 2014;25:45–55. doi: 10.1016/j.cytogfr.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Leask A. Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ Res. 2010;106:1675–1680. doi: 10.1161/CIRCRESAHA.110.217737. [DOI] [PubMed] [Google Scholar]
- 23.Babyatsky MW, Rossiter G, Podolsky DK. Expression of transforming growth factors alpha and beta in colonic mucosa in inflammatory bowel disease. Gastroenterology. 1996;110:975–984. doi: 10.1053/gast.1996.v110.pm8613031. [DOI] [PubMed] [Google Scholar]
- 24.Inoue R, Jensen LJ, Shi J, Morita H, Nishida M, Honda A, Ito Y. Transient receptor potential channels in cardiovascular function and disease. Circ Res. 2006;99:119–131. doi: 10.1161/01.RES.0000233356.10630.8a. [DOI] [PubMed] [Google Scholar]
- 25.Nishida M, Onohara N, Sato Y, Suda R, Ogushi M, Tanabe S, Inoue R, Mori Y, Kurose H. Galpha12/13-mediated up-regulation of TRPC6 negatively regulates endothelin-1-induced cardiac myofibroblast formation and collagen synthesis through nuclear factor of activated T cells activation. J Biol Chem. 2007;282:23117–23128. doi: 10.1074/jbc.M611780200. [DOI] [PubMed] [Google Scholar]
- 26.Onohara N, Nishida M, Inoue R, Kobayashi H, Sumimoto H, Sato Y, Mori Y, Nagao T, Kurose H. TRPC3 and TRPC6 are essential for angiotensin II-induced cardiac hypertrophy. EMBO J. 2006;25:5305–5316. doi: 10.1038/sj.emboj.7601417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du J, Xie J, Zhang Z, Tsujikawa H, Fusco D, Silverman D, Liang B, Yue L. TRPM7-mediated Ca2+ signals confer fibrogenesis in human atrial fibrillation. Circ Res. 2010;106:992–1003. doi: 10.1161/CIRCRESAHA.109.206771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kono T, Kaneko A, Omiya Y, Ohbuchi K, Ohno N, Yamamoto M. Epithelial transient receptor potential ankyrin 1 (TRPA1)-dependent adrenomedullin upregulates blood flow in rat small intestine. Am J Physiol Gastrointest Liver Physiol. 2013;304:G428–G436. doi: 10.1152/ajpgi.00356.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee BH, Hsu WH, Hsu YW, Pan TM. Suppression of dimerumic acid on hepatic fibrosis caused from carboxymethyl-lysine (CML) by attenuating oxidative stress depends on Nrf2 activation in hepatic stellate cells (HSCs) Food Chem Toxicol. 2013;62:413–419. doi: 10.1016/j.fct.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Jiang X, Zhang Y, Li F, Zhu Y, Chen Y, Yang S, Sun G. Allicin as a possible adjunctive therapeutic drug for stage II oral submucous fibrosis: a preliminary clinical trial in a Chinese cohort. Int J Oral Maxillofac Surg. 2015;44:1540–1546. doi: 10.1016/j.ijom.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 31.Kun J, Szitter I, Kemény A, Perkecz A, Kereskai L, Pohóczky K, Vincze A, Gódi S, Szabó I, Szolcsányi J, et al. Upregulation of the transient receptor potential ankyrin 1 ion channel in the inflamed human and mouse colon and its protective roles. PLoS One. 2014;9:e108164. doi: 10.1371/journal.pone.0108164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bertin S, Aoki-Nonaka Y, Lee J, de Jong PR, Kim P, Han T, Yu T, To K, Takahashi N, Boland BS, et al. The TRPA1 ion channel is expressed in CD4+ T cells and restrains T-cell-mediated colitis through inhibition of TRPV1. Gut. 2017;66:1584–1596. doi: 10.1136/gutjnl-2015-310710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romano B, Borrelli F, Fasolino I, Capasso R, Piscitelli F, Cascio M, Pertwee R, Coppola D, Vassallo L, Orlando P, et al. The cannabinoid TRPA1 agonist cannabichromene inhibits nitric oxide production in macrophages and ameliorates murine colitis. Br J Pharmacol. 2013;169:213–229. doi: 10.1111/bph.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riera CE, Menozzi-Smarrito C, Affolter M, Michlig S, Munari C, Robert F, Vogel H, Simon SA, le Coutre J. Compounds from Sichuan and Melegueta peppers activate, covalently and non-covalently, TRPA1 and TRPV1 channels. Br J Pharmacol. 2009;157:1398–1409. doi: 10.1111/j.1476-5381.2009.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koo JY, Jang Y, Cho H, Lee CH, Jang KH, Chang YH, Shin J, Oh U. Hydroxy-alpha-sanshool activates TRPV1 and TRPA1 in sensory neurons. Eur J Neurosci. 2007;26:1139–1147. doi: 10.1111/j.1460-9568.2007.05743.x. [DOI] [PubMed] [Google Scholar]
- 36.Tsuchiya K, Kubota K, Ohbuchi K, Kaneko A, Ohno N, Mase A, Matsushima H, Yamamoto M, Miyano K, Uezono Y, et al. Transient receptor potential ankyrin 1 agonists improve intestinal transit in a murine model of postoperative ileus. Neurogastroenterol Motil. 2016;28:1792–1805. doi: 10.1111/nmo.12877. [DOI] [PubMed] [Google Scholar]
- 37.Furio L, Pampalakis G, Michael IP, Nagy A, Sotiropoulou G, Hovnanian A. KLK5 Inactivation Reverses Cutaneous Hallmarks of Netherton Syndrome. PLoS Genet. 2015;11:e1005389. doi: 10.1371/journal.pgen.1005389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi J, Mori E, Mori Y, Mori M, Li J, Ito Y, Inoue R. Multiple regulation by calcium of murine homologues of transient receptor potential proteins TRPC6 and TRPC7 expressed in HEK293 cells. J Physiol. 2004;561:415–432. doi: 10.1113/jphysiol.2004.075051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi N, Kuwaki T, Kiyonaka S, Numata T, Kozai D, Mizuno Y, Yamamoto S, Naito S, Knevels E, Carmeliet P, et al. TRPA1 underlies a sensing mechanism for O2. Nat Chem Biol. 2011;7:701–711. doi: 10.1038/nchembio.640. [DOI] [PubMed] [Google Scholar]
- 40.Kono T, Shimada M, Yamamoto M, Kaneko A, Oomiya Y, Kubota K, Kase Y, Lee K, Uezono Y. Complementary and synergistic therapeutic effects of compounds found in Kampo medicine: analysis of daikenchuto. Front Pharmacol. 2015;6:159. doi: 10.3389/fphar.2015.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishizuka M, Shibuya N, Nagata H, Takagi K, Iwasaki Y, Hachiya H, Aoki T, Kubota K. Perioperative Administration of Traditional Japanese Herbal Medicine Daikenchuto Relieves Postoperative Ileus in Patients Undergoing Surgery for Gastrointestinal Cancer: A Systematic Review and Meta-analysis. Anticancer Res. 2017;37:5967–5974. doi: 10.21873/anticanres.12043. [DOI] [PubMed] [Google Scholar]
- 42.Yada K, Ishibashi H, Mori H, Morine Y, Zhu C, Feng R, Kono T, Shimada M. The Kampo medicine “Daikenchuto (TU-100)” prevents bacterial translocation and hepatic fibrosis in a rat model of biliary atresia. Surgery. 2016;159:1600–1611. doi: 10.1016/j.surg.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 43.Inoue K, Naito Y, Takagi T, Hayashi N, Hirai Y, Mizushima K, Horie R, Fukumoto K, Yamada S, Harusato A, et al. Daikenchuto, a Kampo medicine, regulates intestinal fibrosis associated with decreasing expression of heat shock protein 47 and collagen content in a rat colitis model. Biol Pharm Bull. 2011;34:1659–1665. doi: 10.1248/bpb.34.1659. [DOI] [PubMed] [Google Scholar]
- 44.Nassini R, Pedretti P, Moretto N, Fusi C, Carnini C, Facchinetti F, Viscomi AR, Pisano AR, Stokesberry S, Brunmark C, et al. Transient receptor potential ankyrin 1 channel localized to non-neuronal airway cells promotes non-neurogenic inflammation. PLoS One. 2012;7:e42454. doi: 10.1371/journal.pone.0042454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moilanen LJ, Laavola M, Kukkonen M, Korhonen R, Leppänen T, Högestätt ED, Zygmunt PM, Nieminen RM, Moilanen E. TRPA1 contributes to the acute inflammatory response and mediates carrageenan-induced paw edema in the mouse. Sci Rep. 2012;2:380. doi: 10.1038/srep00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ehrlich HP, Allison GM, Leggett M. The myofibroblast, cadherin, alpha smooth muscle actin and the collagen effect. Cell Biochem Funct. 2006;24:63–70. doi: 10.1002/cbf.1188. [DOI] [PubMed] [Google Scholar]
- 47.Latella G, Sferra R, Speca S, Vetuschi A, Gaudio E. Can we prevent, reduce or reverse intestinal fibrosis in IBD? Eur Rev Med Pharmacol Sci. 2013;17:1283–1304. [PubMed] [Google Scholar]
- 48.Peyrin-Biroulet L, Fiorino G, Buisson A, Danese S. First-line therapy in adult Crohn’s disease: who should receive anti-TNF agents? Nat Rev Gastroenterol Hepatol. 2013;10:345–351. doi: 10.1038/nrgastro.2013.31. [DOI] [PubMed] [Google Scholar]
- 49.Condino G, Calabrese E, Zorzi F, Onali S, Lolli E, De Biasio F, Ascolani M, Pallone F, Biancone L. Anti-TNF-alpha treatments and obstructive symptoms in Crohn’s disease: a prospective study. Dig Liver Dis. 2013;45:258–262. doi: 10.1016/j.dld.2012.10.009. [DOI] [PubMed] [Google Scholar]