Abstract
Depressive symptoms in parents and in youths have been found to relate to disease comorbidity processes in children, including greater disease-related impairment and poorer clinical outcomes. The current study sought to assess whether coming from a family characterized by more depressive symptoms on average would potentiate the effects of changes in youths’ own negative mood on the expression of two receptor genes relevant to asthma that are the primary targets of asthma medication, such that the combination of low child negative mood in the context of greater parental depressive symptoms would relate to the lowest levels of gene expression. One-hundred-twenty youths with diagnosed asthma and their parents participated every 6 months for 2 years. Parents reported on their depressive symptoms, children reported negative mood symptoms, and youths completed blood draws from which expression of Glucocorticoid Receptor (GR) and Beta2 Adrenergic Receptor (β2-AR) genes was extracted. Multilevel linear modeling revealed significant interactions between average levels of parental depressive symptoms and changes in youths’ negative mood symptoms predicting gene expression, such that youths expressed significantly less GR and β2-AR during times when they experienced more negative mood symptoms, but this was only true if they came from families with higher levels of average parental depressive symptoms. The current study identifies novel and biologically-proximal molecular signaling patterns that connect depressive symptoms to pediatric asthma while also highlighting the important role of family environment for biological processes that may operate within depression comorbidity.
Keywords: Depression, parents-child relationships, asthma, molecular genetics
Symptoms of depression in children not only relate to poorer psychosocial functioning, but also predict worse physical health outcomes (Pinquart & Shen, 2011). For example, depression at age 15 has been shown to prospectively predict worse self- and interviewer-perceived health, higher health care utilization, and greater work impairment due to physical health at age 20 (Keenan-Miller, Hammen, & Brennan, 2007). Not only do children with more depressive symptoms report higher levels of somatic complaints, broadly (Egger, Costello, Erkanli, & Angold, 1999; Pine, Cohen, & Brook, 1996), but comorbid depression in the context of a pediatric illness is reliably predictive of poorer disease-specific functioning. For instance, meta-analytic work finds that adolescent depression is associated with greater treatment nonadherence in youths with diabetes, which in turn relates to worse clinical disease outcomes (Gonzalez et al., 2008). Depressive symptoms are similarly associated with lower quality of life in individuals with pediatric inflammatory bowel disease (Herzer, Denson, Baldassano, & Hommel, 2011) and with chronic pediatric pain (Kashikar-Zuck, Goldschneider, Powers, Vaught, & Hershey, 2001). To better understand the full impact of depressive symptoms on the lives of youths, it is therefore critical for research to identify psychological and biological processes that underlie comorbidity between depression and pediatric chronic illness.
Although functional impairment associated with depressive symptoms is evident across a variety of diseases, pediatric asthma provides a particularly relevant disease context in which to examine mechanisms and consequences of depression comorbidity. Asthma is a chronic respiratory disorder that is characterized by inflammation and obstruction of the airways. As a disease, it is especially appropriate to examine in relation to psychological comorbidity because it is one of the most common chronic illnesses of childhood (National Center for Health Statistics, 2013), is well understood (Wright, 2011), has previously shown robust connections to psychological phenomena like stress (Bloomberg & Chen, 2005), and operates on a relatively fast time-scale that is conducive to more temporally-precise assessments (Skoner, 2002). Moreover, children with asthma have a two-fold higher prevalence of depressive/anxiety symptoms compared to children without asthma (Katon et al., 2007). In turn, depressive symptoms are associated with more frequent hospital visits (Ahmedani, Peterson, Wells, & Williams, 2013), greater medication non-compliance (DiMatteo, Lepper, & Croghan, 2000), greater social and educational impairment (Gutstadt et al., 1989), and increased risk of dying from asthma-related causes (Strunk, Mrazek, Fuhrmann, & LaBrecque, 1985).
To understand how these associations between asthma and depressive symptoms occur biologically, an examination of molecular signaling pathways may provide insight into important and proximal processes. Briefly, although an individual’s DNA sequence serves as a blueprint that informs downstream cell functioning, not all genes are active in any given moment. Rather, the process of gene expression entails the synthesis of RNA molecules, which are then translated into proteins that are used to create structures within cells and to carryout functions by cells. Importantly, the transcription of many genes are believed to be sensitive to social and emotional conditions, such that expression is increased or decreased in response to experiential input (Cole et al., 2007).
Of particular relevance to the pathophysiology of asthma are glucocorticoid receptors (GR) and β2-adrenegeric receptors (β2-AR). GR is critical to anti-inflammatory signaling cascades that regulate the activity of immune cells, antibody generation, and cytokine production, whereas β2-AR binds with epinephrine, which, among other functions, influences bronchodilation and smooth muscle functioning. These receptors are also the primary targets of medications for asthma. Corticosteroids, which act on GR, typically serve as controller medications for asthma, while β2-AR-agnonists are typically prescribed as rescue medications to be administered during acute exacerbations and act on β2-AR (Busse & Lemanske, 2001). Individuals who express less of either receptor would be expected to show greater asthma symptoms and less responsivity to asthma medications. Importantly, previous research suggests that the expression of these receptors is sensitive to psychological experiences. For example, Miller and Chen (2006) demonstrated that children with asthma who experienced both acute and chronic stress showed 5.5 times less GR mRNA and 9.5 times less β2-AR mRNA than those without similar stress exposure. Specific to depression, biologically-plausible explanations for connections between mental health and GR and β2-AR expression include heightened activation of stress-responses systems and inflammatory processes that are shared by asthma and depression (Van Lieshout, Bienenstock, & MacQueen, 2009), such as those that account for well-documented associations between depression and cortisol secretion profiles in medically-healthy populations (Adam et al. 2010). Another possibility is that behavioral changes associated with depression, such as less adherence to medication, may effect expression of GR and β2-AR (Grenard et al., 2011). It is important to note that these genes, particularly GR, have also been implicated in mood disorders (for a review, see Pariante & Lightman, 2008). Thus, it is also possible that associations between depression and GR expression are due to reverse directionality or are bi-directional.
Although children’s depressive symptoms, such as negative mood, may directly predict gene expression in pediatric asthma, elements of the family context may also contribute to youths’ gene expression profiles by modifying the effect of children’s psychopathology symptoms. During childhood, parents can play a critical role in preventing, reducing, or overcoming depressive problems, such that parenting interventions are commonly utilized in the treatment of youth depression (Compas et al., 2010). In turn, these dynamics may also moderate disease processes that are affected by depression. This possibility is supported by substantial research documenting connections between family functioning and youth asthma. For example, Marin, Chen, Munch, and Miller (2009) found that the effects of acute stress on the production of asthma-related cytokines among children with pediatric asthma were magnified for children who experienced high levels of chronic family stress, but were buffered for children with low levels of family stress. Similarly, a negative family emotional climate has been shown to predict youths’ asthma severity by affecting children’s depressive symptoms and potentiating emotional triggering of asthma (Wood et al., 2006).
One powerful contributor to problematic family environments is parental depression. Indeed, mothers with depression reliably demonstrate more negative and coercive behaviors, greater disengagement, and lower levels of warmth and positivity toward their children than non-depressed mothers, and these impairments endure even once depression recedes (Lovejoy, Graczyk, O’Hare, & Neuman, 2000; Thomas et al., 2014). For this reason, it may be useful to consider exposure to parental depressive symptoms in the aggregate across a given period of time, and its effects on children’s functioning. Moreover, parental depression confers multifactorial risk, both through a presence of aversive parenting behaviors as well as through an absence of parental support (Sellers et al., 2014). For example, even if parents overcome a tendency toward punitive parenting when depressed, children may still face a less supportive home environment that fails to help them regulate their own mood difficulties. This difficulty for depressed parents to act as a buffer is supported by a longitudinal study comparing children from mothers with affective disorders to those from healthy mothers, which found that children with symptomatic mothers showed more subsequent depressive symptoms in response to stressful experiences than children who experienced stressful events but who had mothers without depressive symptoms (Hammen, Burge, & Adrian, 1991).
For children with chronic illnesses, these environmental features may have repercussions for youths’ biological functioning. In a sample of children with and without asthma, higher levels of parental depressive symptoms predicted increases in children’s eosinophil cationic protein—an asthma-relevant inflammatory marker—over a six-month period, and this was not accounted for by children’s own depressive symptoms (Wolf, Miller, & Chen, 2008). Parental depressive symptoms have also been shown to predict more hospitalizations (Lange et al., 2011), greater use of the emergency department (Bartlett, Kolodner, & Butz, 2001), and more difficulties with using inhaler medication properly (Bartlett et al., 2004) among children with asthma. The extent to which parental depressive symptoms would interact with children’s own affective problems to predict asthma-relevant gene expression remains unknown, however.
To address this gap, the current study utilized five waves of longitudinal data across a two-year period to test whether average parental depressive symptoms would moderate the effect of changes in youths’ negative mood symptoms on the expression of GR and β2-AR genes in the leukocytes of youths with physician-diagnosed asthma. These genes were chosen because they are the primary targets of asthma medication and thus represent biologically-proximal mediators of comorbidity. We hypothesized that children from family contexts generally characterized by higher levels of parental depressive symptoms would demonstrate gene expression profiles that were more sensitive to changes in their own negative mood symptoms, predicting that these youths would show significantly less expression of GR and of β2-AR during periods when they experienced more negative mood symptoms than average, whereas children from families with lower average parental depressive symptoms would not show differences in their gene expression during times of greater negative mood symptoms. We anticipated that these associations would emerge above and beyond demographic features of parents and youths (including gender, socioeconomic status, and age), leukocyte distributions, asthma medication use, and asthma severity. We also tested a reverse-directionality model in which changes in gene expression (and their interaction with average parental depressive symptoms) predicted youth depressive symptoms.
Methods
Participants
Youths ages 9- to 18-years-old with physician-diagnosed asthma were recruited to participate with one parent, as part of a larger study, through newspaper and community advertisements and through asthma clinics in a large North American city. Youths and parents were required to be fluent in English and youths were required to have received a diagnosis of asthma from a physician, to be free of acute respiratory illnesses at the time of their first visit, to have been free of oral steroids for at least 2 weeks preceding their baseline visit, and to be free of medication for chronic disorders besides asthma. One-hundred-twenty youths and their parents met these criteria and enrolled in the larger study. Reflecting gender differences in the prevalence of pediatric asthma (Almqvist, Worm, Leynaert, for the working group of GA2LEN WP 2.5 Gender, 2007), 67% of youths in the current sample were male. Sixty-three percent of children identified as White and were on average 12.61 years old (SD=2.63). Eighty-three percent of parents were female and average family income was in the $50,000–74,999 Canadian dollar range.
Procedure
Parents provided written consent and youths provided written assent, overseen by the Institutional Review Board of the University of British Columbia. Youths and parents came to the lab for a baseline assessment, during which participants reported on their demographic information and parents completed a questionnaire about their depressive symptoms. Six months later, parents and youths returned to the lab, during which parents again reported their depressive symptoms and youths completed a measure of negative mood, reported on their asthma medication use, and underwent a blood draw. The assessments of parent symptoms, youth mood symptoms, medication, and blood were repeated every six months for an additional year and a half, resulting in biological data at up to four time points per youth plus one additional baseline assessment of parent symptoms (a two year period in total). The majority of dyads (75.5%) completed all five possible visits and 94.5% completed two or more visits. Analyses were based on all available data at each time-point. There were no significances differences on any study variables between families who provided complete data at all time points and those with any missing data.
Measures
Parent Depressive Symptoms
At each of the 5 visits, parents completed the Center for Epidemiological Studies—Depression Scale (Radloff, 1977), a widely-used 20-item questionnaire that asks participants to rate on a 0–3 scale how often they experienced various depressive symptoms during the past week, with higher scores indicating more depression symptomatology (Cronbach’s α= .90 in current sample). The reliability and validity of this measure have been robustly demonstrated in both community and clinical samples (Lewinsohn, Seeley, Roberts, & Allen, 1997; Shinar, Gross, Price, Banko, & Bolduc, 1986). In the present study, we were interested in how living in a family environment generally characterized by different levels of parental depressive symptoms would moderate the effects of experiencing negative moods on children’s gene expression, guided by research documenting the enduring and widespread effects of current as well as previous parental depressive episodes (Lovejoy et al., 2000). To that end, we treated parental depressive symptoms as a largely stable factor, averaging CES-D scores across all available visits. This decision was supported by the significant correlations between scores at every visit and the high reliability of the total score across the five visits (Cronbach’s α=.83). Parental depressive symptoms were measured as a continuous variable, but for interpretation purposes, follow-up analyses plotted associations at the 25th percentile (“low parental depressive symptoms”) and 75th percentile (“high parental depressive symptoms”) of the measure.
Youth Negative Mood Symptoms
At visits 2–5, youths completed the negative mood symptom subscale from the Child Depression Inventory (Kovacs, 1992; the full CDI was not administered in order to reduce participant burden). These six items assessed the extent to which children experienced aspects of negative mood over the past two weeks, specifically, feeling sad, worrying about bad things, feeling like crying, things bothering them, and having difficulty making up their mind (Cronbach’s α= .69 in current sample). The CDI has been shown to have acceptable reliability and validity, including in children as young as 8-years-old (Smucker, Craighead, & Craighead, 1986). Scores on this measure were centered around the participant’s average, such that higher scores indicate elevations in negative mood for that visit.
Gene Expression
At visits two through five, 2.5 mL of whole blood was drawn into PAXgene Blood RNA tubes through antecubital venipuncture to measure the expression of messenger RNA (mRNA) for the α isoform of the Glucocorticoid Receptor (GR) and for β2-Adrenergic Receptor (β2-AR). Total RNA was extracted using PAXgene Blood RNA Kits (Pre-Analytix, Hombrechtikon, Switzerland) and real-time reverse transcription-polymerase chain reaction was executed using commercially-available one-step assays (TaqMan Gene Expression Assay developed in partnership with Applied Biosystems and based on RefSeq NM_000176 for GR-α; TaqMan Gene Expression Assay #Hs00240532 for β2-AR; Applied Biosystems). Values of each target mRNA were adjusted for expression of a housekeeping gene, 18S, using the delta CT method. Results are expressed as relative quantities of mRNA on a log 2 base scale, where higher values indicate greater expression of the target mRNA and each unit difference in relative quantity indicates a twofold difference in expression.
Covariates
To account for the possibility that differences in demographic characteristics might relate to differential gene expression patterns, child age, race (dummy coded for racial minority status), and gender, along with parent gender and family income were assessed at baseline and included as dyad-level covariates (level 2).
To assess whether any observed associations might be accounted for by disease-related factors or by the composition of the circulating leukocyte pool, asthma severity, use of asthma medications in the past week, and leukocyte populations were also assessed. Here, asthma severity was included as a covariate at level 2 and was calculated based on guidelines from the National Asthma Education and Prevention Program/Expert Panel Report 2, in which ratings from 1 to 4 are determined based on medication use and asthma symptom frequency, where 1= mild intermittent asthma and 4= severe persistent asthma (Bacharier et al., 2004). To index medication use, at visits 2–5, children reported on the number of days in the preceding 2 weeks that they took an inhaled corticosteroid medication and the number of days in the preceding 2 weeks that they took a beta-agonist medication, which were included as visit-level (level 1) covariates. Lastly, cell subset counts for monocytes, neutrophils, and lymphocytes were obtained from a complete blood count with differential at each visit (ADVIA 70 Hematology System; GMI Inc., Holliston, MA) and included as level 1 covariates.
Analytic Strategy
For our primary analyses, we were interested whether being in a family characterized by higher average parental depressive symptoms would moderate the effects of changes in children’s negative mood on youths’ expression of GR-α and β2-AR. To test our hypothesis that children experiencing worse mood would show less gene expression particularly if they had parents with high levels of depressive symptoms, we conducted a series of multilevel models to estimate fixed effects using Hierarchical Linear and Nonlinear Modeling (HLM) software (Version 6.08; Raudenbush, Bryk, & Congdon, 2004). At Level-1, representing the within-person portion of the model, each target gene was separately modeled by youth negative mood (person-centered), along with the covariates of days of inhaled corticosteroid medication use in past week (person-centered), days of beta agonist medication use in past week (person-centered), lymphocyte count (person-centered), monocyte count (person-centered), neutrophil count (person-centered) and a random error term. At Level 2, representing the between-person portion of the model, parents’ average depressive symptom score (grand-centered), along with covariates of child gender, child age (grand-centered), child minority racial status, parent gender, family income category (grand-centered), and asthma severity rating (grand-centered) were used to model the intercept and slopes of Level 1 predictors. A random effect term was included in the Level 2 model of the intercept. A portion of one model is presented below.
The main coefficient of interest was the cross-level interaction between parent depression and youth negative mood. A significant interaction term would indicate that parental depression moderated the strength of the association between children’s negative mood on the target gene’s expression. If a significant interaction term emerged, simple slopes analyses were conducted following techniques developed by Preacher, Curran, and Bauer (2006), examining the slope of youth negative mood on gene expression at the 25th percentile (i.e., low parental depression), and 75th percentiles (i.e., high parental depression) of the sample distribution of parental depressive symptoms. Given previous work positing that GR may play a causal role in youth depressive symptoms, models were re-run to examine reverse-directionality in which youths’ negative mood was predicted from changes in GR or β2-AR expression, alone and in interaction with average parental depressive symptoms, adjusting for child age, parent and child gender, family income, minority status, and asthma severity.
Results
Descriptive statistics of study characteristics are presented in Table 1. Results of the multilevel model predicting GR-α expression revealed that, although there was not a main effect of parental depression nor a significant main effect of youth negative mood symptoms, there was a significant cross-level interaction between parent depression and youth mood symptoms wherein the association between visits with higher youth negative mood symptoms and reduced gene expression became increasingly negative at higher levels of parental depressive symptomatology, as displayed in Figure 1 and Table 2. Tests of simple slopes revealed that the association between negative mood and GR-α expression was marginally significant for youths in families with high parental depression (b=−.44, SE=.24 p=.078) but was not significant for dyads with low parental depression (b=−.09, SE=.22, p=.675).
Table 1.
Descriptive Characteristics of the Sample
| Variable | Mean | SD |
|---|---|---|
| Youth Age (baseline) | 12.61 | 2.63 |
| Youth Gender | 67% Female | |
| Race/Ethnicity | 67% White | |
| Parent Gender | 83% Female | |
| Income Category (range 2–9) | 5.42 | 1.89 |
| Parental Depressive Symptoms (range 0–33) | 9.94 | 7.30 |
| Youth Negative Mood Symptoms (range 0–8) | 1.37 | 1.50 |
| Days of Inhaled Corticosteroid (past 2 weeks) | 5.58 | 6.23 |
| Days of Beta-Agonist Medication (past 2 weeks) | 4.30 | 5.61 |
| Neutrophils (× 109 cells per L) | 3.61 | 1.65 |
| Lymphocytes (× 109 cells per L) | 2.44 | 0.77 |
| Monocytes (× 109 cells per L) | 0.49 | 0.18 |
| GR-α (relative expression; log2) | 5.21 | 1.37 |
| β2-AR (relative expression; log2) | 5.71 | 2.91 |
Figure 1. Interaction between Average Parental Depressive Symptoms and Within-Person Changes in Youth Negative Mood Predicting Expression of Glucocorticoid Receptor in Youths.
Note. Low parental depressive sx=25th percentile of sample distribution; high parental depressive sx=75th percentile of sample distribution. GR values are expressed on a log2 scale.
Table 2.
Abbreviated Results Displaying Main Effects and Cross-Level Interaction between Youth Negative Mood and Parental Depressive Symptoms
| Coefficient | Standard Error | P-value | |
|---|---|---|---|
| Model of GR-α | |||
| Intercept | 5.11 | .18 | <.001 |
| Parent Depressive Symptom Average | .00 | .02 | .951 |
| Asthma Severity | −.012 | .15 | .411 |
| Youth Age | .07 | .05 | .149 |
| Youth Gender | .10 | .28 | .717 |
| Parent Gender | −.56 | .33 | .094 |
| Racial Minority Status | −.311 | .27 | .257 |
| Income Category | −.01 | .07 | .876 |
| Child Negative Mood Symptoms | −.31 | .22 | .173 |
| Parent Depressive Symptom Average | −.04 | .02 | .034 |
| Days of Inhaled Corticosteroid (past 2 weeks) | −.03 | .09 | .709 |
| Days of Beta-Adrenergic-Agonist (past 2 weeks) | .13 | .08 | .098 |
| Neutrophils | .46 | .21 | .032 |
| Lymphocytes | .35 | .48 | .462 |
| Monocytes | −.09 | 2.07 | .964 |
|
| |||
| Model of β2-AR | |||
| Intercept | 5.56 | .38 | <.001 |
| Parent Depressive Symptom Average | .00 | .04 | .913 |
| Asthma Severity | −.18 | .34 | .599 |
| Youth Age | .21 | .11 | .063 |
| Youth Gender | −.26 | .67 | .697 |
| Parent Gender | −.52 | .69 | .453 |
| Racial Minority Status | −.75 | .61 | .225 |
| Income Category | −.06 | .17 | .740 |
| Child Negative Mood Symptoms | −1.17 | .59 | .058 |
| Parent Depressive Symptom Average | −.21 | .06 | .003 |
| Days of Inhaled Corticosteroid (past 2 weeks) | .32 | .29 | .277 |
| Days of Beta-Adrenergic-Agonist (past 2 weeks) | .29 | .26 | .277 |
| Neutrophils | 1.92 | .63 | .005 |
| Lymphocytes | 1.83 | 1.54 | .246 |
| Monocytes | 1.00 | 5.84 | .866 |
Note. Right-justified text indicates intercepts of level-1 variables and indented text indicates cross-level interactions with level-2 variables. To conserve space, only main effects and the focal cross-level interaction between youth negative mood symptoms and average parental depressive symptoms are displayed. However, all level-2 variables were included as predictors of all level-1 slopes in the full models. Level-1 variables are person-centered, whereas level-2 variables are grand-centered. Gender was coded such that the majority gender was the referent group; youth gender was coded with 0=male and 1=female, whereas parent gender was coded with 0=female and 1=male. Racial minority status was coded where 0=majority and 1=minority.
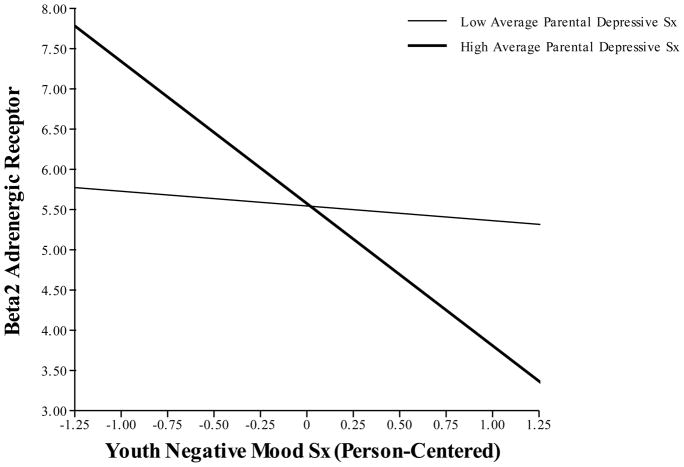
The results of the multilevel model predicting β2-AR revealed a marginally significant main effect of youth mood, which was qualified by a significant interaction between parental depression and child negative mood, wherein children who experienced more negative mood in the context of greater parental depression expressed less β2-AR (see Figure 2). As before, main effects of parental depressive symptoms were not significant. Tests of simple slopes showed that the association between mood and gene expression was not significant for dyads with low parental depressive symptoms (b=−.18, SE=.55, p=.741), but were significant for children whose parent has high levels of depressive symptoms (b=−1.76, SE=.67, p=.015).
Figure 2. Interaction between Average Parental Depressive Symptoms and Within-Person Changes in Youth Negative Mood Predicting Expression of β2-Adrenergic Receptor in Youths.
Note. Low parental depressive sx=25th percentile of sample distribution; high parental depressive sx=75th percentile of sample distribution. β2-AR values are expressed on a log2 scale.
Post-hoc analyses did not find support for reverse directionality for gene expression predicting youth negative mood. Changes in GR were not related to youth negative mood alone (b=−.07, SE=.09 p=.453) nor in interaction with average parental depressive symptoms (b=−.01, SE=.01, p=.455). Similarly, there was not a main effect of β2-AR predicting youth negative mood symptoms (b=.01, SE=.05 p=.774) nor an interaction between β2-AR and average parental depressive symptoms (b=−.01, SE=.01, p=.303).
Taken together, these analyses suggest that parental depressive symptoms potentiate the effect of increases in youth’s negative mood on asthma-relevant gene expression, and these effects occur above and beyond contributions of leukocyte subtypes, medication use, demographic characteristics, and asthma severity.
Discussion
The current study demonstrated an interactive effect of parent and youth depressive symptoms on children’s asthma-relevant gene expression over a two-year period, suggesting that these mental health processes may affect inflammatory signaling in pediatric asthma. Specifically, it found that the effect of youths’ negative mood symptoms on their expression of GR and β2-AR genes was potentiated by their parents’ level of depressive symptoms, such that during periods when children reported more negative mood symptoms than their average, they expressed significantly less GR- α and β2-AR genes, but this was only true for children who came from families characterized by higher average levels of parental depressive symptoms. Moreover, these associations emerged above and beyond contributions of sociodemographic factors, medication use, asthma severity, and the distribution of leukocyte populations. Together, the present research identifies a biologically-plausible way that psychopathology symptoms in children and their families may lead to worse health processes for children.
That only the combination of youth negative mood in the context of greater average parental depressive symptoms related to less gene expression is consistent with prior research findings that reduced expression of GR and β2-AR genes in children with asthma was strongest for those experiencing both acute and chronic stress, but that acute stressors alone did not predict gene expression (Miller & Chen, 2006). It is also in keeping with robust bodies of work documenting the role of parents in modifying youths’ psychopathology symptoms as well as physical health. For example, parental psychopathology symptoms have been shown to potentiate effects of exposure to community violence on youths’ PTSD symptoms (Self-Brown, LeBlanc, & Kelley, 2006). High levels of chronic family stress have been shown to similarly intensify associations between acute stress and children’s mitogen-stimulated production of asthma-relevant cytokines IL-4, IL-5, and IFN-γ (Marin et al., 2009).
These are several explanations for how these associations may develop. One possibility is that parental depression interferes with a mother’s or father’s ability to act supportively or buffer their children when they experience their own depressive symptoms. Likewise, when children experience more depressive symptoms, they may become less able to cope with the hostility or negative affect that accompanies their parent’s depression. These problems may then lead to greater psychological distress on the part of the child, impacting the functioning of their sympathetic nervous system and hypothalamic-pituitary-adrenal axis and resulting in changes in epinephrine and cortisol secretion, for example (Gotlib, Joormann, Minor, & Hallmayer, 2008; Miller, Chen, & Zhou, 2007), in turn altering inflammatory signaling processes (Miller, Cohen, & Ritchey, 2002) and disease dynamics (Miller, Chen, & Cole, 2009). An alternative explanation is that associations may be due to lower adherence with medications. However, if this were the case, our associations would not be expected to emerge when statistically accounting for youths’ exposure to corticosteroid and beta agonist medications. It remains possible, though, that children and parents with depressive symptoms may be less effective in how they administer medication—for example, not taking rescue inhalers until symptoms become extreme or not spacing maintenance medications appropriately. A final explanation for the patterns of results is that congruence between mood symptoms in parents and children may indicate shared genetic liability for depression, such as carrying the short allele for the promotor region of the serotonin transporter gene (5-HTTLPR). Previous work has suggested that carriers of the short allele are more biologically sensitive to environmental input in ways that can relate to depression (Caspi, Hariri, Holmes, Uher, & Moffitt, 2010; Vrshek-Schallhorn et al., 2014). In this way, children may show greater changes in mood symptoms over time as they react to varying situations. It is possible that this sensitivity then contributes to changes in gene expression relevant to physical health.
The current work adds to existing literature on mental and physical health comorbidity by finding evidence of molecular signaling patterns that link youth and family mental health to biological processes relevant to pediatric asthma. In doing so, it identifies a novel aspect of the biological cascades that may contribute to why children with depressive symptoms have worse asthma-related problems, such as more frequent hospital visits (Ahmedani et al., 2013) and increased mortality due to asthma (Strunk et al., 1985). Furthermore, these findings may have important clinical implications. First, our results suggest that it may be important to assess both parent and child mental health symptoms during routine pediatric asthma care, rather than focusing exclusively on the mental health of the presenting patient. Second, our work reaffirms the value of considering parenting- and mental health-focused interventions for the treatment of physical health problems. For example, a recent randomized-control trial suggested that family therapy can be effective in reducing pediatric asthma symptoms (Ng, Li, Lou, Tso, & Wan, 2008). Moreover, by improving both mental and physical health, such interventions may be especially cost-effective, and thus more easily justifiable when clinical and monetary resources are limited.
There are several important limitations to acknowledge in the present study. For example, parental depressive symptoms and youth negative mood symptoms were measured using self-report questionnaires rather than diagnostic interviews or clinician-administered measures. Similarly, there was a somewhat low internal consistency for the measure of child negative mood. Although the self-report measures are well-validated, future work would benefit from more rigorous assessment of psychopathology symptoms. Another limitation is that this set of analyses did not focus on how the observed associations related to history of asthma, or other elements of asthma management, such as exposure to allergens. In addition, the current study was observational, making it difficult to determine causality. For example, it is possible that children’s asthma or negative mood may contribute to parent’s depressive symptoms. We also were unable to differentiate environmental versus genetic vulnerability. Another limitation is that the study was made up of largely mothers and White families, which may limit generalizability. In addition, children were not able to participate in the current study if they were on a medication for depression or other psychiatric disorder, which likely restricted range. It is also important to acknowledge that while effects may be statistically significant, it is unclear whether they are clinically significant. Lastly, the present study focused on two target genes that have been implicated in asthma and asthma-management. However, this approach of limiting focus to the expression of two genes has the potential to overlook other associations that might emerge using genome-wide analysis.
The current research also raises questions for future work. For example, additional research should probe which aspects of parental or youth depressive symptoms most contribute to the pattern of associations. For instance, is it a lack of support from parents, greater parent-child conflict, children’s impaired emotion regulation, or greater family unpredictability that is most relevant to gene expression? Moreover, given that maternal and paternal depression appear to have different associations with youth mental health (Pilowsky et al., 2014), it will also be important for future work to include both parents to explore gender differences for these patterns of results. Additionally, it will be critical to test these processes within other pediatric disease models to examine whether parent and child depressive symptoms relate to the expression of other genes. By identifying common and disease-specific associations, future research can better understand processes associated with depression and depression comorbidity. Finally, given that pediatric diseases, including asthma, occur across periods of tremendous social-emotional and physical change, future research should more closely examine developmental shifts in symptoms and gene expression. Possible dynamics to explore would be whether associations are stronger for younger children and whether associations change once children move away from home. As children age, it is possible that the mental health of other relationship partners, such as close friends or romantic partners, may become more relevant to youths’ mental health and gene expression than parental depressive symptoms.
Regardless of these limitations and unanswered questions, the current work is the first to document interactive associations between parent- and youth-depressive symptoms on gene expression in a sample of children with asthma. As such, it identifies novel and biologically-proximal molecular signaling patterns that connect depressive symptoms to pediatric asthma while also highlighting the important role of family environment for biological processes that may operate within depression comorbidity.
Supplementary Material
Acknowledgments
Support for this research was provided by the National Heart, Lung, and Blood Institute grant R01-HL108723 and National Heart, Lung, and Blood Institute grant R01-HL073975.
Footnotes
The authors declare that they have no conflict of interest.
Contributor Information
Erika M. Manczak, Stanford University
Bryn Dougherty, Northwestern University.
Edith Chen, Northwestern University.
References
- Adam EK, Doane LD, Zinbarg RE, Mineka S, Craske MG, Griffith JW. Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology. 2010;35(6):921–931. doi: 10.1016/j.psyneuen.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmedani B, Peterson E, Wells K, Williams L. Examining the relationship between depression and asthma exacerbations in a prospective follow-up study. Psychosomatic Medicine. 2013;75(3):305–310. doi: 10.1097/PSY.0b013e3182864ee3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almqvist C, Worm M, Leynaert B for the working group of GA2LEN WP 2.5. Impact of gender on asthma in childhood and adolescence: a GA2LEN review. Allergy. 2007 doi: 10.1111/j.1398-9995.2007.01524.x. 070907221144001. [DOI] [PubMed] [Google Scholar]
- Bacharier L, Strunk R, Mauger D, White D, Lemanske R, Sorkness C. Classifying asthma severity in children: Mismatch between symptoms, medication use, and lung function. American Journal of Respiratory and Critical Care Medicine. 2004;170(4):426–432. doi: 10.1164/rccm.200308-1178OC. [DOI] [PubMed] [Google Scholar]
- Bartlett S, Kolodner K, Butz A. Maternal depressive symptoms and emergency department use among inner-city children with asthma. Archives of Pediatric and Adolescent Medicine. 2001;155:347–353. doi: 10.1001/archpedi.155.3.347. [DOI] [PubMed] [Google Scholar]
- Bartlett S, Krishnan J, Riekert K, Butz A, Malveaux F, Rand C. Maternal depressive symptoms and adherence to therapy in inner-city children with asthma. Pediatrics. 2004;113(2):229–237. doi: 10.1542/peds.113.2.229. [DOI] [PubMed] [Google Scholar]
- Bloomberg G, Chen E. The relationship of psychologic stress with childhood asthma. Immunology and Allergy Clinics of North America. 2005;25(1):83–105. doi: 10.1016/j.iac.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Busse W, Lemanske R. Advances in immunology: Asthma. The New England Journal of Medicine. 2001;344(5):350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- Cole S, Hawkley L, Arevalo J, Sung C, Rose R, Cacioppo J. Social regulation of gene expression in human leukocytes. Genome Biology. 2007;8(9):R189. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compas B, Champion J, Forehand R, Cole D, Reeslund K, Fear J, et al. Coping and parenting: Mediators of 12-month outcomes of a family group cognitive–behavioral preventive intervention with families of depressed parents. Journal of Consulting and Clinical Psychology. 2010;78(5):623–634. doi: 10.1037/a0020459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMatteo M, Lepper H, Croghan T. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Archives of Internal Medicine. 2000;160(14):2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- Egger H, Costello E, Erkanli A, Angold A. Somatic complaints and psychopathology in children and adolescents: Stomach aches, musculoskeletal pains, and headaches. Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38(7):852–860. doi: 10.1097/00004583-199907000-00015. [DOI] [PubMed] [Google Scholar]
- Gonzalez J, Peyrot M, McCarl L, Collins E, Serpa L, Mimiaga M, Safren S. Depression and diabetes treatment nonadherence: A meta-analysis. Diabetes Care. 2008;31(12):2398–2403. doi: 10.2337/dc08-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib I, Joormann J, Minor K, Hallmayer J. HPA axis reactivity: A mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biological Psychiatry. 2008;63(9):847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenard J, Munjas B, Adams J, Suttorp M, Maglione M, McGlynn E, Gellad W. Depression and medication adherence in the treatment of chronic diseases in the United States: A meta-analysis. Journal of General Internal Medicine. 2011;26(10):1175–1182. doi: 10.1007/s11606-011-1704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutstadt L, Gillette J, Mrazek D, Fukuhara J, LaBrecque J, Strunk R. Determinants of school performance in children with chronic asthma. American Journal of Diseases of Children. 1989;143(4):471–475. doi: 10.1001/archpedi.1989.02150160101020. [DOI] [PubMed] [Google Scholar]
- Hammen C, Burge D, Adrian C. Timing of mother and child depression in a longitudinal study of children at risk. Journal of Consulting and Clinical Psychology. 1991;59(2):341–345. doi: 10.1037//0022-006x.59.2.341. [DOI] [PubMed] [Google Scholar]
- Herzer M, Denson L, Baldassano R, Hommel K. Patient and parent psychosocial factors associated with health-related quality of life in pediatric inflammatory bowel disease. Journal of Pediatric Gastroenterology and Nutrition. 2011;52(3):295–299. doi: 10.1097/MPG.0b013e3181f5714e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashikar-Zuck S, Goldschneider K, Powers S, Vaught M, Hershey A. Depression and functional disability in chronic pediatric pain. The Clinical Journal of Pain. 2001;17:341–349. doi: 10.1097/00002508-200112000-00009. [DOI] [PubMed] [Google Scholar]
- Katon W, Lozano P, Russo J, McCauley E, Richardson L, Bush T. The prevalence of DSM-IV anxiety and depressive disorders in youth with asthma compared with controls. Journal of Adolescent Health. 2007;41(5):455–463. doi: 10.1016/j.jadohealth.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan-Miller D, Hammen C, Brennan P. Health outcomes related to early adolescent depression. Journal of Adolescent Health. 2007;41(3):256–262. doi: 10.1016/j.jadohealth.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M. Children’s depression inventory. North Tonawanda, NY: Multi-Health System; 1992. [Google Scholar]
- Lange N, Bunyavanich S, Silberg J, Canino G, Rosner B, Celedón J. Parental psychosocial stress and asthma morbidity in Puerto Rican twins. Journal of Allergy and Clinical Immunology. 2011;127(3):734–740e7. doi: 10.1016/j.jaci.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn P, Seeley J, Roberts R, Allen N. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychology and Aging. 1997;12(2):277–287. doi: 10.1037//0882-7974.12.2.277. [DOI] [PubMed] [Google Scholar]
- Lovejoy M, Graczyk P, O’Hare E, Neuman G. Maternal depression and parenting behavior: A meta-analytic review. Clinical Psychology Review. 2000;20(5):561–592. doi: 10.1016/s0272-7358(98)00100-7. [DOI] [PubMed] [Google Scholar]
- Marin T, Chen E, Munch J, Miller G. Double-exposure to acute stress and chronic family stress is associated with immune changes in children with asthma. Psychosomatic Medicine. 2009;71(4):378–384. doi: 10.1097/PSY.0b013e318199dbc3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Chen E. Life stress and diminished expression of genes encoding glucocorticoid receptor and β2-adrenergic receptor in children with asthma. Proceedings of the National Academy of Sciences. 2006;103(14):5496–5501. doi: 10.1073/pnas.0506312103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Chen E, Zhou E. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133(1):25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Miller G, Cohen S, Ritchey A. Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid-resistance model. Health Psychology. 2002;21(6):531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Miller G, Chen E, Cole S. Health psychology: Developing biologically plausible models linking the social world and physical health. Annual Review of Psychology. 2009;60(1):501–524. doi: 10.1146/annurev.psych.60.110707.163551. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics (U.S.). Division of Health Interview Statistics. Vital and Health Statistics. 10. 258. 2013. Summary Health Statistics for U.S. Children: National Health Interview Survey, 2012. [Google Scholar]
- Ng S, Li A, Lou V, Tso I, Wan P. Incorporating family therapy into asthma group intervention: A randomized waitlist-controlled trial. Family Process. 2008;47:115–130. doi: 10.1111/j.1545-5300.2008.00242.x. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends in Neurosciences. 2008;31(9):464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Pilowsky DJ, Wickramaratne P, Poh E, Hernandez M, Batten LA, Flament MF, et al. Psychopathology and functioning among children of treated depressed fathers and mothers. Journal of Affective Disorders. 2014;164:107–111. doi: 10.1016/j.jad.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine D, Cohen P, Brook J. The association between major depression and headache: results of a longitudinal epidemiologic study in youth. Journal of Child and Adolescent Psychopharmacology. 1996;6(3):153–164. doi: 10.1089/cap.1996.6.153. [DOI] [PubMed] [Google Scholar]
- Pinquart M, Shen Y. Depressive symptoms in children and adolescents with chronic physical illness: An updated meta-analysis. Journal of Pediatric Psychology. 2011;36(4):375–384. doi: 10.1093/jpepsy/jsq104. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of educational and behavioral statistics. 2006;31(4):437–448. [Google Scholar]
- Radloff L. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Raudenbush SW, Bryk AS, Congdon R. HLM 6 for Windows [Computer software] Lincolnwood, IL: Scientific Software International; 2004. [Google Scholar]
- Self-Brown S, LeBlanc M, Kelley M. Effects of community violence exposure and parental mental health on the internalizing problems of urban adolescents. Violence and Victims. 2006;21(2):183–198. doi: 10.1891/vivi.21.2.183. [DOI] [PubMed] [Google Scholar]
- Sellers R, Harold GT, Elam K, Rhoades KA, Potter R, Mars B, et al. Maternal depression and co-occurring antisocial behaviour: Testing maternal hostility and warmth as mediators of risk for offspring psychopathology. Journal of Child Psychology and Psychiatry. 2014;55(2):112–120. doi: 10.1001/archpsyc.1990.01810180089012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinar D, Gross C, Price T, Banko M, Bolduc P. Screening for depression in stroke patients: the reliability and validity of the Center for Epidemiologic Studies Depression Scale. Stroke. 1986 doi: 10.1161/01.str.17.2.241. [DOI] [PubMed] [Google Scholar]
- Skoner D. Outcome measures in childhood asthma. Pediatrics. 2002;109:393–398. [PubMed] [Google Scholar]
- Smucker M, Craighead W, Craighead L. Normative and reliability data for the Children’s Depression Inventory. Journal of Abnormal Child Psychology. 1986;14(1):25–39. doi: 10.1007/BF00917219. [DOI] [PubMed] [Google Scholar]
- Strunk R, Mrazek D, Fuhrmann G, LaBrecque J. Physiologic and psychological characteristics associated with deaths due to asthma in childhood: a case-controlled study. Jama. 1985;254(9):1193–1198. [PubMed] [Google Scholar]
- Thomas SR, O’Brien KA, Clarke TL, Liu Y, Chronis-Tuscano A. Maternal depression history moderates parenting responses to compliant and noncompliant behaviors of children with ADHD. Journal of Abnormal Child Psychology. 2014;43(7):1257–1269. doi: 10.1016/j.cpr.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lieshout R, Bienenstock J, MacQueen G. A review of candidate pathways underlying the association between asthma and major depressive disorder. Psychosomatic Medicine. 2009;71(2):187–195. doi: 10.1097/PSY.0b013e3181907012. [DOI] [PubMed] [Google Scholar]
- Wolf J, Miller G, Chen E. Parent psychological states predict changes in inflammatory markers in children with asthma and healthy children. Brain, Behavior, and Immunity. 2008;22(4):433–441. doi: 10.1016/j.bbi.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood B, Lim J, Miller B, Cheah P, Simmens S, Stern T, et al. Family emotional climate, depression, emotional triggering of asthma, and disease severity in pediatric asthma: Examination of pathways of effect. Journal of Pediatric Psychology. 2006;32(5):542–551. doi: 10.1093/jpepsy/jsl044. [DOI] [PubMed] [Google Scholar]
- Wright R. Epidemiology of stress and asthma: From constricting communities and fragile families to epigenetics. Immunology and Allergy Clinics of North America. 2011;31(1):19–39. doi: 10.1016/j.iac.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.