Abstract
1,4-Benzodiazepine N-nitrosoamidines have been used as scaffolds for the preparation of different tricyclic derivatives. Replacement of the N-nitrosoamidine moiety through treatment with the nucleophiles acetylhydrazine, aminoacetaldehyde dimethylacetal and 1-amino-2-propanol, followed by an acid-catalyzed cyclization step, afforded triazolo and imidazobenzodiazepines 1, 6, and 7, respectively, in good yields. When acetylhydrazine is used as a nucleophile, the overall process provides an alternative route to alprazolam (1b) and triazolam (1c), respectively.
Keywords: N-Nitrosoamidines; 1,4-benzodiazepines; midazolam; alprazolam; triazolam
Introduction
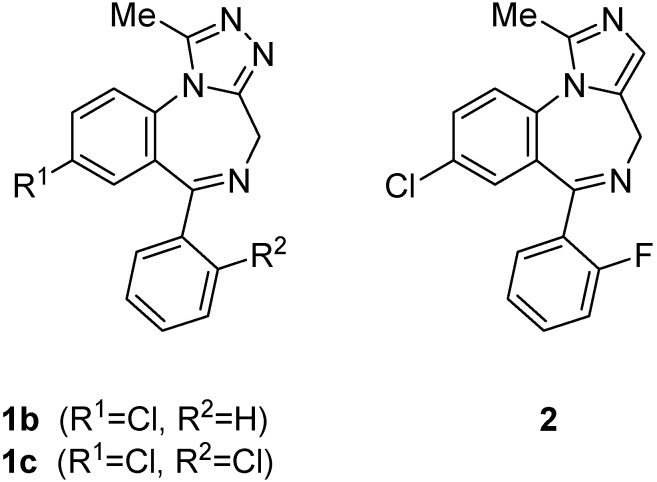
The wide spectrum of pharmacological effects exhibited by benzodiazepines makes them one of the most versatile classes of drugs used in psychopharmacology [1]. Among them, 1,4-benzodiazepines fused to a five-membered heterocyclic ring are the most commonly prescribed group of drugs for the treatment of CNS disturbances [2]. Triazolobenzodiazepines such as alprazolam (1b) or triazolam (1c) and imidazobenzodiazepines such as midazolam (2) (Figure 1) see extensive clinical use as anxiolytic and anesthetic agents, respectively. Moreover, appropriate modifications of these systems have been found to produce compounds with qualitatively different pharmacological profiles [3].
Figure 1.
.
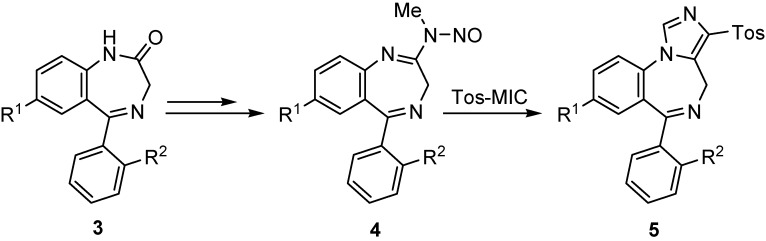
In the 1,4-benzodiazepine series chemical activation of cyclic secondary amides 3 via transformation to N-nitrosoamidines 4 has been described as a useful approach to the functionalization of position 2 of the bicyclic core of the heterocycle [4]. In this context, we have previously described the use of these 1,4-benzodiazepine-derived N-nitrosoamidines 4 as synthetic equivalents of imidoyl chlorides in their reaction with Tos-MIC to form 3-(4-tosyl)imidazo[1,5-a][1,4]benzodiazepines 5 (Scheme 1) [5].
Scheme 1.
.
As an extension of this previous work, we describe herein the use of this strategy for the preparation of several tricyclic benzodiazepines with structures closely related to those of compounds 1b, 1c, and 2, using compounds 4 as starting materials.
Results and Discussion
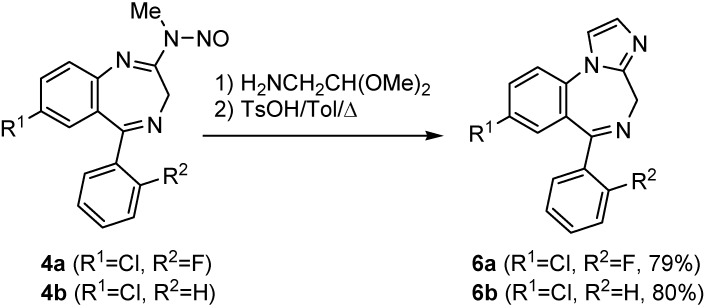
Treatment of N-nitrosoamidines 4 [6] with aminoacetaldehyde dimethylacetal afforded the corresponding amidines in an addition-elimination sequence. These amidine derivatives were heated in toluene at 80º C in the presence of two equivalents of p-toluenesulfonic acid (TsOH) to furnish imidazo[1,2-a][1,4]benzodiazepines 6 in very good yields (Scheme 2) [7]. The use of this methodology in the preparation of imidazosugar derivatives, which show glycosidase inhibitor activity, has been recently described [8].
Scheme 2.
.
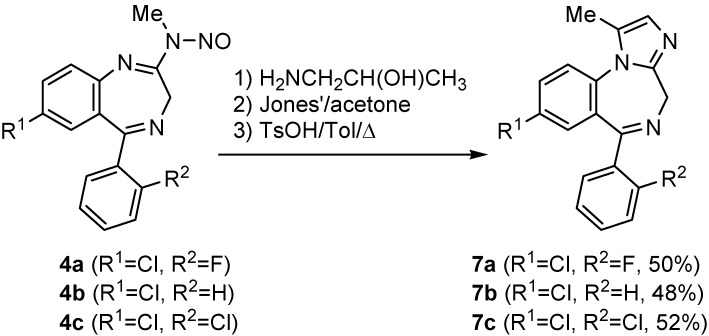
The N-nitrosoamidine functionality in compounds 4 can also be replaced through reaction with 1-amino-2-propanol. The hydroxyl functionality present in this newly created amidine was subsequently oxidized to the corresponding methyl ketone through treatment with Jones’ reagent. p-Toluenesulfonic acid-mediated cyclization under the conditions described above afforded tricyclic benzodiazepines 7 in moderate yields (Scheme 3) [9].
Scheme 3.
.
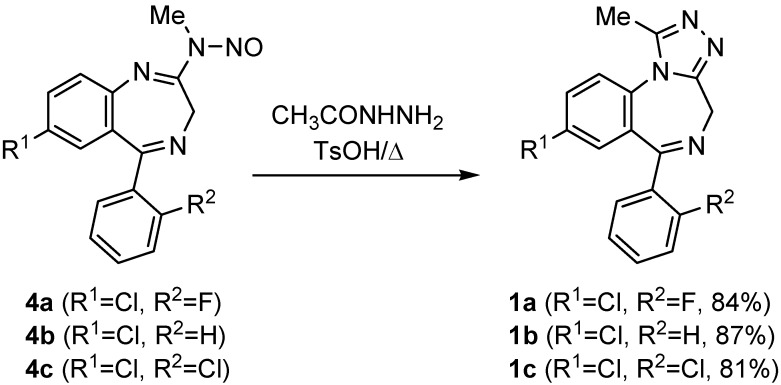
Finally, treatment of N-nitrosoamidines 4 with acetylhydrazine gave rise to the corresponding amidines, which were then cyclized upon heating in dimethylformamide (DMF) in the presence of TsOH to afford triazole-fused 1,4-benzodiazepines 1 in very good yields (Scheme 1). Although this reaction has previously been reported in the literature for the formation of the 4-oxide derivative of 1b [4b], the final tricyclic system could not be isolated, but rather appeared together with another uncyclized product. The use of TsOH in the cyclization step greatly increases the efficiency of this process as it enables the complete conversion of the starting material, thereby improving the final yield. The overall process constitutes an efficient alternative protocol for the preparation of alprazolam (1a) and triazolam (1b) (Scheme 4).
Scheme 4.
.
Conclusions
In summary, the syntheses of several tricyclic benzodiazepine derivatives through the reactions of benzodiazepine N-nitrosoamidines with nitrogen nucleophiles are described. The initially formed amidines were subsequently cyclized in the presence of TsOH to the corresponding triazolo- and imidazo[1,2-a][1,4]benzodiazepines in good yields. Although these heterocyclic systems have been previously described, the use of compounds 4 as precursors offers an efficient alternative for their preparation.
Experimental
General
All reactions were carried out under argon or nitrogen atmospheres. Solvents were purified prior to use: toluene was distilled from sodium; CH2Cl2 and DMF were distilled from calcium hydride. All reagents were used as received. The reactions were monitored with the aid of thin-layer chromatography (TLC) on 0.25-mm precoated silica gel plates. Visualization was carried out with UV light and aqueous ceric ammonium molybdate solution or potassium permanganate stain. Flash column chromatography was performed with the indicated solvents on silica gel 60 (particle size 0.040-0.063 mm). 1H- and 13C-NMR spectra were recorded on a 300 MHz Bruker AC300 spectrometer. Compounds 1a-c [10], 5a,b [11] and 6a,b [10a-c] all showed NMR spectra identical to those previously described in the literature.
General procedure for the preparation of triazolobenzodiazepines 1
A solution of N-nitrosoamidine 4 (2 mmol) and TsOH (0.19 g, 1 mmol) in DMF (10 mL) was heated at 120º C for 1 h. The reaction was cooled to room temperature (r.t.), quenched with saturated sodium bicarbonate (20 mL), and extracted with CH2Cl2. The combined organic layers were dried, concentrated, and subjected to flash chromatography (4:1 EtOAc-MeOH) to afford the final products 1 in the yields shown in Scheme 4.
General procedure for the preparation of imidazobenzodiazepines 6
A solution of N-nitrosoamidine 4 (2 mmol) in aminoacetaldehyde dimethyl acetal (5 mL) was stirred at r.t. for 24 h. The acetal, used as solvent, was evaporated in vacuo. The residue was dissolved in toluene (10 mL), TsOH (0.76 g, 4 mmol) was added, and the resulting solution was heated at 80ºC for 14 h. The reaction was quenched with aqueous sodium bicarbonate (20 mL) and extracted with CH2Cl2 (3 x 20 mL); the combined organic layers were dried and concentrated in vacuo. Flash chromatography of the crude mixture (EtOAc) afforded the desired tricyclic systems 6 in the yields shown in Scheme 2.
General procedure for the preparation of imidazobenzodiazepines 7
A solution of N-nitrosoamidine 4 (2 mmol) in 1-amino-2-propanol (5 mL) was stirred at r.t. for 24 h. The reaction was then quenched with a saturated sodium bicarbonate solution (30 mL), extracted with CH2Cl2 (3 x 20 mL) and the combined organic layers were washed with water (2 x 20 mL). The residue was concentrated, dried, redissolved in acetone, and treated with Jones’ reagent (1 mL). The reaction mixture was stirred at r.t. for 1 h and quenched with methanol (10 mL). Solvents were eliminated in vacuo and the crude reaction mixture was dissolved in CH2Cl2 (30 mL), filtered through a pad of Celite®, washed with aqueous sodium bicarbonate, dried, and concentrated in vacuo. The residue was once again dissolved in toluene (20 mL), treated with TsOH (0.76 g, 4 mmol) and heated at 80º C for 14 h. The reaction was cooled to r.t., quenched again with sodium bicarbonate, and after aqueous workup and flash chromatography (4:1 EtOAc-MeOH), the final compounds 6 were obtained in the overall yields shown in Scheme 3.
Acknowledgements
This research was supported by the European Community (Feder Project 1FD97-0869) and the Generalitat Valenciana (GR03-193). C. P. expresses his gratitude for a Ramón y Cajal contract.
Footnotes
Sample Availability: Not available.
References and Notes
- 1.Reviews: Fielding S., LaI H. Anxiolytics. Futurea; New York: 1979. Möhler H., Okada T. Science. 1977;198:849. doi: 10.1126/science.918669.
- 2. Vida J. A. In: Medicinal Chemistry. Wolf M. W., Burger A., editors. Wiley; New York: 1981. p. 787. Landquist J. K. In: Comprehensive Heterocyclic Chemistry. Katritzky A. R., Rees C. W., editors. Vol 1. Pergamon Press; Oxford: 1984. Ch. 1/06. Sharp J. T. In: Comprehensive Heterocyclic Chemistry. Katritzky A. R., Rees C. W., editors. Vol 7. Pergamon Press; Oxford: 1984. Ch. 5/18. Hester J. B., Jr. In: Antianxiety Agents. Berger J. G., editor. Wiley; New York: 1986. p. 51. Fryer R. I. In: Comprehensive Medicinal Chemistry. Hansch C., editor. Vol 3. Pergamon Press; New York: 1990. p. 539.
- 3.a) Hester J. B., Jr. J. Heterocycl. Chem. 1980;17:575. doi: 10.1002/jhet.5570170331. [DOI] [Google Scholar]; b) Hester H. B., Jr., Rudzik A. D., Voigtlander P. F. V. J. Med. Chem. 1980;23:392. doi: 10.1021/jm00178a009. [DOI] [PubMed] [Google Scholar]; c) Hester H. B., Jr., Rudzik A. D., Voigtlander P. F. V. J. Med. Chem. 1980;23:402. doi: 10.1021/jm00178a010. [DOI] [PubMed] [Google Scholar]; d) Hester H. B., Jr., Rudzik A. D., Voigtlander P. F. V. J. Med. Chem. 1980;23:643. doi: 10.1021/jm00180a012. [DOI] [PubMed] [Google Scholar]; e) Gall M., Kamdar B. V. J. Med. Chem. 1981;24:1575. [Google Scholar]; f) Walser A., Fryer R. I. J. Heterocycl. Chem. 1983;20:551. doi: 10.1002/jhet.5570200313. [DOI] [Google Scholar]; g) Gall M., Kamdar B. V. J. Heterocycl. Chem. 1988;25:1649. doi: 10.1002/jhet.5570250610. [DOI] [Google Scholar]; k) Banks W. L., Digenis G. A. Tetrahedron Lett. 1989:6473. doi: 10.1016/S0040-4039(01)88997-8. [DOI] [Google Scholar]; h) Hester J. B., Ludens J. H., Emmert D. E., West B. E. J. Med. Chem. 1989;32:1157. doi: 10.1021/jm00126a003. [DOI] [PubMed] [Google Scholar]; i) Walser A., Flynn T., Mason C., Crowley H., Maresca C., Yaremko B., O´Donnel M. J. Med. Chem. 1991;34:1209. doi: 10.1021/jm00107a048. [DOI] [PubMed] [Google Scholar]
- 4. Walser A., Fryer R. I., Sternbach L. H. J. Heterocycl. Chem. 1974;11:619. doi: 10.1002/jhet.5570110432. Walser A., Fryer R. I. J. Org. Chem. 1975;40:153. doi: 10.1021/jo00890a001. Walser A., Benjamin L. E., Sr., Flynn T., Mason C., Schwartz R., Fryer R. I. J. Org. Chem. 1978;43:936. doi: 10.1021/jo00399a029.
- 5.del Pozo C., Macías A., Alonso E., González J. Synthesis. 2004:2697. [Google Scholar]
- 6.Amides 4 and 1,4-benzodiazepine N-nitrosoamidines 5 were prepared according to the procedure described in the literature: a) ref. 4c Bock M. G., Di Pardo R. M., Evans B. E., Rittle K. E., Jobey D. F., Freidinger R. M., Hirshfield J., Springer J. P. J. Org. Chem. 1987;52:3232. doi: 10.1021/jo00391a010.
- 7.Although the synthesis of 5a starting from the corresponding thioamide of 3 via cyclization with acetic acid has already been described, the use of this modified protocol substantially increases the final yields of the overall process: Maffrand J. P., Eloy F. F. Tetrahedron Lett. 1973;36:3449. doi: 10.1016/S0040-4039(01)86939-2.
- 8. Granier T., Panday N., Vasella A. Helv. Chim. Acta. 1997;80:979. doi: 10.1002/hlca.19970800329. Panday N., Granier T., Vasella A. Helv. Chim. Acta. 1998;81:475. doi: 10.1002/hlca.19980810303. Panday N., Vasella N. Synthesis. 1999:1459. doi: 10.1055/s-1999-3654.
- 9.The synthesis of these analogs of midazolam 2 through condensation of the corresponding thioamide with propargylamine and further cyclization in acidic media has already been described. The current protocol can be viewed as an alternative for preparing these systems
- 10. Hester J. B., Jr., Upjohn Co. 3903103. US Pat. 1975 Hester J. B., Jr., Upjohn Co. 4000153. US Pat. 1976 Hester J., Jr., Upjohn Co. 3993660. US Pat. 1976
- 11. Hester J. B., Jr., Hanze A. R., Upjohn Co. 3927016. US Pat. 1975 Hester J. B., Jr., Hanze A. R., Upjohn Co. 3933794. US Pat. 1976 Hester J. B., Jr., Hanze A. R., Upjohn Co. 3917627. US Pat. 1975 Gall M., Upjohn Co. 3941803. US Pat. 1976