Abstract
The behavioural responses of the haematophagous bug Triatoma infestans towards some previously identified components of its faeces: 4-methylquinazoline, 2,4-dimethylquinazoline and their mixtures were evaluated using a video tracking system. Fifth instar nymphs and females but not males were significantly attracted to polyethylene glycol formulations of 4-methyl + 2,4-dimethylquinazoline (50 μg each). Fifth instar nymphs were also attracted to 4-methylquinazoline alone (50 μg) but females were only attracted by the mixture of both methyl quinazolines (50 μg each). Syntheses of both methyl quinazolines were carried out starting from 2-aminoacetophenone by modifying the conditions of reported procedures.
Keywords: 4-Methylquinazoline; 2,4-dimethylquinazoline; aggregation behaviour; Triatoma infestans
Introduction
The haematophagous bug Triatoma infestans (Klug) is the major vector of Chagas disease in South America. The aggregation behaviour elicited from conspecific nymphs and adults by its feces is well known [1,2]. This activity has been attributed to a number of nitrogenous compounds emitted by the faeces. Ammonia elicited electrophysiological and behavioural responses in laboratory bioassays [3,4]. In concordance with the observations of other authors for haematofagous insects, we have reported a very dose dependent response of T. infestans nymphs towards different salts used as sources of ammonia [5].
Other nitrogenous compounds such as 4-methyl- and 2,4-dimethylquinazoline were reported to be released from faeces of T. infestans [4]. However, the authors were unable to demonstrate that these compounds could elicit attraction from immature stadiums when tested at doses of 0.1 – 100 ng. In the present work, 4-methyl- and 2,4-dimethylquinazoline were synthesized and the attractant effect of these compounds on fifth instar nymphs and adult T. infestans was assessed at different doses.
Sensitive and selective means of monitoring for T. infestans is central to any vector control campaign [6]. However, this is currently achieved by manual surveys and the use of unbaited refuge boxes [7,8]. Identification of bioactive compounds could be very useful for incorporation in a more sensitive monitor or control device for this species, the principal vector of Chaga’s Disease in South America [9].
Results
Fifth instar nymphs and females were found to be significantly attracted (P < 0.05) to blends of 50 μg of 4-methyl- plus 50 μg 2,4-dimethylquinazoline in solutions of polyethylene glycol 400 (Table 1). No significant differences (P > 0.05) were observed when the behaviour of males was evaluated.
Table 1.
Behavioural response of fifth instar nymphs and adults of T. infestans to a 4-methyl + 2,4-dimethylquinazoline blend in an arena bioassay.
| Behavioural response | Control | 4-methylquinazoline + 2,4-dimethylquinazoline | |
| (5 + 5) ng | (50 + 50) μg | ||
|
Fifth instar nymphs Positive recordings (%) |
30 |
60 |
100 |
| Mean number of visits (± SE) | 0.4 ± 0.2a | 0.8 ± 0.3a | 2.2 ± 0.3b |
|
Females Positive recordings (%) |
30 |
60 |
70 |
| Mean number of visits | 0.4 ± 0.2a | 1.2 ± 0.4a | 2.4 ± 0.7b |
|
Males Positive recordings (%) |
30 |
40 |
60 |
| Mean number of visits (± SE) | 0.7 ± 0.4 a | 0.9 ± 0.5a | 1.2 ± 0.4a |
Values followed by the same letter were not significantly different after the Tukey-Kramer test (P > 0.05).
The behavioural response of each methyl quinazoline was then tested only on fifth instar nymphs and females, which were attracted by the blend. Just 4-methylquinazoline seems to be responsible for the attractive effects of the quinazoline blend. In fact, 2,4-dimethylquinazoline did not elicit any significant effect neither on fifth instar nymphs nor females (P > 0.05) (Table 2 and Table 3). In contrast, when the effectiveness against T. infestans females was analysed, no significant attraction (P > 0.05) was observed for the individual components of the methylquinazoline mixture. Both compounds seem to be necessary to elicit a significant effect on females (Table 2 and Table 3).
Table 2.
Behavioural response of fifth stadium nymphs of T. infestans to 4-methyl quinazoline and 2,4-dimethyl quinazoline in an arena bioassay
| Behavioural response | Control | 4-methyl quinazoline (50 μg) |
2,4-dimethyl quinazoline (50 μg) |
|---|---|---|---|
| Positive recordings (%) | 25 | 65 | 35 |
| Mean number of visits (± SE) | 0.2 ± 0.1 a | 1.5 ± 0.2 b | 0.7 ± 0.3 a |
Values followed by the same letter were not significantly different after the Tukey-Kramer test (P > 0.05).
Table 3.
Behavioural response of females of T. infestans to 4-methyl quinazoline and 2,4-dimethylquinazoline in an arena bioassay
| Behavioural response | Control | 4-methyl quinazoline (50 μg) |
2,4-dimethyl quinazoline (50 μg) |
|---|---|---|---|
| Positive recordings (%) | 30 | 70 | 80 |
| Mean number of visits (± SE) | 0.2 ± 0.1 a | 1.0 ± 0.3 a | 0.9 ± 0.2 a |
Values followed by the same letter were not significantly different after the Tukey-Kramer test (P > 0.05).
Discussion
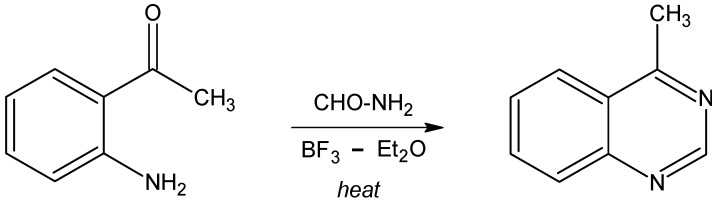
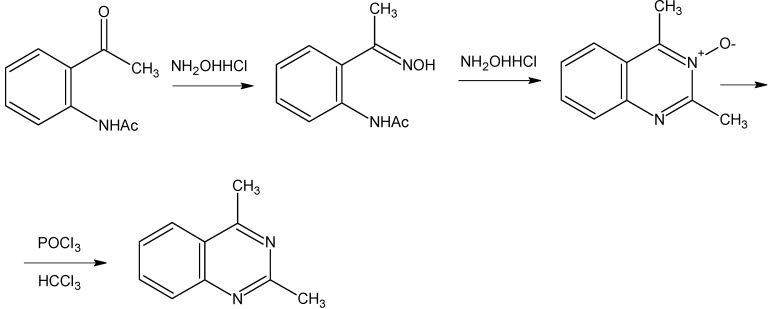
The preparation of 4-methylquinazoline was carried out by condensation of aminoacetophenone with formamide in the presence of boron trifluoride etherate as catalyst [10], as shown in Scheme 1. The reaction yield has been increased from 55% to 75% by increasing the amount of catalyst used and final purification by flash silica chromatography. Likewise, synthesis of 2,4-dimethylquinazoline was performed according to [11], as shown in Scheme 2. The corresponding N-oxide was prepared by condensation of the oxime of N-acetylaminoacetophenone with hydroxylamine hydrochloride, followed by reduction of this compound with phosphorus oxychloride or iron powder. In this case, the yields obtained in the different steps were similar to those previously reported [11].
Scheme 1.
Scheme 2.
Lorenzo et al. [1] demonstrated that every stadium of T. infestans was attracted to and arrested by their own faeces. In addition, it has been reported that the faeces release ammonia which elicited electrophysiological responses from the antennae of T. infestans [12] and attracted nymphs in a servosphere bioassay [3].
In this work the attractiveness of blends of the previously identified fecal components 4-methyl- and 2,4-dimethylquinazoline on fifth instar nymphs and females was established. 4-methylquinazoline seems to be responsible for the effects observed in nymphs. In contrast, for females and according to the test conditions, both compounds seem to be necessary to elicit this behaviour.
In concordance with the results previously reported by López and Morgan [4] we could not establish an attractant effect when these compounds were assessed at low doses. However, in that work [4], mature and the closer to maturity nymphal instars were studied. Differential responsiveness could be hypothesised to be a consequence of the physiological function of these compounds. Faeces have been pointed out as markers of T. infestans refuges [13]. These kinds of signals should be more relevant for more mature stadiums, notably females responsible for the colonisation process.
Synthetic 4-methyl and 2,4-dimethylquinazoline appear as promising compounds to be included in monitor and control devices currently under development for T. infestans, the most important vector of Chagas disease in Argentina and much of South America [9].
Experimental
General
All chemicals were analytical grade from Aldrich Co., Saint Louis, MO (USA) and used without further purification. NMR spectra were recorded on a Varian Gemini 200 spectrometer (USA).
Syntheses of 4-methylquinazoline and 2,4-dimethylquinazoline.
For the preparation of 4-methylquinazoline a modification of the reported method [10] was used (Scheme 1). Thus, a solution of 2-aminoacetophenone (3 g) in freshly distilled formamide (60 mL) containing boron trifluoride etherate (1 mL) was heated at 150°C for 6 hr, while the complete disappearance of the starting product was followed by TLC on silica gel eluting with a mixture of CH2Cl2-MeOH (95/5). The reaction mixture was cooled to room temperature, extracted with benzene (3 x 75 mL), dried (Na2SO4) and evaporated. The residue was purified by column chromatography on flash silica gel (35-75 mesh), eluting the reaction products first with CH2Cl2 and with 95:5 CH2Cl2-MeOH to give a yellow oil (2.4 g) with the expected spectral features of 4-methylquinazoline. Bulb-to-bulb distillation of this oil at 50-60 °C/0.5 torr. (lit. [4] 85 °C/1 torr) afforded a colourless oil which crystallised on standing in the refrigerator without solvent. The 1H-NMR spectrum agreed with the structure of the expected product: δ4 (CDCl3) 9.18 (1H, s), 8.12 (1H, d) 8.04 (1H, d), 7.9 (1H, t) 7.66 (1H, t) and 2.97 (3H, s).
2,4-Dimethylquinazoline was prepared according to [11] (Scheme 2). Thus, a solution of 2-aminoacetophenone (3 g) in acetic anhydride (30 mL) was heated at 50 °C for 30 min. Evaporation under reduced pressure afforded a solid residue which was dissolved in methylene chloride and washed three times with a saturated solution of sodium bicarbonate. The organic layer was dried (Na2SO4) and evaporated under reduced pressure to give 3.9 g of the expected acetate. Treatment as reported [11] of this acetate (8.4 g) with hydroxylamine hydrochloride afforded a 2:1 mixture of oxime and N-oxide, as deduced by 1H-NMR analyses. A mixture of this product (6.5 g) and hydroxylamine hydrochloride (2,4 g) in ethanol (50 mL) was refluxed for 3 hr, while the complete disappearance of the initially formed oxime was monitored by TLC on silica gel (eluting with a 98:2 mixture of CH2Cl2-MeOH). The solvent was removed under reduced pressure and the residue was filtered through a silica column (6 x 4 cm) eluting with 98:2 CH2Cl2-MeOH. After evaporation of the solvents the N-oxide of 2,4-dimethyl-quinazoline was obtained (4.4 g, 75% yield, mp 96-7 °C, lit. [11] 103 °C); 1H-NMR: δ (CDCl3) 7.92 (1H, d) 7.86 (1H, d) 7.72 (1H, t), 7.63 (1H, t) and 2.9 (s, 6H). For reduction of this N-oxide to 2,4-dimethylquinazoline, two different methods were used:
Method A: A mixture of N-oxide (1 g) and phosphorus oxychloride (1.8 mL) in chloroform (8 mL) was heated at reflux for 15 min. The deep red reaction mixture was allowed to cool and diluted with H2O (8 mL). After treatment with 20% aqueous NaOH solution to a basic pH and repeated extraction with chloroform, the combined organic extracts were washed with H2O, dried (Na2SO4) and evaporated under reduced pressure to yield a red oil (850 mg). TLC analysis of this oil revealed the absence of starting N-oxide and the presence of three products. Column chromatography (3 x 10 cm) with flash silica gel eluting with methylene chloride afforded a pure product (160 mg, 18%), identified by 1H-NMR as the desired 2,4-dimethylquinazoline (see below).
Method B. To a solution of N-oxide (4.5 g) in AcOH (27 mL) and H2O (4.5 mL) iron powder (1.4 g) was added and the mixture was stirred at 80°C for 20 h. The solvent was removed under reduced pressure and the residue was washed with aqueous sodium bicarbonate solution. The aqueous phase was repeatedly extracted with CH2Cl2 (5 x 20 mL). The combined organic extracts were dried (Na2SO4) and evaporated. The oily residue (4 g) was purified by column chromatography (4 x 15 cm) with flash silica gel, eluting with 9:1 CH2Cl2-n-hexane to give 2,4-dimethylquinazoline (2.5 g, 65%). Bulb to bulb microdistillation afforded a colourless oil which crystallized on standing in the refrigerator (m.p. 70 °C, lit. [10] 72 °C). 1H-NMR δ (CDCl3). 8.1 (1H, d) 7.95 (1H, d) 7.85 (1H, t), 7.58 (1H, t), 2.94(3H, s) and 2.86 (3H, s).
Biological material
Fifth instar nymphs, females and males of T. infestans were kept at the Centro de Investigaciones de Plagas e Insecticidas (Buenos Aires, Argentina) in Perspex® containers (25 x 25 x 25 cm) at 27 oC, 70% RH, and with a 12:12 (L:D) h photoperiod. The insects were fed on pigeon blood every seven days. They were starved for seven days before being utilised for bioassays.
Bioassay
The behavioral response of T. infestans to the synthetic compounds was tested using an open arena bioassay. Individual T. infestans were placed in a circular polypropylene arena (18 cm diameter, 15 cm high) with polished walls. The floor of the arena was covered with filter paper to allow unimpeded insect movement. Each arena was surrounded with matte black cardboard (20 cm high) to reduce the effect of extraneous light interfering with insect behaviour. The temperature in the arenas was maintained at 26°C and diffuse lighting was provided by a 40 W fluorescent tube placed below the arena.
An insect was placed in the arena and allowed to acclimatise for 30 minutes before the bioassay. In this time the insects typically adopted a thigmotactic behaviour, coming to rest in contact with the arena walls. Ten µL of polyethylene glycol containing the test compound(s) was then placed in the middle of the arena and the insect response monitored for 30 min. Ten µL of polyethylene glycol alone were used as control. Each test was replicated at least 12 times using a different insect in each replicate.
The insect behaviour was monitored during 30 min using a Videomex-V (Columbus, OH, USA) video recording system. Each arena was divided into a circular central zone (4 cm in diameter) and outer zones using Multiple Zone Motion Monitor software (Columbus, OH, USA).
The presence of the insect was identified as a white image over a black background (pixels ‘on’). To evaluate the approach of the insect to the source of compound, two parameters were analysed: (a) percentage of positive recordings, defined as the percent of independent replicates when the area occupied in the central zone during the bioassay was different from zero; (b) mean number of visits to the source, the total number of visits to the central zone divided by the number of replicates.
Acknowledgements
This investigation received financial support from the Agencia Nacional de Promoción de Ciencia y Técnica of Argentina, the Consejo Nacional de Investigaciones Científicas y Técnicas of Argentina (CONICET) and the European Communities Programme on Scientific and Technical Cooperation with Developing Countries. The authors also thank Dra. Delmi Canale (Insectario del Servicio Nacional de Chagas, Córdoba, Argentina) for supplying the insects; the Editor and two anonymous reviewers for their suggestions and comments, that helped us to improve this article.
Footnotes
Sample Availability: Contact the authors.
References
- 1.Lorenzo Figueras A.N., Kenigsten A., Lazzari C.R. Aggregation in the haematophagous bug Triatoma infestans: Chemical signals and temporal pattern. J. Insect Physiol. 1994;40:311–316. [Google Scholar]
- 2.Schofield C.J., Patterson J.W. Assembly pheromone of Triatoma infestans and Rhodnius prolixus nymphs (Hemiptera: Reduviidae) J. Med. Entomol. 1977;13:727–734. doi: 10.1093/jmedent/13.6.727. [DOI] [PubMed] [Google Scholar]
- 3.Taneja J., Guerin P.M. Ammonia attracts the haematophagous bug Triatoma infestans: behavioral and neurophysiological data on nymphs. J. Comp. Physiol. A. 1997;181:21–34. doi: 10.1007/s003590050089. [DOI] [Google Scholar]
- 4.López L.C., Morgan E.D. Chemical investigation of aggregation behavior of Triatoma bugs (Hemiptera: Reduviidae) J. Chem. Ecol. 1995;21:2069–2078. doi: 10.1007/BF02033863. [DOI] [PubMed] [Google Scholar]
- 5.Alzogaray R.A. Unpublished results.
- 6.[WHO] - World Health Organisation [WHO] Tropical Disease Research: Progress 1995-1996. Thirteenth programme report of the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical diseases. World Health Organisation; Geneva: 1997. [Google Scholar]
- 7.Schofield C.J. Triatominae: Biología y Control. Eurocommunica Publications; Bognor Regis (U.K.): 1994. [Google Scholar]
- 8.Wisnivesky-Colli C., Paulone Y., Pirez A., Chuit R., Gualtieri J., Solarz N., Smith A., Segura E. A new tool for continuo detection of the presence of Triatominae bugs, vectors of Chagas disease, in rural households. Medicina. 1987;47:45–50. [PubMed] [Google Scholar]
- 9.Zerba E.N. Chemical control of Chagas disease vectors. Biom. Environ. Sci. 1989;2:24–29. [PubMed] [Google Scholar]
- 10.Byford A., Goadby P., Hooper M., Kamath H.V., Kulkarni S.N. O-Aminophenyl alkyl-aralkyl ketones and their derivatives. Part V. An efficient synthetic route to some biologically active 4-substitued quinazolines. Indian J. Chem. 1988;27:396–397. [Google Scholar]
- 11.Kovendi A., Kircz M. New synthesis for quinazoline N3-oxides and 1,2-dihydroquinazoline N3-oxides. Chem. Berichte. 1965;98:1049–1059. [Google Scholar]
- 12.Bernard J. Thesis. Univ. Rennes; France: 1974. Etude électrophysiologique de récepteurs impliqués dans l′orientation vers l′hote et dans l′acte hématophage chez un hémiptere: Triatoma infestans. [Google Scholar]
- 13.Lorenzo M.G., Lazzari C.R. The spatial pattern of defecation in Triatoma infestans and the role of faeces as a chemical mark of the refuge. J. Insect Physiol. 1996;42:903–907. [Google Scholar]