Abstract
Background: IgA nephropathy (IgAN) is the most common primary glomerulonephritis and an important cause of end-stage kidney disease. Unlike the slowly progressive course seen among Caucasian and East Asian subjects (actuarial survival 80-85% over 10 years), in India about 30-40% of patients have nephrotic syndrome and renal dysfunction at presentation and a 10-year renal survival of 35%, as reported from a retrospective registry. These observations cannot be entirely attributed to a lack of uniform screening protocols or late referral and attest to the probability that IgAN may not be the same disease in different parts of the world.
Methods: We will prospectively recruit 200 patients with IgAN (the GRACE IgANI— Glomerular Research And Clinical Experiments- Ig A Nephropathy in Indians—cohort) and stratify them into low and high risk of progression based on published absolute renal risk scores. We will test the validity of this risk score in an unselected Indian IgAN population over a 5-year follow-up period. In parallel, we will undertake extensive exploratory serum, urine, renal and microbiome biomarker studies, firstly, to determine if the underlying pathogenic pathways are the same in Indian IgAN compared to those reported in Caucasian and East Asian IgAN. Secondly, we will systematically assess the value of measuring selected biomarkers and adding this data to traditional measures of risk in IgAN to predict kidney failure. We ultimately hope to generate a composite IgAN risk score specific for the Indian population.
Ethics and data dissemination: Approval was obtained from the Institutional Review Board (Silver, Research and Ethics Committee) of the Christian Medical College, Vellore, India (Ref. No. IRB Min. No. 8962 [Other] dated 23.07.2014 and IRB Min. No. 9481 [Other] dated 24.06.2015). It is anticipated that results of this study will be presented at national and international meetings, with reports being published from late 2018.
Keywords: Immunology, Nephrology, IgA nephropathy, Glomerulonephritis, Pathology, South-Asians, Indians, Epidemiology, Protocol
Introduction
Global burden of IgA Nephropathy (IgAN)
IgAN is a frequent cause of end-stage kidney disease (ESKD) in both White and Asian populations. By contrast, IgAN has rarely been detected in Black populations, either from the United States or Africa. The prognosis of patients with normal renal function and proteinuria <1 g per day is excellent, with one study reporting 98% renal survival over 15 years 1. By contrast, 15–25% of patients develop ESKD over 20 years if there is significant proteinuria (>1 g per day) and existing evidence of renal dysfunction 2.
What is known in South Asia and India
Incidence of glomerular disease in the tropics is much greater than in temperate countries. Nephrotic syndrome is 60–100 times more common in India than in the UK and USA 3. Glomerular diseases are often a consequence of interaction between an environmental nephritogenic antigen and a genetically susceptible host. Hence, the high rate of antigenic exposure in the tropics could account for this high incidence of glomerulonephritis. There is no national glomerulonephritis registry in India. Most epidemiological studies are single- or multi-centre retrospective analyses of renal biopsy datasets.
The most common presentation of primary glomerulonephritis in various studies in India is nephrotic syndrome and renal impairment ( Table 1). Asymptomatic urinary abnormalities are reported far less frequently (1.7–9%) 4, 5 when compared to data from registries in other developed nations like Japan and Italy 6, 7. This is perhaps not surprising as there is no universal urine screening program in India, precluding detection of the disease at an early asymptomatic stage. IgAN is the most common primary glomerulonephritis in India, identified in 10–15% of all renal biopsies reported from our institution and elsewhere in India 4, 5, 8, 9. Retrospective studies from renal registries in India report that 30–40% of patients with IgAN have nephrotic syndrome and renal dysfunction at presentation 4, 9– 11. Indian patients present with IgAN approximately over a decade earlier than Caucasian and East Asian patients, whose average age at presentation is 35–40 years 12– 14. These observations cannot be entirely attributed to differences in access to primary care and country-specific screening programs for kidney disease, and attest to the likelihood that IgAN may not be the same disease in different parts of the world.
Table 1. Renal biopsy studies from India.
| Studies | Renal
biopsies, N |
Period, years | IgAN, n (%) | Nephrotic
syndrome, % |
Nephritic
syndrome (%) |
Hypertension, % | Renal
dysfunction, % |
|---|---|---|---|---|---|---|---|
| Bhuyan
8 (1992)
(Delhi, North India) |
1146 | 83 (7.2) | 24 | NA | 39 | 34 | |
| Sehgal
16 (1995)
(Chandigarh, North India) |
106 | - | 11 (10.4) | 11 | NA | NA | NA |
| Muthukumar
11 (2002)
(Chennai, South India) |
NA* | 98 (NA) | 25.6 | 5.1 | 9.2 | 13.5 | |
| Narasimhan
4,
17 (2006)
(CMC Vellore, South India) |
5415 | 1986–2002 | 478 (8.6) | 55 | 16 | 58 | 60 |
| Vanikar
18 (2005)
(Gujarat, Western India) |
4132 | 1998–2004 | 120 (16.2) | NA | NA | NA | NA |
| Chandrika
19 (2007)
(Kerala, South India) |
1592 | 2 years | 227 (14.3) | 36.7 | 18.9 | 3.5 | 5.7 |
| Das
5 (2011)
(Hyderabad, Central India) |
1849 | 1990–2008 | 81 (4.4) | 44.4 | 21 | 65.4 | 39 |
| Siddappa
9 (2013)
(Mangalore, South India) |
400 | 2007–2010 | 31 (7.8) | 35.5 | NA | 45.2 | 38.7 |
| Jeganathan
20 (2013)
Mangalore, South India |
75 | 2 years | 12 (16) | 0 | 83.3 | 16.7 | 0 |
| Golay
21 (2013)
Eastern India |
666 | 2010–2012 | 54 (8.11) | 6.09 | 9.23 | - | 10 |
NA, not applicable.
George et al. 15, at Christian Medical College, Vellore, India (CMC Vellore), reported IgAN in 9.6% of 649 adults with primary glomerulonephritis. Hypertension was detected in 51.6% of patients and renal failure in 32.3%. Follow up was possible in 61.3% for a mean period of 17.3 months and progression to ESKD was noted in 7.9% 15. Muthukumar et al. (Chennai, South India) showed in their cohort of 98 patients that five-year renal survival was 38.5% (CI 24.6%–52.3%) 11.
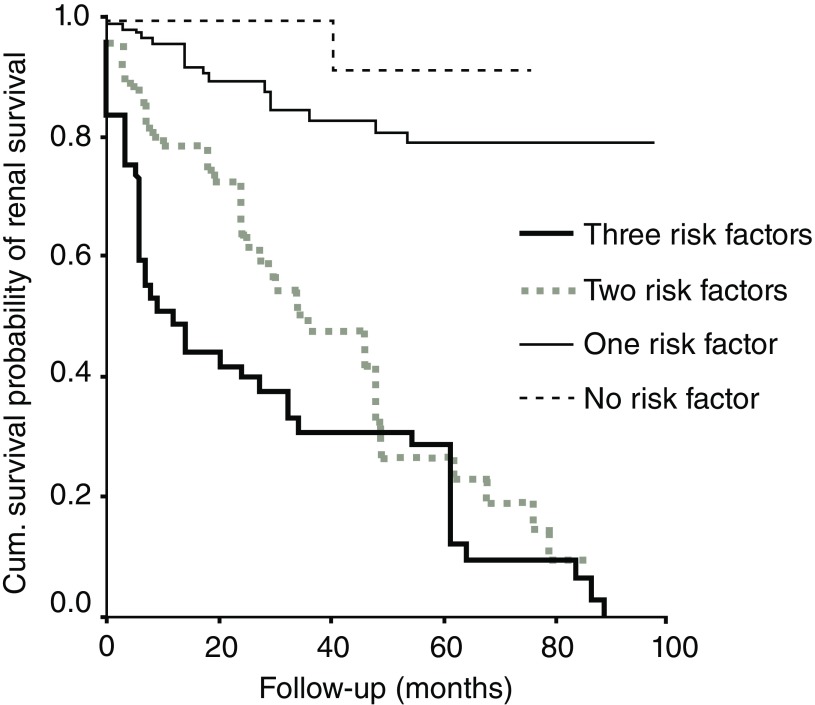
Chacko et al. (CMC Vellore) reported that four factors were significantly associated with ESKD. These were hypertension, nephrotic range proteinuria, degree of sclerosed glomeruli, and interstitial fibrosis at presentation. The mean renal survival time of patients without any of these was 132 months, whereas those with one, two, or three risk factors were 105, 47, and 28 months, respectively ( Figure 1). A total of 46% of patients either progressed to ESKD or had permanent decline in renal function at last follow-up. Overall renal survival at 10 years was only 35% when compared with nearly 90% in similar studies from Singapore, Australia, and France. Also, patients diagnosed from the year 2000 onward had poorer outcomes, than those diagnosed before the year 2000 22, 23.
Figure 1. IgA nephropathy in India is associated with poorer renal survival.
Risk factors: hypertension, proteinuria, interstitial fibrosis, sclerosed glomeruli. Figure has been reproduced with permission from Chacko et al. 23 under a CC-BY-NC-SA license.
Traditional prognostic factors in IgAN
Persistent proteinuria, hypertension, and an increased serum creatinine level at presentation are the strongest clinical predictors of progression in IgAN 1, 2, 24. The Oxford histological classification of IgAN was developed by the International IgA Nephropathy Network in collaboration with the Renal Pathology Society in 2009 25, 26. Retrospective analysis of renal biopsies from 265 patients identified four variables that, independent of one another and clinical features, were correlated with renal outcome: mesangial hypercellularity, segmental sclerosis, endocapillary hypercellularity and tubular atrophy/interstitial fibrosis (MEST) 25, 26. The prognostic power of the Oxford classification has subsequently been validated in a number of different IgAN cohorts from across the globe 27– 29. In 2017, the Oxford classification was amended to include a C (MEST-C) score to highlight the significance of crescents in IgAN 30.
A prediction score called the ‘absolute renal risk score’ (ARR), uses clinical measures and the Oxford classification to estimate the 5-year risk of developing ESKD in IgAN 31. This risk score was developed by Berthoux 32 and validated in Japan with a cohort of 702 IgAN patients 31, but has not been studied in an Indian population, who have a more rapid rate of renal decline.
The pathogenesis of IgAN: A Caucasian perspective
Studies of IgAN have consistently identified changes in the O-glycans attached to the hinge region of the IgA1 immunoglobulin heavy chain 33– 35. These changes are present in both serum and mesangial IgA1 34. This reduction in the number of O-linked galactose residues at the IgA1 hinge has been detected using specific lectin binding assays, fluorophore-assisted carbohydrate electrophoresis and mass spectrometry 36. These IgA1 molecules have been shown to activate human mesangial cells in vitro 37 resulting in the generation of IgG and IgA1 anti-hinge region autoantibodies and circulating immune complexes that leads to activation of the complement system through the lectin and alternative pathways 38, 39. Patients with IgAN have higher levels of poorly galactosylated IgA1 in their serum than healthy subjects 40, 41, and these levels appear to correlate with a higher likelihood of developing progressive renal failure 42. In a study of 97 patients with IgAN of varying severity, titres of IgG anti-hinge region autoantibodies correlated with both the ARR and the risk of ESKD or death 43. There is also an emerging role for circulating secretory IgA in IgAN, with recent studies reporting that secretory IgA can bind to and activate human mesangial cells more strongly than non-secretory IgA 44, 45. Recent data show that IgAN patients have altered fecal microbiota and volatile organic compounds (VOC) profiling, which in turn differed between progressors and non-progressors 46.
There is clear evidence of complement activation within the glomerulus in IgAN, with 60–70% of all IgAN biopsies demonstrating C3 deposition in the mesangium. There is evidence from both immunohistochemical and genetic studies for both activation of the alternative and lectin pathways in IgAN, but little evidence for classical pathway activation 38, 39. Roos et al. examined 60 renal biopsies of IgAN patients and found mannose binding lectin (MBL), a key component of the lectin pathway, co-deposition in 15 (25%) of cases. MBL deposition was associated with more severe renal disease 47.
In this study, we will assess the correlation between clinical and histological features, and measures of immunological activation in a cohort of Indian patients with IgAN. We will use our understanding of the pathogenesis of IgAN in Caucasians and East Asians to determine whether factors such as serum levels of secretory IgA, IgA immune complexes and IgG anti-hinge region autoantibodies can be used to risk stratify patients and monitor response to immunotherapy in an Indian population.
The Kidney Disease Improving Global Outcomes (KDIGO) guidelines on glomerulonephritis 48 do not recommend immunosuppression in most patients with IgAN. However, considering the severe presentation and progression of IgAN in India, the standard protocol for managing patients with IgAN at CMC Vellore is to treat with immunosuppression in those who have a urinary protein excretion >1 g/day with/without renal dysfunction along maximally tolerated inhibition of the renin-angiotensin system (RAS). We give oral prednisolone at 2 mg/kg alternate days (max. 120 mg) for 3 months and then taper and stop over the next 3 months. Mycophenolic acid is added as a second immunosuppressor at 25–30 mg/kg, with monitoring of area under the curve (target 30–50 mg.h/l), if they are persistently nephrotic with or without renal dysfunction and if the therapy is affordable to the patient. The treatment dilemma in IgAN has been highlighted recently 49 and we will carefully monitor the effects of immunosuppression in this cohort.
Protocol
We hypothesise that the natural history and risk of renal progression in Indian patients with IgAN differs from that widely reported for Caucasian and East Asian patients. This may be due to differences in the underlying pathogenic pathways operating in Indian patients. A completed STROBE reporting checklist is available ( Supplementary File 1).
Primary objective
To measure exploratory biomarkers in an incident Indian population with IgAN and look for associations with clinical outcomes at 2 and 5 years of follow-up.
Secondary objectives
To measure exploratory biomarkers in an incident Indian population of IgAN and compare these findings with biomarker studies in Caucasian and East Asian IgAN populations to determine whether pathogenic pathways are similar across ethnicities.
To determine the impact of immunosuppressive agents on the levels of selected biomarkers in an Indian IgAN population.
To develop renal risk score for Indian patients with IgAN to help counsel patients at the time of diagnosis and direct health resources and immunosuppressive therapy to those patients at greatest risk of ESKD.
Patient recruitment
The study will be conducted in the Department of Nephrology, CMC Vellore. CMC Vellore is the largest private not-for-profit tertiary referral hospital in South India and caters to patients mainly from South, North and North-East India. All patients being planned for a renal biopsy at CMC Vellore will be screened and all eligible patients will be approached to participate in the study. The inclusion and exclusion criteria are given in Table 2. These criteria apply to all IgAN and non-IgAN participants. All consenting patients will be recruited prior to the renal biopsy procedure. IgA nephropathy will be diagnosed by the presence of IgA-dominant or co-dominant immune deposits within glomeruli, as shown by immunofluorescent staining of the renal biopsy tissue 25. The non-IgAN kidney disease control arm will be patients with biopsy-proven primary glomerulonephritis other than IgAN. The healthy control group will be matched for age and gender to the IgAN cohort and will be voluntary kidney donors on work-up for kidney donation. Both arms will be matched in terms of sample size. The Caucasian and East Asian patient cohorts will be obtained from the University of Leicester, IgA nephropathy lab database, and the main collaborator for this work is J.B. Details concerning enrollment and ethical approval of this arm have been previously published 50. Some of the initial biomarkers we propose to test in sera are levels of galactose-deficient IgA1, immune complexes, antibody to galactose-deficient IgA1, secretory IgA, immune cell markers among other exploratory biomarkers to be determined during the study. The renal tissue will also be stained for components of the alternative and lectin pathways and for IgA1 components. The study started patient recruitment in March 2015 and ended at the last quarter of 2017. All patients are being followed up prospectively.
Table 2. Inclusion and exclusion criteria for screening patient eligibility prior to recruitment into the GRACE-IgANI cohort study.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Age ≥18 years | Secondary IgA nephropathy: e.g. due to lupus, liver cirrhosis, Henoch-Schonlein
purpura. |
| Primary IgAN diagnosed by renal biopsy | Glomerular filtration rate as estimated by the CKD-EPI equation <10 ml/min/1.73 m 2. |
| Immunosuppression naive for three
months prior to recruitment |
Patients with systemic diseases that can affect the kidneys like diabetes, systemic
lupus erythematosus, presence of HIV, HBsAg, HCV infections, malignancies etc. |
| Willing to come for follow-up visits | Patients with a history of psychological illness or condition which interferes with
their ability to understand or comply with the requirements of the study. |
IgAN, IgA nephropathy; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus.
Outcome measures
Primary outcome
The primary outcome is the evaluation of a range of aforementioned exploratory serum, urine and renal biomarkers and the investigation of their association to the rate of fall in estimated glomerular filtration rate (eGFR) at 2 and 5 years in a low- and high-risk incident Indian IgAN population as defined by ARR scores of <23 and of ≥23 points, respectively, at baseline and for the whole cohort.
Secondary outcomes
-
1.
Evaluation of a range of exploratory serum, urine and renal biomarkers, and to investigate their association to a composite end-point of at-least 50% decline in eGFR and ESKD (eGFR <15 ml/min/1.73 m 2), renal replacement therapy (RRT) or death at 2 and 5 years in a low- and high-risk incident Indian IgAN population as defined by an ARR score of <23 and of ≥23 points, respectively, at baseline and for the whole cohort.
-
2.
To determine the validity of the ARR to predict either the rate of fall in eGFR or a composite end-point of at least 50% decline in eGFR and ESKD (eGFR <15 ml/min/1.73 m 2), RRT or death at 2 and 5 years in an unselected incident Indian population with IgAN.
-
3.
To compare exploratory biomarkers in an incident Indian population of IgAN and compare these findings with biomarker studies in Caucasian and East Asian IgAN populations to determine whether pathogenic pathways are similar across ethnicities.
-
4.
To determine whether the addition of novel biomarker measurements can improve the predictive performance of an RRS in an Indian IgAN population.
-
5.
To determine whether selected biomarker levels measured at baseline and serially during follow-up are influenced by the use of immunosuppression.
-
6.
To develop an RRS for Indian patients with IgAN.
Sample size
Patients will be classified as being at low risk of progressive renal disease if their ARR score is <23 points or high risk if their ARR score of ≥23 points at baseline 31. Based on previous studies in India, we have assumed that the frequency of rapid progressors (>5 ml/min/1.73 m 2/year fall in eGFR) in the low-risk group will be 5%, and in the high-risk group will be 20% 17, 23. So, 176 patients give a power of 80% with an alpha error of 5% for detecting significant difference between proportions. Considering a 15% dropout rate (non-compliance to follow-up visits, unplanned pregnancies), 200 patients were recruited prospectively over 2.5 years (from March 2015 until the last quarter of 2017). We will look for significant differences among the exploratory biomarkers between the two risk groups and as independent variables to predict progressive decline in renal function and a composite end-point of at least 50% decline in eGFR and ESKD (eGFR <15 ml/min/1.73 m 2), renal replacement therapy (RRT) or death. We would like to find the best cut-off of the ARR score using received operating characteristic (ROC) curve methods. The best threshold will be obtained using likelihood ratio statistics, that is, by aiming to have high specificity with a high positive predictive value. However, the utility of the ROC will be decided based on the AUC. As part of the study apart from evaluating whether a different ARR threshold improves precision, we will also study whether addition of new biomarkers to an optimised Indian RRS improves the predictive value in an Indian population—this will include the C score of the new MEST-C score and exploratory biomarkers measured at baseline. At the same time, we will evaluate the accuracy in the Caucasian cohort and determine the additive value of additional measures to help predict Caucasian and Indian risk separately. In order to validate the usefulness of the threshold, another prospective validation cohort with a known threshold will be set up and followed to study the prognosis. However, in order to establish reliable CIs for the validity statistics bootstrap method (with 10,000 resampling) will be done in this cohort. We will include an equivalent number of healthy and disease controls to match the sample size of IgA patients. They will not be followed up longitudinally and will only be included in cross-sectional analyses at baseline.
Study procedures and timeline
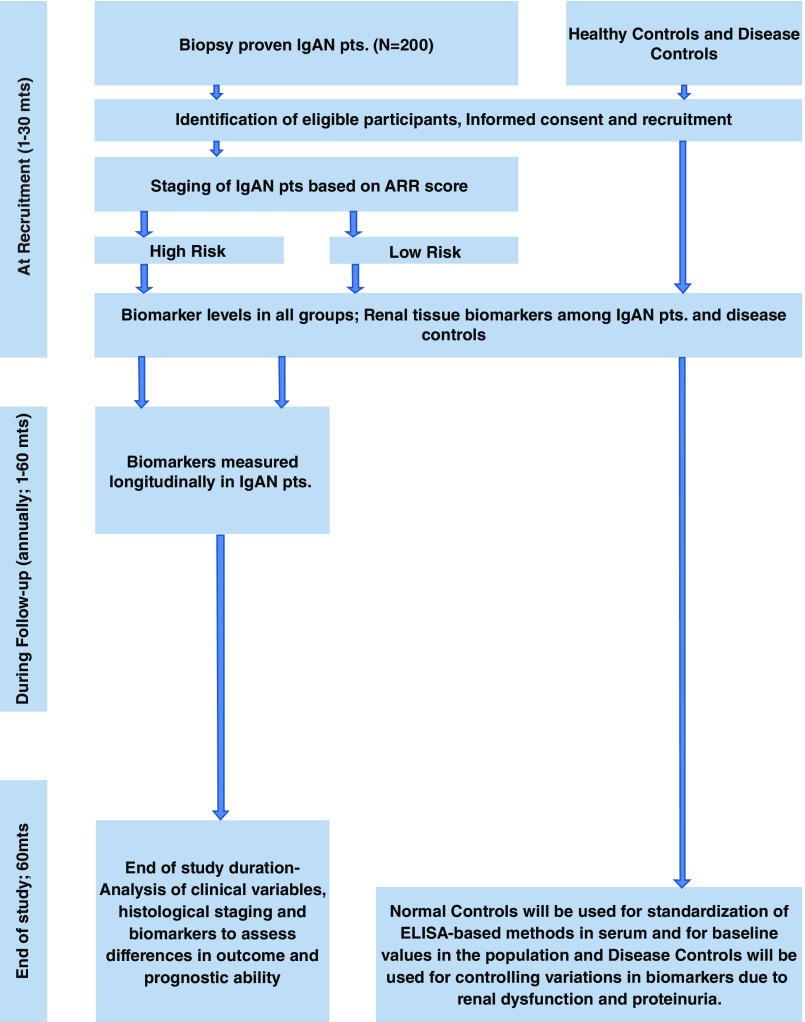
This is a prospective longitudinal cohort study with consecutive recruitment of incident IgAN patients. The baseline assessments will include the collection of demographic details, a physical examination, standard clinical laboratory tests and collection of biological samples (DNA, serum, plasma, renal tissue, urine and faeces) for future biomarker and microbiomic analysis 46. Patients will be followed at least annually (usually 2–3 visits per year) and at each visit standard laboratory testing, alongside the collection of biological samples for exploratory analysis, will be undertaken. The study co-ordinators will also contact patients telephonically or through postal/email correspondence to assess their adherence to medications, optimise the doses of RAS blockers according to the self-reported blood pressure and renal function tests. There will be a pre-specified interim analysis of the cohort once all patients have completed 2 years of follow-up. All patients will be followed-up for 5 years. Timelines for recruitment and follow-up visits are summarized in Figure 2.
Figure 2. Flow diagram for recruitment and follow-up visits.
Statistical analysis
Demographic data on patients will be gathered and suitable comparisons will be made to ensure that the groups are well-matched. All outcome measures will undergo appropriate omnibus tests to investigate statistical differences between groups and within groups. Risk factors for progressive disease in an Indian population will be evaluated using a Cox proportional hazard model with a stepwise backward elimination method and then compared with those comprising the ARR score derived from a Japanese cohort. To create a renal prediction risk score specifically for Indian IgAN (either a modified ARR or completely new score), incorporating measures of exploratory biomarkers, the score for each variable will be weighted by the regression coefficients calculated using the relevant Cox model. In addition to this, we will look at associations between outcome measures and investigate potential interactions between variables to generate hypotheses for future studies. All statistical analyses will be done using SPSS version 21 and GraphPad (Prism) version 7.
Patient and public involvement
This observational study has been designed based on the retrospective data from our institute 22, 23 and the felt need of all stakeholders for urgent research in this area. The patients and the public were not involved in the design or conduct of this study, but they are the intended benefactors of this research. The results will be disseminated electronically to all participants in the study.
Strengths and limitations
Strengths of this protocol include the fact that this is a prospective longitudinal design with consecutive recruitment, reducing recruitment bias and enabling longitudinal biomarker evaluations. To our knowledge, this is also the first South Asian IgAN cohort to be established. In addition, bio-banking of clinical samples to a standardised protocol for future research will enable us to test emerging hypotheses on a well-established cohort.
Limitations of this protocol include the fact that pediatric (those aged <18 years) cases are excluded and limits the results interpretation to only adult patients. Additionally, participants with advanced kidney disease (eGFR <10 ml/min/1.73 m 2) are excluded so study conclusions may not reflect this subset of individuals.
Ethics and dissemination
The full protocol was presented to the Institutional Review Board (Silver, Research and Ethics Committee) of the Christian Medical College, Vellore, India. Approval was provided vis. Ref. No. IRB Min. No. 8962 [Other] dated 23.07.2014 and Ref. No. IRB Min. No. 9481 [Other] dated 24.06.2015.
The study was submitted for external peer review as part of an application to the Wellcome Trust/DBT India Alliance and the lead author was awarded the Wellcome Trust/DBT India Alliance Early Career Fellowship [grant number IA/CPHE/14/1/501501].
Written informed consent has been obtained from participants in line with recommendations set out in E6(R1) of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Good Clinical Practice guidelines. Original consent forms will be stored, and records and data will be managed to ensure confidentiality per the recommendations of the Indian Council of Medical Research (ICMR) Ethical Guidelines for Biomedical Research on Human Subjects. Material transfer agreement to the collaborators’ laboratory has been approved by the ICMR.
Dissemination plan
We plan to present all data at national and international conferences. Furthermore, we will also publish the findings in international journals. We expect to publish our initial findings during late 2018. Following the publication of our results, anonymised participant level data will be available for collaborations, on request. The GRACE-IgANI study is registered with the ISRCTN ( ISRCTN36834159) and the registration status is retrospective as patient recruitment started before registration.
Data availability
All data underlying the results are available as part of the article and no additional source data are required.
Acknowledgements
We acknowledge Prof. Gagandeep Kang (fellowship sponsor for grant number IA/CPHE/14/1/501501 awarded to SA); Prof. Jayaprakash Muliyil (statistical review at project conception); and Rajanbabu Franklin and Laisa Arul Gladis (associate research officers in the project). This trial has been registered with an International Standard Randomised Controlled Trial Number: ISRCTN36834159.
Funding Statement
This work is supported by the Wellcome Trust/DBT India Alliance Fellowship (grant number 501501). S.A. is a Wellcome Trust/DBT India Alliance Early Career Fellow (Clinical and Public Health). Initial small funding was by a major fluid research internal grant [8962 (Other) dated 23.07.2014] from Christian Medical College, Vellore-632004, India.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved]
Supplementary material
Supplementary File 1. Completed STROBE checklist for this study.References
- 1. Bailey RR, Lynn KL, Robson RA, et al. : Long term follow up of patients with IgA nephropathy. N Z Med J. 1994;107(976):142–4. [PubMed] [Google Scholar]
- 2. Alamartine E, Sabatier JC, Guerin C, et al. : Prognostic factors in mesangial IgA glomerulonephritis: an extensive study with univariate and multivariate analyses. Am J Kidney Dis. 1991;18(1):12–9. 10.1016/S0272-6386(12)80284-8 [DOI] [PubMed] [Google Scholar]
- 3. Chugh KS, Sakhuja V: Glomerular diseases in the tropics. Am J Nephrol. 1990;10(6):437–50. 10.1159/000168167 [DOI] [PubMed] [Google Scholar]
- 4. Narasimhan B, Chacko B, John GT, et al. : Characterization of kidney lesions in Indian adults: towards a renal biopsy registry. J Nephrol. 2006;19(2):205–10. [PubMed] [Google Scholar]
- 5. Das U, Dakshinamurty KV, Prayaga A: Pattern of biopsy-proven renal disease in a single center of south India: 19 years experience. Indian J Nephrol. 2011;21(4):250–7. 10.4103/0971-4065.85482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nationwide and long-term survey of primary glomerulonephritis in Japan as observed in 1,850 biopsied cases. Research Group on Progressive Chronic Renal Disease. Nephron. 1999;82(3):205–13. 10.1159/000045404 [DOI] [PubMed] [Google Scholar]
- 7. Gesualdo L, Di Palma AM, Morrone LF, et al. : The Italian experience of the national registry of renal biopsies. Kidney Int. 2004;66(3):890–4. 10.1111/j.1523-1755.2004.00831.x [DOI] [PubMed] [Google Scholar]
- 8. Bhuyan UN, Dash SC, Srivastava RN, et al. : IgA associated glomerulonephritis. J Assoc Physicians India. 1992;40(5):310–3. [PubMed] [Google Scholar]
- 9. Siddappa S, Kowsalya R, Mythri KM: IgA nephropathy in a tertiary care center from south India. Indian J Nephrol. 2011;21(4):230–4. 10.4103/0971-4065.82635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mittal N, Joshi K, Rane S, et al. : Primary IgA nephropathy in north India: is it different? Postgrad Med J. 2012;88(1035):15–20. 10.1136/postgradmedj-2011-130077 [DOI] [PubMed] [Google Scholar]
- 11. Muthukumar T, Fernando ME, Jayakumar M: Prognostic factors in immunoglobulin-A nephropathy. J Assoc Physicians India. 2002;50:1354–9. [PubMed] [Google Scholar]
- 12. Prakash S, Kanjanabuch T, Austin PC, et al. : Continental variations in IgA nephropathy among Asians. Clin Nephrol. 2008;70(5):377–84. 10.5414/CNP70377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Berthoux FC, Mohey H, Afiani A: Natural history of primary IgA nephropathy. Semin Nephrol. 2008;28(1):4–9. 10.1016/j.semnephrol.2007.10.001 [DOI] [PubMed] [Google Scholar]
- 14. Hall YN, Fuentes EF, Chertow GM, et al. : Race/ethnicity and disease severity in IgA nephropathy. BMC Nephrol. 2004;5:10. 10.1186/1471-2369-5-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. George J, Ninan VT, Thomas PP, et al. : Primary IgA nephropathy in adults. J Assoc Physicians India. 1993;41(8):489–91. [PubMed] [Google Scholar]
- 16. Sehgal S, Datta BN, Sakhuja V, et al. : Primary IgA nephropathy: a preliminary report. Indian J Pathol Microbiol. 1995;38(3):233–7. [PubMed] [Google Scholar]
- 17. Balakrishnan N, John GT, Korula A, et al. : Spectrum of biopsy proven renal disease and changing trends at a tropical tertiary care centre 1990 – 2001. Indian J Nephrol. 2013;13:29–35. Reference Source [Google Scholar]
- 18. Vanikar AV, Kanodia KV, Patel RD, et al. : Primary IgA nephropathy in Western India. Indian J Nephrol. 2005;15:227–231. Reference Source [Google Scholar]
- 19. Chandrika BK: IgA nephropathy in Kerala, India: a retrospective study. Indian J Pathol Microbiol. 2009;52(1):14–6. 10.4103/0377-4929.44954 [DOI] [PubMed] [Google Scholar]
- 20. Jeganathan J, Kumar S, Khalid M, et al. : Pattern of glomerular diseases in a tertiary care center in South India: a prospective study. Saudi J Kidney Dis Transpl. 2013;24(1):168–71. 10.4103/1319-2442.106363 [DOI] [PubMed] [Google Scholar]
- 21. Golay V, Trivedi M, Abraham A, et al. : The spectrum of glomerular diseases in a single center: A clinicopathological correlation. Indian J Nephrol. 2013;23(3):168–75. 10.4103/0971-4065.111833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chacko B, John GT, Neelakantan N, et al. : Presentation, prognosis and outcome of IgA nephropathy in Indian adults. Nephrology (Carlton). 2005;10(5):496–503. 10.1111/j.1440-1797.2005.00445.x [DOI] [PubMed] [Google Scholar]
- 23. Chacko B, John GT, Neelakantan N, et al. : Primary IgA Nephropathy: A ten-year analysis on the renal outcomes and a model for estimating risk of progression. Indian J Nephrol. 2004;14:163–71. Reference Source [Google Scholar]
- 24. Reich HN, Troyanov S, Scholey JW, et al. : Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol. 2007;18(12):3177–83. 10.1681/ASN.2007050526 [DOI] [PubMed] [Google Scholar]
- 25. Working Group of the International IgA Nephropathy Network and the Renal Pathology Society, Roberts ISD, Cook HT, et al. : The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009;76(5):546–56. 10.1038/ki.2009.168 [DOI] [PubMed] [Google Scholar]
- 26. Working Group of the International IgA Nephropathy Network and the Renal Pathology Society, Cattran DC, Coppo R, et al. : The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76(5):534–45. 10.1038/ki.2009.243 [DOI] [PubMed] [Google Scholar]
- 27. Shi SF, Wang SX, Jiang L, et al. : Pathologic predictors of renal outcome and therapeutic efficacy in IgA nephropathy: validation of the oxford classification. Clin J Am Soc Nephrol. 2011;6(9):2175–84. 10.2215/CJN.11521210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alamartine E, Sauron C, Laurent B, et al. : The use of the Oxford classification of IgA nephropathy to predict renal survival. Clin J Am Soc Nephrol. 2011;6(10):2384–8. 10.2215/CJN.01170211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Herzenberg AM, Fogo AB, Reich HN, et al. : Validation of the Oxford classification of IgA nephropathy. Kidney Int. 2011;80(3):310–7. 10.1038/ki.2011.126 [DOI] [PubMed] [Google Scholar]
- 30. Haas M, Verhave JC, Liu ZH, et al. : A Multicenter Study of the Predictive Value of Crescents in IgA Nephropathy. J Am Soc Nephrol. 2017;28(2):691–701. 10.1681/ASN.2016040433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tanaka S, Ninomiya T, Katafuchi R, et al. : Development and validation of a prediction rule using the Oxford classification in IgA nephropathy. Clin J Am Soc Nephrol. 2013;8(12):2082–90. 10.2215/CJN.03480413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Berthoux F, Mohey H, Laurent B, et al. : Predicting the risk for dialysis or death in IgA nephropathy. J Am Soc Nephrol. 2011;22(4):752–61. 10.1681/ASN.2010040355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Coppo R, Amore A: Aberrant glycosylation in IgA nephropathy (IgAN). Kidney Int. 2004;65(5):1544–7. 10.1111/j.1523-1755.2004.05407.x [DOI] [PubMed] [Google Scholar]
- 34. Julian BA, Novak J: IgA nephropathy: an update. Curr Opin Nephrol Hypertens. 2004;13(2):171–9. [DOI] [PubMed] [Google Scholar]
- 35. Suzuki H, Moldoveanu Z, Hall S, et al. : IgA nephropathy: characterization of IgG antibodies specific for galactose-deficient IgA1. Contrib Nephrol. 2007;157:129–33. 10.1159/000102454 [DOI] [PubMed] [Google Scholar]
- 36. Pouria S, Barratt J: Secondary IgA nephropathy. Semin Nephrol. 2008;28(1):27–37. 10.1016/j.semnephrol.2007.10.004 [DOI] [PubMed] [Google Scholar]
- 37. Kim MJ, McDaid JP, McAdoo SP, et al. : Spleen tyrosine kinase is important in the production of proinflammatory cytokines and cell proliferation in human mesangial cells following stimulation with IgA1 isolated from IgA nephropathy patients. J Immunol. 2012;189(7):3751–8. 10.4049/jimmunol.1102603 [DOI] [PubMed] [Google Scholar]
- 38. Bene MC, Faure GC: Composition of mesangial deposits in IgA nephropathy: complement factors. Nephron. 1987;46(2):219. 10.1159/000184350 [DOI] [PubMed] [Google Scholar]
- 39. Rauterberg EW, Lieberknecht HM, Wingen AM, et al. : Complement membrane attack (MAC) in idiopathic IgA-glomerulonephritis. Kidney Int. 1987;31(3):820–9. 10.1038/ki.1987.72 [DOI] [PubMed] [Google Scholar]
- 40. Hiki Y, Odani H, Takahashi M, et al. : Mass spectrometry proves under- O-glycosylation of glomerular IgA1 in IgA nephropathy. Kidney Int. 2001;59(3):1077–85. 10.1046/j.1523-1755.2001.0590031077.x [DOI] [PubMed] [Google Scholar]
- 41. Allen AC, Bailey EM, Brenchley PE, et al. : Mesangial IgA1 in IgA nephropathy exhibits aberrant O-glycosylation: observations in three patients. Kidney Int. 2001;60(3):969–73. 10.1046/j.1523-1755.2001.060003969.x [DOI] [PubMed] [Google Scholar]
- 42. Zhao N, Hou P, Lv J, et al. : The level of galactose-deficient IgA1 in the sera of patients with IgA nephropathy is associated with disease progression. Kidney Int. 2012;82(7):790–6. 10.1038/ki.2012.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Berthoux F, Suzuki H, Thibaudin L, et al. : Autoantibodies targeting galactose-deficient IgA1 associate with progression of IgA nephropathy. J Am Soc Nephrol. 2012;23(9):1579–87. 10.1681/ASN.2012010053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Oortwijn BD, Eijgenraam JW, Rastaldi MP, et al. : The role of secretory IgA and complement in IgA nephropathy. Semin Nephrol. 2008;28(1):58–65. 10.1016/j.semnephrol.2007.10.007 [DOI] [PubMed] [Google Scholar]
- 45. Oortwijn BD, van der Boog PJ, Roos A, et al. : A pathogenic role for secretory IgA in IgA nephropathy. Kidney Int. 2006;69(7):1131–8. 10.1038/sj.ki.5000074 [DOI] [PubMed] [Google Scholar]
- 46. De Angelis M, Montemurno E, Piccolo M, et al. : Microbiota and metabolome associated with immunoglobulin A nephropathy (IgAN). PLoS One. 2014;9(6):e99006. 10.1371/journal.pone.0099006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Roos A, Rastaldi MP, Calvaresi N, et al. : Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J Am Soc Nephrol. 2006;17(6):1724–34. 10.1681/ASN.2005090923 [DOI] [PubMed] [Google Scholar]
- 48. Beck L, Bomback AS, Choi MJ, et al. : KDOQI US commentary on the 2012 KDIGO clinical practice guideline for glomerulonephritis. Am J Kidney Dis. 2013;62(3):403–41. 10.1053/j.ajkd.2013.06.002 [DOI] [PubMed] [Google Scholar]
- 49. Feehally J: Immunosuppression in IgA Nephropathy: Guideline Medicine Versus Personalized Medicine. Semin Nephrol. 2017;37(5):464–77. 10.1016/j.semnephrol.2017.05.019 [DOI] [PubMed] [Google Scholar]
- 50. Gale DP, Molyneux K, Wimbury D, et al. : Galactosylation of IgA1 Is Associated with Common Variation in C1GALT1. J Am Soc Nephrol. 2017;28(7):2158–2166. 10.1681/ASN.2016091043 [DOI] [PMC free article] [PubMed] [Google Scholar]