Abstract
3,5-Disubstituted hydantoin (1,3-imidazolidinedione) derivatives 5a-h were prepared by base induced cyclization of the corresponding N-(1-benzotriazolecarbonyl)-l- and d--amino acid amides 4a-h. Compounds 5a-h were evaluated for their cytostatic and antiviral activities. Among all the compounds evaluated, 3-benzhydryl-5-isopropyl hydantoin (5a) showed a weak but selective inhibitory effect against vaccinia virus (EC50 = 16 μg/mL; selectivity index: 25). 3-Cyclohexyl-5-phenyl hydantoin (5g) showed inhibitory activity against cervical carcinoma (HeLa, IC50 = 5.4 μM) and breast carcinoma (MCF-7, IC50 = 2 μM), but also cytotoxic effects towards human normal fibroblasts (WI 38). On the contrary, the 3-benzhydryl-5-phenyl substituted hydantoin derivative 5h showed moderate inhibitory activity towards HeLa, MCF-7, pancreatic carcinoma (MiaPaCa-2), lung carcinoma (H 460) and colon carcinoma (SW 620) (IC50 = 20−23 μM), but no effect on WI 38.
Keywords: Hydantoin, l- and d-amino acids, antiviral activity, antitumoral activity
Introduction
Hydantoin (1,3-imidazolidinedione) derivatives display diverse and interesting pharmacological properties. Several such derivatives (phenytoin, mephenythoin, norantoin, methetoin, ethotoin, fosphenytoin) are well-known anticonvulsive drugs [1,2]. Other 5-substituted hydantoins like 5,5-dithienylhydantoin, 5,5-dipyridylhydantoin, spirothiohydantoin, thiohydantoin and dithiohydantoins also possess anticonvulsive activity [3]. Hydantoin derivatives can also be found as antiarrhythmics (azimilide), antimicrobial agents (nitrofurantoin), skeletal muscle relaxants (dantrolene) and nonsteroidal antiandrogens (nilutamide), while allantoin is used as a keratolytic, astringent, wound remedy, antacid and antipsoriatic drug [2]. Hydantoins also exhibit antidepressant, antiviral and antithrombotic activities, as well as inhibitory activity against some enzymes (human aldose reductase and human leucocyte elastase) [4]. Finally, some herbicides (spirohydantoin, thioxohydantocidin), fungicides (clodantoin) and insecticides also have the hydantoin skeleton in their structure [5,6]. During our previous research a series of hydantoins were synthesized and evaluated for their antiviral and cytostatic activities [7,8]. The best pharmacological activities were achieved with lipophilic hydantoin derivatives bearing cycloalkyl, phenyl or benzhydryl substituents. This led us to prepare and pharmacologically evaluate a series of the novel structural analogues of these lead compounds in order to assess their antiviral and antitumoral potential. The results are reported in this paper.
Results and Discussion
Chemistry
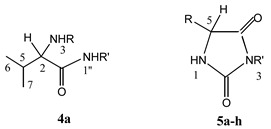
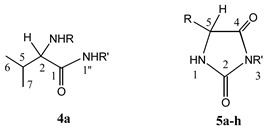
A series of 3,5-disubstituted hydantoins 5a-h were prepared by intramolecular cyclization of N-[(1-benzotriazolecarbonyl (Btc)]-amino acid amides 4a-h, following our previously described procedures [7,8,9] (Scheme 1). All these hydantoins are new compounds, except 5d and 5g, whose syntheses have been previously described [9,10,11], although their cytostatic and antiviral activities have not been reported until now. Synthesis of the N-Btc-amino acid amides 4a-h and their precursors N-Btc-amino acid derivatives 2a-d and the corresponding chloride derivatives 3a-d were also described in our previous reports [12]. Syntheses of the compounds 4a-h and 5a-h were carried out under mild reaction conditions and the products were obtained in high yields. In the preparation of the amides 4a-h, an equimolar ratio of the N-Btc-amino acid chloride derivatives 3a-d and the corresponding amine was crucial in order to avoid formation of ureido amides. Our previous research results had shown that 3-cyclohexanemethyl-5-phenylhydantoin possessed marked antiviral activity (i.e. against cytomegalo-virus) [7]. Based on that lead compound we have now prepared some novel hydantoins containing one or two lipophilic substituents at positions 3 and 5. Three amino acids of the l-series (l-valine, l-leucine and l-phenylalanine) and one d-amino acid (d-phenylglycine) and highly lipophilic amines (cyclopentyl-, cyclohexyl-, cyclohexanemethyl- or benzhydrylamines) were used as the starting reagents. As the chiral centre was not involved in chemical transformations of the l- and d-amino acid precursors (Scheme 1) these configurations should be retained in the target hydantoin molecules [7]. The validity of this assumption has been proven by X-ray crystallographic analysis of the stereostructure of the hydantoin derivative of l-valine [8].
Scheme 1.
Synthesis of hydantoin derivatives of l- and d-amino acids, 5a-f and 5g,h, respectively
The structures of the newly synthesized compounds were deduced from the analysis of their 1H- and 13C-NMR and IR spectra, and confirmed by elemental analysis. The IR spectra of the hydantoin derivatives (Table 1) showed characteristic bands at 3421−3227 (NH), 1775−1760 and 1718−1698 (CO) cm-1. The chemical shifts in 1H- and 13C-NMR spectra (Table 2 and Table 3) are in concordance with the proposed structures of the novel compounds and also consistent with previously described analogous hydantoin derivatives [7].
Table 1.
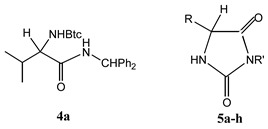
Analytical data of the novel N-Btc-l-valine benzhydrylamide (4a) and 3,5-disubstituted hydantoin derivatives 5a-h.
| Compd. | R | R' | Yield (%) | mp (°C) | Molecular formula (Mr) | IR (KBr) νmax (cm-1) |
|---|---|---|---|---|---|---|
| 4a | − | − | 48 | 155−160 | C25H25N5O2 (427.25) | 3287, 2966, 1713, 1650, 1556, 1524 |
| 5a | 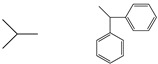 |
86 | oil | C19H20N2O2 (308.37) | 3249, 3063, 3030, 2963, 2930, 1772, 1711, 1417 | |
| 5b | 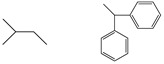 |
88 | 139−141 | C20H22N2O2 (322.40) | 3421, 3265, 3110, 2956, 1773, 1711, 1422 | |
| 5c |  |
73 | 121−124 | C15H18N2O2 (258.32) | 3258, 3091, 2959, 2868, 1760, 1718, 1431 | |
| 5d |  |
85 | 119−120 | C16H20N2O2 (272.34) | 3270, 3093, 2926, 2856, 1768, 1707, 1436 | |
| 5e |  |
80 | 150−153 | C17H22N2O2 (286.37) | 314, 3030, 2924, 2852, 1768, 1698, 1454, 1428 | |
| 5f | 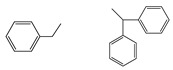 |
29 | 76 (decomp.) | C23H22N2O2 (356.42) | 3269, 3029, 2924, 1774, 1711, 1419 | |
| 5g |  |
37 | 180−184 | C15H18N2O2 (258.32) | 3227, 3098, 3037, 2927, 2856, 1765, 1705, 1429 | |
| 5h | 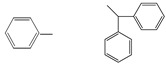 |
24 | 172−177 | C22H18N2O2 (342.39) | 3230, 3093, 2825, 1774, 1712, 1454, 1423 | |
Table 2.
1H-NMR spectral data of the novel N-Btc-l-valine benzhydrylamide (4a) and 3,5-disubstituted hydantoin derivatives 5a-h.
| Compd. | R | R' | 1H-NMR (DMSO- d6) δ ppm |
|---|---|---|---|
| 4a |  |
9.19 (d, 1H, 3, J = 8.4 Hz), 8.68 (d, 1H, 1'', J = 8.5 Hz), 8.25−7.14 (m, 14H, arom.), 6.20 (d, 1H, 2'', J = 8.5 Hz), 4.49 (d, 1H, 2, J = 7.9 Hz), 2.34−2.23 (m, 1H, 5), 0.96−0.70 (m, 6H, 6, 7) | |
| 5a |  |
8.40 (s, 1H, 1), 7.38−7.23 (m, 10H, arom.), 6.35 (s, 1H, 2''), 4.07−4,06 (d, 1H, 5, J = 3.3 Hz), 2,08−1,97 (m, 1H, 6, J = 3.4 Hz), 0.93−0.91 (d, 3H, 7,J = 6.9 Hz), 0.72−0.70 (d, 3H, 8, J = 6.8 Hz) | |
| 5b |  |
8.51 (s, 1H, 1), 7.38−7.22 (m, 10H, arom.), 6.34 (s, 1H, 2''), 4.20−4.16 (q, 1H, 5, J = 4.1 Hz), 1.79−1.74 (m, 1H, 7, J = 4.7 Hz), 1.56−1.36 (m, 2H, 6,J = 4.7 Hz), 0.88−0.85 (2d, 6H, 8, 9, J = 4.1 Hz) | |
| 5c | 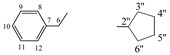 |
8.18 (s, 1H, 1), 7.28−7.13 (m, 5H, arom.), 4.30−4.27 (t, 1H, 5, J = 4.4 Hz), 4.09−4.02 (m, 1H, 2'', J = 7.7 Hz), 2.94−2.93 (d, 2H, 6, J = 4.2 Hz), 1.67−1.42 (m, 8H, 3''−6'') | |
| 5d | 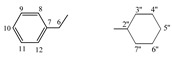 |
8.15 (s, 1H, 1), 7.28−7.13 (m, 5H, arom.), 4.30−4.27 (t, 1H, 5, J = 4.4 Hz), 3.52−3.44 (m, 1H, 2'', J = 3.5 Hz), 2.94−2.92 (t, 2H, 6, J = 3.5 Hz), 1.85−0.94 (m, 10H, 3''−7'') | |
| 5e | 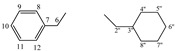 |
8.21 (s, 1H, 1), 7.27−7.14 (m, 5H, arom.), 4.39−4.36 (t, 1H, 5, J = 4.2 Hz), 3.05−2.87 (m, 4H, 6, 2''), 1.61−0.50 (m, 11H, 3''−8'') | |
| 5f | 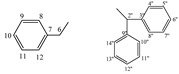 |
8.44 (s, 1H, 1), 7.30−6.73 (m, 15H, arom.), 6.13 (s, 1H, 2''), 4.53−4.50 (t, 1H, 5, J = 4.3 Hz), 3.07−2.94 (m, 2H, 6) | |
| 5g | 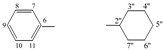 |
8.64 (s, 1H, 1), 7.44−7.29 (m, 5H, arom.), 5.13 (s, 1H, 5), 3.81−3.70 (m, 1H, 2'', J = 3.8 Hz), 2.12−1,02 (m, 10H, 3''−7'') | |
| 5h | 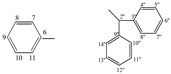 |
8.91 (s, 1H, 1), 7.43−7.20 (m, 15H, arom.), 6.39 (s, 1H, 2''), 5.34 (s, 1H, 5) | |
Table 3.
13C-NMR spectral data of the novel N-Btc-l-valine benzhydrylamide (4a) and 3,5-disubstituted hydantoin derivatives 5a-h.
| Compd. | R | R' | 13C-NMR (DMSO-d6) δ ppm |
|---|---|---|---|
| 4a | 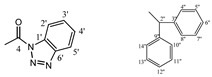 |
169.29 (1), 148.59 (4), 145.45 (1'), 142.00 (3'', 9''), 131.17 (6'), 130.08 (5'), 128.30 (5'', 7'', 11'', 13''), 128.15 (4'', 8''), 127.24 (10'', 14''), 127.15 (6''), 126.99 (12''), 125.61 (4'), 119.82 (3'), 113.37 (2'), 63.90 (2), 56.04 (2'') 30.77 (5), 19.17 (6), 18.20 (7) | |
| 5a | 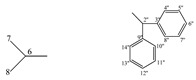 |
173.63 (4), 156.87 (2), 138.63 (3''), 138.37 (9''), 128.56(4'', 8''), 128.37 (5'', 7''), 128.32 (10'', 14''), 128.22 (11'',13''), 127.64 (6''), 127.53''), 61.06 (2''), 57.14 (5), 29.92 (6), 18.64 (7), 15.84 (8) | |
| 5b | 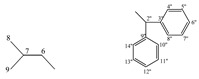 |
174.34 (4), 156.22 (2), 138.30 (3''), 138.21 (9''), 128.15 (4'', 8'', 10'', 14''), 128.12 (5'', 7'', 11'', 13''), 127.33 (6'', 12''), 56.85 (2''), 54.44 (5), 40.75 (6), 23.96 (7), 22.98 (8), 21.27 (9) | |
| 5c |  |
175.20 (4), 158.25 (2), 136.80 (7), 131.64 (8, 12), 129.81 (9, 11), 128.55 (10), 58.11 (5), 51.90 (2''), 38.30 (6), 30.30 (6''), 30.02 (3''), 26.39 (4'', 5'') | |
| 5d |  |
173.20 (4), 156.22 (2), 134.78 (7), 129.76 (8, 12), 127.82 (9, 11), 126.60 (10), 56.04 (5), 49.64 (2''), 36.28 (6), 28.58 (3''), 28.39 (7''), 25.18 (4'', 6''), 24.68 (5'') | |
| 5e |  |
173.27 (4), 156.60 (2), 134.78 (7), 129.80 (8, 12), 127.82 (9, 11), 126.58 (10), 56.71 (5), 43.37 (2''), 35.95 (6), 35.66 (3''), 29.79 (4''), 29.52 (8''), 25.65 (5''), 25.17 (7''), 25.12 (6'') | |
| 5f | 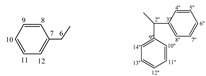 |
172.87 (4), 155.72 (2), 138.01 (3''), 137.95 (9''), 134.65 (7), 126.78−130.01 (8−12, 4''−8'', 10''−14'') 56.62 (5, 2''), 35.78 (6) | |
| 5g |  |
172.36 (4), 156.61 (2), 135.91 (6), 128.62 (7, 11), 128.23 (8, 10), 126.68 (9), 59.08 (5), 50.22 (2''), 28.92(3''), 28.83 (7''), 25.27 (4''), 25.24 (6''), 24.72 (5'') | |
| 5h | 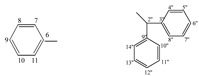 |
172.35 (4), 156.54 (2), 138.96 (3''), 138.30 (9''), 135.85 (6), 128.94−126.87 (7−11, 4''−8'', 10''−14''), 59.66 (2''), 57.35 (5) | |
Biological Results
The activities of the novel 3,5-disubstituted hydantoins 5a-h were evaluated against herpes simplex virus-1 (HSV-1 KOS strain ), herpes simplex virus-2 (HSV-2 strain G), herpes simplex virus-1 (ACV-resistant KOS strain), vaccinia virus, vesicular stomatitis virus, Coxsackie virus B4, respiratory syncytial virus, parainfluenza-3 virus, reovirus-1, Sindbis virus and Punta Toro virus. These activities were compared with those of acyclovir, ganciclovir and ribavirin (Table 4).
Table 4.
Antiviral activity of the novel 3,5-disubstituted hydantoins 5a-h.
| Compd. | MCCa | EC50b | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1c | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
| 5a | 400 | > 80 | 80 | 80 | 16 | 80 | > 80 | > 80 | > 80 | > 80 | 48 | > 80 |
| 5b | 80 | > 16 | > 16 | > 16 | > 16 | > 16 | > 16 | > 16 | > 16 | > 16 | 16 | > 16 |
| 5c | > 400 | > 400 | 240 | > 80 | 240 | > 400 | 240 | > 400 | > 400 | > 400 | 240 | > 400 |
| 5d | 80 | > 16 | > 16 | > 16 | > 16 | > 80 | 80 | > 80 | > 80 | > 80 | > 80 | > 80 |
| 5e | 400 | > 80 | > 80 | > 80 | > 80 | > 16 | > 16 | > 16 | > 16 | > 16 | > 16 | > 16 |
| 5f | 80 | > 16 | > 16 | > 16 | 16 | > 16 | 16 | > 16 | 16 | 16 | 16 | > 16 |
| 5g | 80 | > 16 | > 16 | > 16 | > 16 | > 80 | > 80 | > 80 | > 80 | > 80 | > 80 | > 80 |
| 5h | 80 | > 16 | > 16 | > 16 | > 16 | > 16 | 16 | > 16 | 16 | 16 | 16 | > 16 |
| acyclovir | > 100 | 0.11 | 0.18 | 13.51 | 67.5 | > 100 | ndd | nd | nd | nd | nd | nd |
| ganciclovir | > 25 | 0.02 | 0.02 | 0.2 | 15.3 | > 25 | nd | nd | nd | nd | nd | nd |
| ribavirin | > 100 | > 100 | 73.3 | > 100 | 24.4 | > 100 | > 100 | > 100 | 73.3 | 73.3 | 24.4 | 73.3 |
a MCC – minimum cytotoxic concentration (μg mL−1) that causes a microscopically detectable alternation of cell morphology; b EC50 – effective concentration (μg mL−1) required to reduce virus plaque formation by 50%. Virus input was 100 plaque forming units (PFU); c Viruses: 1 – HSV-1 (KOS), 2 – HSV-2 (G), 3 – HSV-1 TK KOS ACVr, 4 – Vaccinia virus, 5 – Vesicular stomatitis virus, 6 – Coxsackie virus B4, 7 – Respiratory syncytial virus, 8 – Parainfluenza-3 virus, 9 – Reovirus-1, 10 – Sindbis virus, 11 – Punta Toro virus; d Not determined.
Structurally related hydantoin derivatives 5f and 5h exhibited similar inhibitory effects against the evaluated viruses. Thus, these compounds showed some antiviral activity against Coxsackie virus, parainfluenza-3 virus, reovirus-1 and Sindbis virus (EC50 = 16 μg mL−1); which is 5-fold lower than the minimum cytotoxic concentration (MCC). 3-Benzhydryl-5-isopropyl hydantoin (5a) showed inhibitory effects against vaccinia virus (EC50 = 16 μg mL−1), at 25-fold lower concentration than the MCC.
The 3,5-disubstituted hydantoin derivatives 5a-h were also evaluated for their activities against malignant tumor cell lines: murine leukemia (L1210) and human T-lymphocytes (Molt4/C8 and CEM), cervical carcinoma (HeLa), breast carcinoma (MCF-7), pancreatic carcinoma (MiaPaCa-2), lung carcinoma (H 460), colon carcinoma (SW 620), as well as normal fibroblasts (WI 38) (Table 5).
Table 5.
Inhibitory effects of the novel 3,5-disubstituted hydantoins 5a-h on the growth of human malignant tumor cell lines and normal diploid fibroblasts (WI 38).
| Compds | Tumor cell growth a | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1b | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 5a | 102 ± 3 | 183 ± 38 | 83 ± 4 | > 100 | 36 ± 13 | 47 ± 5 | 65 ± 35 | 62 ± 20 | 77 ± 23 |
| 5b | 57 ± 21 | 232±194 | 148 ± 95 | 47 ± 44 | 16 ± 0.6 | 46 ± 29 | 43 ± 1.5 | 31 ± 27 | 42 ± 2 |
| 5c | > 500 | > 500 | 406 ± 16 | > 100 | 63 ± 14 | > 100 | > 100 | > 100 | > 100 |
| 5d | 303 ± 9 | 317 ± 12 | 266 ± 15 | 37 ± 141 | 18 ± 1 | 81 ± 10 | > 100 | 78 ± 21 | 55 ± 14 |
| 5e | 312±128 | > 500 | 246 ± 85 | 38 ± 8 | 22 ± 2 | 70 ± 19 | > 100 | 74 ± 25 | 42 ± 2 |
| 5f | 42 ± 2 | 53 ± 2 | 48 ± 4 | 13 ± 1.4 | 11 ± 1 | 14 ± 0.9 | 19 ± 4 | 15 ± 2 | 13 ± 5 |
| 5g | 172 ± 52 | 277 ± 45 | 146 ± 14 | 5.4 ± 13 | 2 ± 1.6 | 58 ± 42 | 58 ± 25 | 60 ± 35 | 1.5 ± 0.3 |
| 5h | 46 ± 4 | 52 ± 5 | 50 ± 4 | 21 ± 3 | 20 ± 10 | 22 ± 0.7 | 23 ± 0.7 | 21 ± 0.9 | > 100 |
| HU | ndc | nd | nd | 42 ± 38 | > 100 | > 100 | > 100 | > 100 | > 100 |
| 5-FU | nd | nd | nd | 4 ± 1.5 | 15 ± 2 | 10 ± 3 | 2 ± 0.7 | 9 ± 2 | 14 ± 12 |
| Cis | nd | nd | nd | 4 ± 3 | 12 ± 6 | 5 ± 1 | 0.3 ± 0.1 | 8 ± 6 | 20 ± 19 |
a IC50– 50% inhibitory concentration or compound concentration (μM) required to inhibit tumor cell proliferation by 50%; b Tumor cells: 1 – L1210, 2 – MoltC4/C8, 3 – CEM, 4 – HeLa, 5 – MCF-7, 6 – MiaPaCa-2, 7 – H 460, 8 – SW 620, 9 – normal diploid fibroblasts WI 38; c Not determined; HU – hydroxyurea, 5-FU – 5-fluorouracil, Cis – cisplatin.
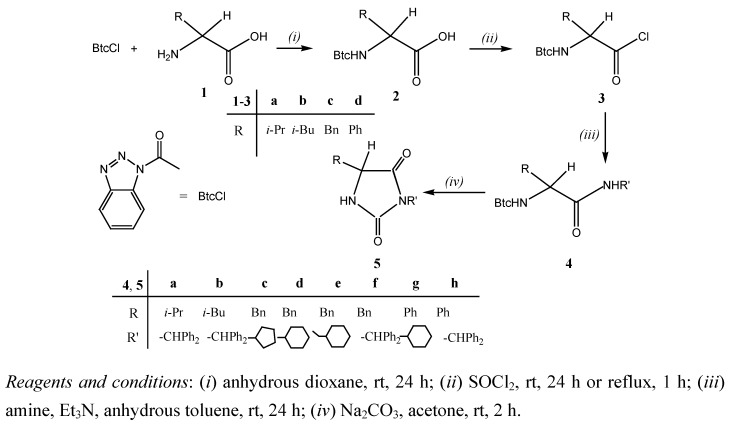
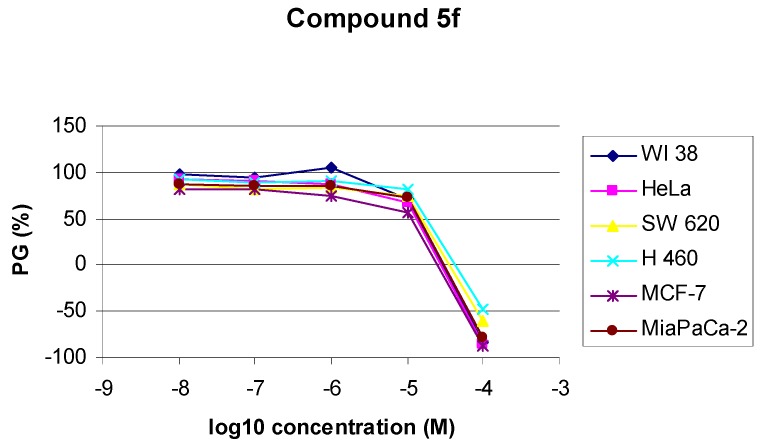
Amongst all evaluated compounds, 3-cyclohexyl-5-phenyl hydantoin (5g) showed rather marked inhibitory activity against HeLa (IC50 = 5.4 μM) and MCF-7 (IC50 = 2 μM) cell lines, but also high inhibitory effects on normal fibroblasts WI 38 (Figure 1).
Figure 1.
Dose-response profile for compounds 5f,5g and 5h tested in vitro on various human cell lines *.
Similarly, 3-benzhydryl-5-benzyl hydantoin (5f) showed moderate inhibitory activity against all human tumor cell lines examined and also normal fibroblasts (WI 38). By contrast, the 3-benzhydryl-5-phenyl substituted hydantoin derivative 5h showed a rather marked inhibitory activity against HeLa (IC50 = 21 μM), MCF-7 (IC50 = 20 μM), MiaPaCa-2 (IC50 = 22 μM), H 460 (IC50 = 23 μM) and SW 620 (IC50 = 21 μM), and no cytotoxic effect on WI 38 fibroblasts.
Conclusions
In summary, the hydantoin derivatives presented herein showed certain antiviral and antitumor activity. Derivatives 5f and 5h exhibited weak inhibitory effects against the evaluated viruses and hydantoin 5a showed a weak but selective inhibitory effect against vaccinia virus. With regards to their antitumor activity, hydantoin 5g showed rather marked inhibitory activity against HeLa and MCF-7 cell lines, while 5h showed inhibitory activity towards several tumor cell lines and no cytotoxic effects on normal cells. Further optimization of the hydantoin derivatives as potential antiviral and cytostatic agents is in progress.
Experimental
General
Melting points were determined on a Boëtius Micro Heating Stage and are uncorrected. IR spectra were recorded on a FTIR Perkin Elmer Paragon 500 spectrometer. 1H- and 13C-NMR spectra were recorded on a Varian Gemini 300 spectrometer, operating at 300 and 75.5 MHz for the 1H- and 13C- nuclei, respectively. Samples were measured in DMSO-d6 solutions at 20 ºC in 5 mm NMR tubes. Chemical shifts (δ) in ppm were referred to TMS. Precoated silica gel 60 F254 plates (Merck) were used for thin-layer chromatography, with 1:1 cyclohexane/ethyl acetate as the eluent. Spots were visualized by short-wave UV light and iodine vapour.
Chemistry
N-Btc-amino acid amides 4a-h
The starting compounds for the hydantoin preparations, namely N-Btc-l-leucine benzhydrylamide (4b), N-Btc-l-phenylalanine cyclopentylamide (4c), N-Btc-l-phenylalanine cyclohexylamide (4d), N-Btc-l-phenylalanine cyclohexanemethylamide (4e), N-Btc-l-phenylalanine benzhydrylamide (4f), N-Btc-d-phenylglycine cyclohexylamide (4g), N-Btc-d-phenylglycine benzhydrylamide (4h) were prepared by procedures described previously [7,9], whereas the analogous l-valine benzhydrylamide (4a) is a new compound.
N-Btc-l-valine benzhydrylamide (4a). To a cold solution of N-Btc-l-valine chloride 3a [9] (1.122 g, 4 mmol) in toluene (40 mL), a solution of benzhydrylamine (0.732 g, 4 mmol) and triethylamine (0.404 g, 4 mmol) in toluene (30 mL) was added dropwise. The reaction mixture was stirred at room temperature for 24 h. Product 4a, which precipitated together with TEA·HCl, was filtered off and dissolved in a dichloromethane/water mixture. The organic layers were extracted with diluted hydrochloride acid (w = 1%) and water, dried over sodium sulfate and evaporated in vacuo. The residue was triturated with ether to give the analytically pure 4a. The analytical, 1H- and 13C-NMR spectral data for 4a are given in Table 1, Table 2 and Table 3.
General method for the synthesis of 3,5-disubstituted hydantoins 5a-h
To a solution of N-(1-benzotriazolecarbonyl)-amino acid derivatives 4a-h (1.5 mmol) in acetone (60 mL), 5% sodium carbonate solution (5 mL) was added. The reaction mixture was stirred 2 h at room temperature. Acetone was evaporated in vacuo and the precipitated products 5a-h were filtered off, washed with water and recrystallized from acetone and water. The following hydantoins were prepared in this fashion: 3-benzhydryl-5-isopropyl hydantoin (5a), 3-benzhydryl-5-isobutyl hydantoin (5b), 3-cyclopentyl-5-benzyl hydantoin (5c), 3-cyclohexyl-5-benzyl hydantoin (5d), 3-cyclohexane-methyl-5-benzyl hydantoin (5e), 3-benzhydryl-5-benzyl hydantoin (5f), 3-cyclohexyl-5-phenyl hydantoin (5g) and 3-benzhydryl-5-phenyl hydantoin (5h). Their analytical data and yields are presented in Table 1, while 1H- and 13C-NMR data are given in Table 2 and Table 3, respectively.
Antiviral Activity Assays
Antiviral activity against HSV-1, HSV-2, vaccinia virus, vesicular stomatitis virus, Coxsackie virus B4, respiratory syncytial virus, parainfluenza-3 virus, reovirus-1, Sindbis virus and Punta Toro virus was determined as described previously [13,14]. Antiviral activity was expressed as the EC50 or concentration required to reduce virus-induced cytopathogenicy by 50%. EC50 values were calculated from graphic plots of the percentage of cytopathogenicity as a function of concentration of the compounds.
Antitumoral Activity Assays
The HeLa (cervical carcinoma), MCF-7 (breast carcinoma), MiaPaCa-2 (pancreatic carcinoma), H 460 (lung carcinoma), SW 620 (colon carcinoma) and WI 38 (diploid fibroblasts) cells were cultured as monolayers and maintained in Dulbecco's modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin in a humidified atmosphere with 5% CO2 at 37˚C. Antitumoral activity against L1210 (murine leukemia), Molt4/C8 and CEM (human T-lymphocytes) cell lines was measured essentially as originally described for the mouse leukemia (L1210) cell line [15].
The growth-inhibitory activity of HeLa, MCF-7, MiaPaCa-2, H 460, SW 620 and WI 38 cells was assessed according to a slightly modified procedure performed at the National Cancer Institute, Developmental Therapeutics Program [16]. The cells were seeded into a series of standard 96-well microtiter plates on day 0. Test agents were then added in five, 10-fold dilutions (10-8 to 10-4 M) and incubated for a further 72 hours. Working dilutions were freshly prepared on the day of testing. The solvent (DMSO) was also tested for eventual inhibitory activity by adjusting its concentration to be the same as for the tested concentrations. After 72 hours of incubation the cell growth rate was evaluated by MTT assay [17] which detects dehydrogenase activity in viable cells. The absorbance (OD, optical density) was measured by a microplate reader at 570 nm. The percentage of growth (PG) of the cell lines was calculated according to one or the other of the following two expressions:
If (mean ODtest – mean ODtzero) ≥ 0, then:
PG = 100 x (mean ODtest – mean ODtzero) / (mean ODctrl – meanODtzero)
If (mean ODtest – mean ODtzero) < 0, then:
G = 100 x (mean ODtest – mean ODtzero) / ODtzero
where mean ODtzero = the average of optical density measurements before exposure of cells to the test compound, mean ODtest = the average of optical density measurements after the desired period of time and mean ODctrl = the average of optical density measurements after the desired period of time with no exposure of cells to the test compound.
Each test point was measured in quadruplicate in three individual experiments. The results are expressed as IC50, a concentration necessary for 50% of inhibition. Each result is a mean value from three separate experiments. The IC50 values for each compound were calculated from dose-response curves using linear regression analysis by fitting the test concentrations that gave PG values above and below the reference value (i.e. 50%).
Acknowledgements
Support for this study by the Ministry of Science, Education and Sports of the Republic of Croatia (Projects No. 0006543, 0125003 and 0098093) is gratefully acknowledged.
Footnotes
Sample Availability: Samples of the compounds are available from authors.
References
- 1.Williams D.A., Lemke T.L. Foye's Principles of Medicinal Chemistry. 5th ed. Lippincott Williams & Wilkins; Philadelphia: 2002. [Google Scholar]
- 2.Kleemann A., Engel J., Kutscher B., Reichert D. Pharmaceutical Substances, Synthesis, Patents, Applications. 4th ed. Thieme; Stuttgart: 2001. [Google Scholar]
- 3.Malawska B. New anticonvulsant agents. Curr. Topics Med. Chem. 2005;5:69–85. doi: 10.2174/1568026053386944. [DOI] [PubMed] [Google Scholar]
- 4.Fiallo M.M.L., Kozlowski H., Garnier-Suillerot A. Mitomycin antitumor compounds. Part 1. CD studies on their molecular structure. Eur. J. Pharm. Sci. 2001;12:487–494. doi: 10.1016/S0928-0987(00)00200-1. [DOI] [PubMed] [Google Scholar]
- 5.Gregoriou M., Noble M.E.M., Watson K.A., Garman E.F., Krulle T.M., Delafuente C., Fleet G.W.J., Oikonomakos N.G., Johnson L.N. The structure of a glycogen phosphorylase glucopyranose spirohydantoin complex at 1.8 angstrom resolution and 100 k – the role of the water structure and its contribution to binding. Protein Sci. 1998;7:915–927. doi: 10.1002/pro.5560070409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiozaki M. Syntheses of hydantocidin and C-2 thioxohydantocidin. Carbohyd. Res. 2002;337:2077–2088. doi: 10.1016/S0008-6215(02)00217-3. [DOI] [PubMed] [Google Scholar]
- 7.Opačić N., Barbarić M., Zorc B., Cetina M., Nagl A., Frković D., Kralj M., Pavelić K., Balzarini J., Andrei G., De Clercq E., Raić-Malić S., Mintas M. The novel l- and d-amino acid derivatives of hydroxyurea and hydantoins: synthesis, X-ray crystal structure study, and cytostatic and antiviral activity evaluations. J. Med. Chem. 2005;48:475–482. doi: 10.1021/jm040869i. [DOI] [PubMed] [Google Scholar]
- 8.Opačić N., Zorc B., Cetina M., Mrvoš-Sermek D., Raić-Malić S., Mintas M. Synthesis and X-ray crystal structure study of the hydroxyurea and hydantoin derivatives of l-valine. J. Peptide Res. 2005;66:85–93. doi: 10.1111/j.1399-3011.2005.00276.x. [DOI] [PubMed] [Google Scholar]
- 9.Zorc B., Butula I. Reaktionen mit N-(1-Benzotriazolylcarbonyl)-aminosauren. I. Synthese von Hydantoinen und Hydantoinsauren-amiden. Croat. Chem. Acta. 1981;54:441–449. [Google Scholar]
- 10.Maslova G.A., Strukov I.T. A new method of preparation of 3,5-disubstituted hydantoins. Zh. Obshch. Khim. 1964;34:3506. [Google Scholar]
- 11.Schön I., Friss J., Kisfaludy L. Side reactions during the removal of protecting groups of N-(protected aminoacyl)urea derivatives. Acta Chim. Acad. Sci. Hung. 1978;98:215–223. [Google Scholar]
- 12.Butula I., Zorc B., Vela V. Reaktionen mit 1-Benzotriazol carbonsaurechlorid. VII. Die Umsetzung mit Aminosauren. Croat. Chem. Acta. 1981;54:435–440. [Google Scholar]
- 13.De Clercq E., Holý A., Rosenberg I., Sakuma T., Balzarini J., Maudgal P.C. A novel selective broad-spectrum anti-DNA virus agent. Nature. 1986;323:464–467. doi: 10.1038/323464a0. [DOI] [PubMed] [Google Scholar]
- 14.Balzarini J., Naesens L., Slachmuylders J., Niphuis H., Rosenberg I., Holý A., Schellekens H., De Clercq E. 9-(2-Phosphonylmethoxyethyl)adenine (PMEA) efficiently inhibits retrovirus replication in vitro and simian immunodeficiency virus infection in Rhesus monkeys. AIDS. 1991;5:21–28. doi: 10.1097/00002030-199101000-00003. [DOI] [PubMed] [Google Scholar]
- 15.De Clercq E., Balzarini J., Torrence P.F., Mertes M.P., Schmidt C.L., Shugar D., Barr P.J., Jones A.S., Verhelst G., Walker R.T. Thymidylate synthetase as target enzyme for the inhibitory activity of 5-substituted 2'-deoxyuridines on mouse leukemia L1210 cell growth. Mol. Pharmacol. 1981;19:321–330. [PubMed] [Google Scholar]
- 16.Boyd M. R., Paull K. D. Some practical considerations and applications of the National Cancer Institute In vitro anticancer drug discovery screen. Drug Dev. Res. 1995;34:91–109. doi: 10.1002/ddr.430340203. [DOI] [Google Scholar]
- 17.Mossman T.J. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Immun. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]