Abstract
The rearrangement under oxidative conditions of 3-(benzyloxy)-tetrahydro-2,6,6-trimethyl-2H-pyran-2-carbaldehydes to afford a chiral protected tetrahydrofuran lactol is described.
Keywords: Tetrahydropyrans, tetrahydrofurans, oxidative rearrangement
Introduction
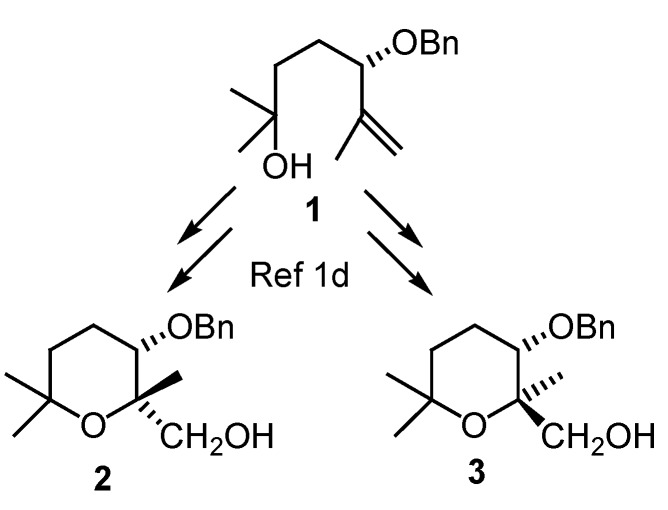
During the last few years our group has been interested in the synthesis of chiral tetrahydropyrans [1] due to their presence in many biological active natural products [2]. In a recent paper we reported the influence of the protecting group on the cyclization of δ-hydroxyepoxides for the synthesis of tetrahydropyrans [1d]. With this method, we were able to synthesize the diastereoisomeric tetrahydropyrans 2 and 3 in chiral form in good yield (Scheme 1).
Scheme 1.
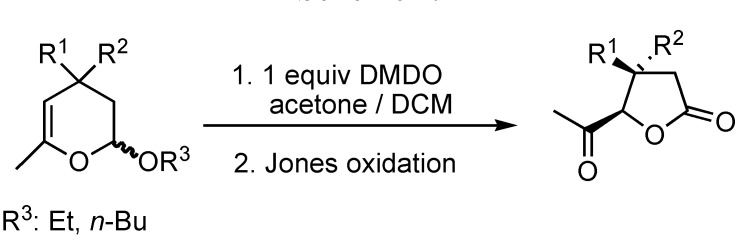
Recently Armstrong et al. have described the ring contraction of 2-alkoxy-3,4-dihydro-2H-pyrans into 4,5-cis-disubstituted tetrahydropyranones by oxidative rearrangement (Scheme 2) [3]. These results prompted us to communicate our findings in this area.
Scheme 2.
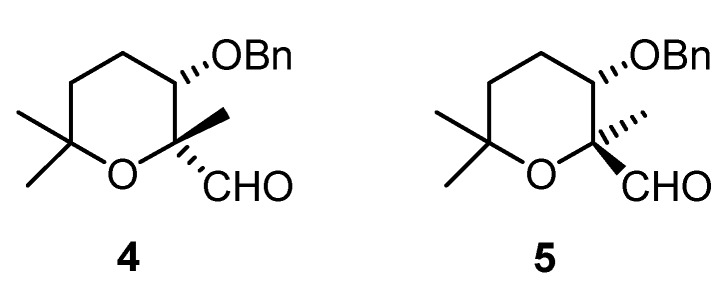
The asymmetric epoxidation of unfunctionalized olefins using sugar ketone derivatives as catalysts is an emerging area in organic chemistry [4]. On the other hand, aldehydes have not been used very often as catalysts in this kind of reactions since they are prone to Oxone® oxidation to give the corresponding acids. Zhao has demonstrated that enantioselective epoxidation could be achieved with Oxone® and an aldehyde bearing an oxygenated functionalization in α-position [5]. Having established an easy procedure for the synthesis of tetrahydropyrans 2 and 3, we decided to use them as precursors for aldehydes 4 and 5 (Figure 1), which can be used as catalysts for enantioselective epoxidation following Zhao’s methodology.
Figure 1.
Results and Discussion
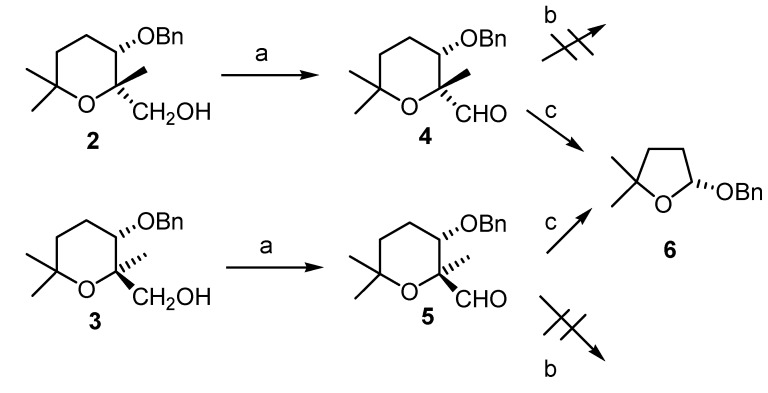
When compounds 2 and 3 were submitted separately to Ley´s oxidation conditions [6] the aldehydes 4 and 5 were obtained in good yields (Scheme 3).
Scheme 3.
a) TPAP, NMO, CH2Cl2, sieves mol, 2 to 4 62%, 3 to 5, 73%; b) Shi epoxidation conditions [7]: Oxone® (5 equiv), aq. K2CO3, Na2EDTA (0.4mM, aq.sol.), Buffer pH=10.5, (Bu4N+)2SO4, CH3CN, 0%. c) Yang epoxidation conditions [8] (pH= 7.5): Oxone® (10 eq.), NaHCO3 (15 eq.), Na2EDTA (0.4mM, aq.sol.), CH3CN/H2O, 60%.
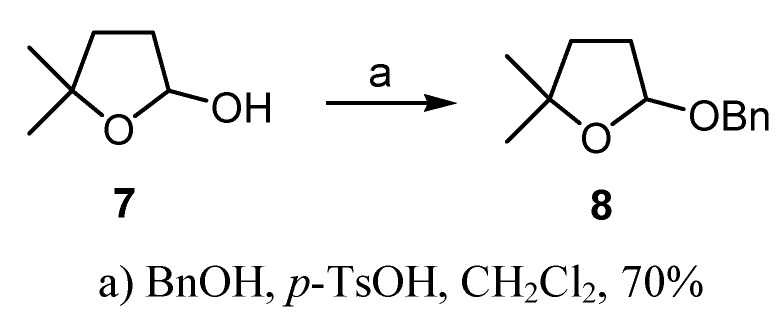
When compounds 4 and 5 were used as catalysts for the epoxidation of trans-stilbene under Shi conditions [7], no epoxidation was observed and the starting material was recovered. On the other hand, when 4 and 5 were used as catalysts for asymmetric epoxidation of the same trans-stilbene substrate under Yang’s conditions [8] only a 6% yield of trans-stilbene oxide was obtained with 0% e.e., but tetrahydrofuran 6 was obtained in good yield (60%). This compound showed optical rotation, so we decided to check its enantiomeric purity by chiral HPLC. For this purpose, the racemic derivative 8 [1b] was obtained by treatment of lactol 7, previously obtained in our group, with benzyl alcohol in acidic media [9] (Scheme 4). From the HPLC analysis, it could be seen that lactol 6 was obtained in an e.e. of 78% [10].
Scheme 4.
a) BnOH, p-TsOH, CH2Cl2, 70%
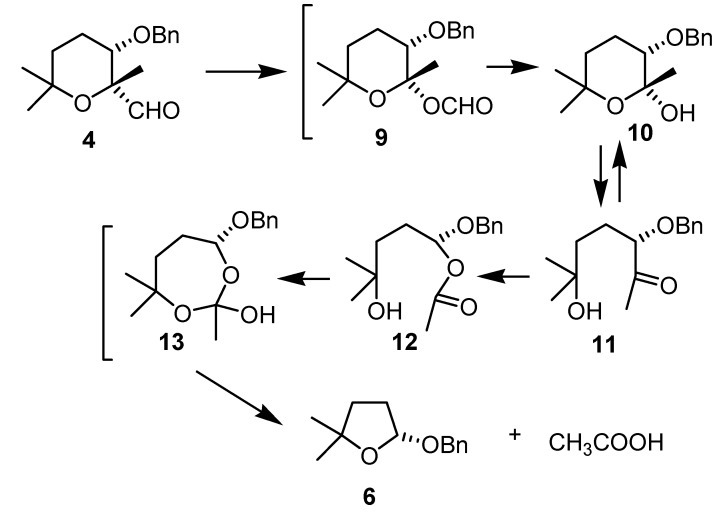
These results could be rationalised by a Baeyer-Villiger oxidation [11] of the aldehyde to give, after hydrolysis, lactol 10. This lactol will be in equilibrium with the corresponding ketone 11, which can undergo another Baeyer-Villiger oxidation to give the acetate 12. This second oxidation is favoured by the presence of an oxygenated function in the α-position [12]. The rearrangement to the lactol 6 maintaining the chirality could be understood through the intermediacy of a hemiorthoester 13, which is the compound that finally rearranges to afford lactol 6. Exactly the same results were obtained with aldehyde 5, when submitted to the same reaction conditions (Scheme 5).
Scheme 5.
Proposed rearrangement from aldehyde 4 to lactol 6.
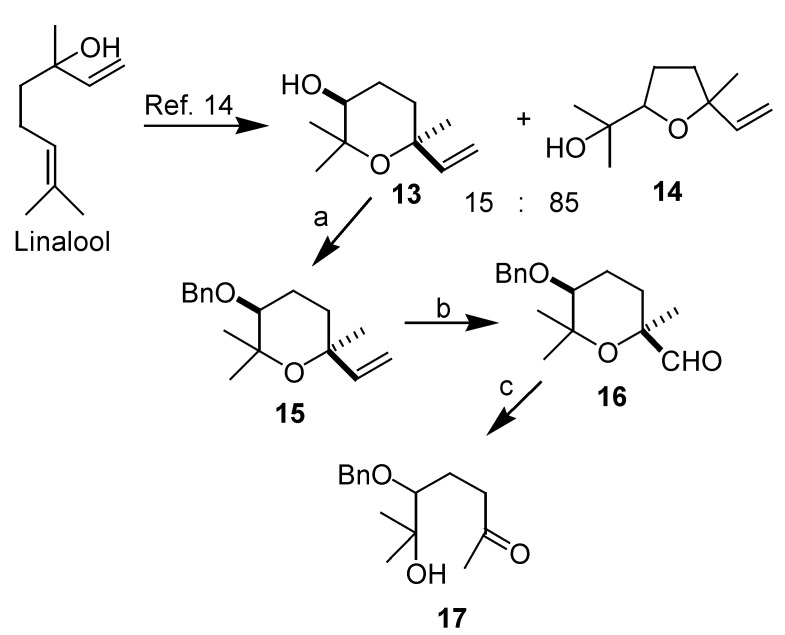
In order to check the influence of the benzyloxy substituent at the 3 position in the rearrangement, an aldehyde analog of 4 and 5, but with the benzyloxy substituent in C-5, was synthesised and submitted to the same oxidizing conditions (Scheme 6).
Scheme 6.
a) NaH, BnBr, THF, 90%; b) 1.- OsO4, NMO, THF/H2O. 2.- NaIO4, THF/H2O, 84%; c) Yang epoxidation conditions [8] (pH= 7.5): Oxone® (10 eq.), NaHCO3 (15 eq.), Na2EDTA (0.4mM, aq.sol.), CH3CN/H2O.
Epoxidation of racemic linalool gave an 81% yield of a 15:85 mixture of tetrahydropyrans and tetrahydrofurans [13], from which cis-tetrahydropyran 13 was isolated. This compound was submitted to benzylation under standard conditions (NaH, BnBr) thus giving 15 in good yield [14]. Cleavage of the double bond by dihydroxylation with OsO4 and subsequent treatment with NaIO4 [15], gave the required aldehyde 16 in good yield. When this aldehyde was submitted to Yang epoxidation conditions, methylketone 17 was obtained, so in this case, only one Baeyer- Villiger oxidation has occurred and it can be concluded that a hydroxyl function next to the aldehyde is necessary in order to produce the observed rearrangement.
Conclusions
A new rearrangement under oxidative conditions is described for the transformation of 3-(benzyloxy)-tetrahydro-2,6,6-trimethyl-2H-pyran-2-carbaldehydes into chiral tetrahydrofuranlactols. Because both enantiomeric forms can be obtained using the appropriate Sharpless epoxidation conditions, this methodology opens new ways for obtaining chiral synthons.
Experimental
General
Unless otherwise stated, all chemicals were purchased as the highest purity commercially available and were used without further purification. IR spectra were recorded on an AVATAR 370 FT-IR Thermo Nicolet spectrophotometer. 1H- and 13C-NMR spectra were recorded in CDCl3 using a Varian 200 VX instrument operating at 200 and 50.3 MHz for 1H and 13C, respectively. Chemical shifts are reported in δ ppm referenced to the residual CHCl3 peak at δ 7.26 ppm and δ 77.0 ppm, for 1H and 13C, respectively, and coupling constants (J) are given in Hz. HRMS were recorded on a QSTAR XL spectrometer using electrospray ionization (ESI) technique. Optical rotations were determined on a Perkin-Elmer 241 polarimeter in 1 dm cells. Diethyl ether and THF were distilled from sodium and dichloromethane was distilled from calcium hydride under an Ar atmosphere.
(2R,3S)-3-(benzyloxy)-tetrahydro-2,6,6-trimethyl-2H-pyran-2-carbaldehyde (4)
To a stirred solution of 2 (103 mg, 0.39 mmol), N-methylmorpholine-N-oxide (158 mg, 1.17 mmol) and 3Å molecular sieves in dry CH2Cl2 (4 mL), a catalytic amount of tetrapropylammonium perruthenate was added. After stirring the reaction mixture at room temperature for 1 hour, it was filtered through a short pad of silica washing with CH2Cl2 to obtain 65 mg (0.25 mmol, 64%) of aldehyde 4. [α]D20 +65.5o (c 0.9, CHCl3); IR (film): 2970-2870,1736, 1452, 1370, 1258, 1111, 737, 698 cm-1. 1H NMR: δ = 9.71 (1H, s, CHO), 7.35-7.30 (5H, m, -Ph), 4.58 (1H, d, J = 11.8 Hz, O-CH2-Ph, HA), 4.37 (1H, d, J = 11.8 Hz, O-CH2-Ph, HB), 3.48 (1H, dd, J1 = 2.69, J2 = 5.92 Hz, H-3), 1.9-1.3 (4H, m, H-4 and H-5), 1.28 (3H, s, H-9), 1.27 (6H, s, H-7 and H-8); 13C-NMR: δ = 20.5 (C-4), 22.5 (C-9), 29.1 (C-7), 30.8 (C-8), 32.0 (C-5), 71.3 (O-CH2-Ph), 72.4 (C-6), 78.1 (C-3), 80.0 (C-2), 127.6 (Phorto), 127.6 (Phpara), 128.3 (Phmeta), 138.2 (Phipso), 205.4 (C-10); HRMS-ESI: [M+Na]+ calcd for C16H22O3Na, 285.1461; found, 285.1474.
(2S,3S)-3-(benzyloxy)-tetrahydro-2,6,6-trimethyl-2H-pyran-2-carbaldehyde (5).
To a stirred solution of 3 (70 mg, 0.26 mmol), N-methylmorpholine-N-oxide (107 mg, 0.79 mmol) and 3Å molecular sieves in dry CH2Cl2 (2.5 mL), a catalytic amount of tetrapropylammonium perruthenate was added. After stirring the reaction mixture at room temperature for 1 hour, it was filtered through a short pad of silica which was washed with CH2Cl2 to obtain 51 mg (0.19 mmol, 73%) of aldehyde 5. [α]D20 + 76.31º (c 0.9, CHCl3); IR (film): 2970-2870, 1736, 1452, 1370, 1258, 1213, 1111, 1026, 737, 698 cm-1; 1H-NMR: δ= 9.56 (1H, s, H-10), 7.35-7.30 (5H, m, -Ph), 4.63 (1H, d, J = 11.8 Hz, O-CH2-Ph, HA), 4.46 (1H, d, J = 11.8 Hz, O-CH2-Ph, HB), 3.75 (1H, bs, H-3), 2.0-1.5 (4H, m, H-4 and H-5), 1.30 (3H, s, H-9), 1.20 and 1.15 (3H each, s, H-7 and H-8); 13C-NMR: δ = 19.6 (C-9), 20.5 (C-4), 26.7 (C-7), 30.4 (C-5), 31.3 (C-8), 71.3 (O-CH2-Ph), 71.5 (C-3), 73.1 (C-6), 81.1 (C-2), 127.5 (Phpara), 127.7 (Phorto), 128.3 (Phmeta), 138.5 (Phipso), 204.4 (C-10); HRMS-ESI: [M+Na]+ calcd for C16H22O3Na, 285.1461; found, 285.1457.
(R)-2-(Benzyloxy)-tetrahydro-5,5-dimethylfuran (6)
To a stirred solution of aldehyde 4 (13 mg, 0.05 mmol) in CH3CN (0.8 mL) at room temperature, aq. Na2EDTA (0.5 mL of 0.4 mM solution) were added. Then, a mixture of Oxone® (150 mg, 0.25 mmol KHSO5) and NaHCO3 (66 mg, 0.77 mmol) was added in small portions over 30 minutes. The reaction mixture was stirred for 12 hours, and then diluted with H2O (10 mL) and extracted with EtOAc (3 x 15 mL). The organic layers were combined, washed with brine and dried over anhydrous Na2SO4. After filtering and evaporating the solvents, the crude was purified by flash chromatography (hexane/EtOAc, 95:5) to obtain 6 mg (0.03 mmol, 60%) of 6. [α]D20 79.4 o (c 0.51, CHCl3); IR (film): 2970, 2924, 1454, 1361, 1262, 1138, 1091, 1042, 1026, 1008, 962 cm-1; 1H-NMR: δ = 7.34-7.25 (5H, m, -Ph), 5.15-5.17 (1H, m, H-2), 4.75 (1H, d, J = 11.7 Hz, O-CH2-Ph, HA), 4.42 (1H, d, J = 11.7 Hz, O-CH2-Ph, HB), 2.07-1.72 (4H, m, H-3 and H-4), 1.40 (3H, s, Me-6), 1.24 (3H, s, Me-7); 13C-NMR: δ = 29.1 (C-7), 30.3 (C-6), 33.3 (C-3), 36.5 (C-4), 68.7 (O-CH2-Ph), 82.7 (C-2), 103.7 (C-5), 127.6 (-Ph), 128.1 (-Ph), 128.5 (-Ph), 138.7 (Phipso); HRMS-ESI: (m/z) [M+Na]+ calcd for C13H18O2Na, 229.1199; found, 229.1200.
(±)-2-(Benzyloxy)-tetrahydro-5,5-dimethylfuran (8)
To a stirred solution of 7 (348 mg, 3.0 mmol) in dry CH2Cl2, benzyl alcohol (3.24 g, 30.0 mmol) and a catalytic amount of p-TsOH were added. The reaction mixture was stirred at room temperature for 30 minutes and then quenched by addition saturated aqueous solution of NaHCO3 (20 mL) and CH2Cl2 (100 mL). The phases were separated and the organic one was washed with brine and dried over anhydrous Na2SO4. After filtering and evaporating the solvents, the crude was purified by flash chromatography (hexane/EtOAc, 95:5) to obtain 430 mg (1.96 mmol, 70%) of 8, the racemate of compound 6.
3-(benzyloxy)-tetrahydro-2,2,6-trimethyl-6-vinyl-2H-pyran (15)
A solution of 13 (130 mg, 0.76 mmol) in THF (1 mL) was added over a suspension of NaH in THF (2 mL) at 0ºC. Then, BnBr (0.18 mL, 1.53 mmol) was added over the mixture and it was left stirring at room temperature for 12 hours. The reaction was quenched by addition of H2O and then it was extracted with EtOAc (3 x 70 mL). The organic layers were combined, washed with brine and dried over anhydrous Na2SO4. After filtering and evaporating the solvents, the crude was purified by flash chromatography (hexane/EtOAc, 95:5) to obtain 180 mg (0.70 mmol, 90%) of 15. IR (film): 2974, 2942, 2871, 1496, 1454, 1365, 1238, 1164, 1092, 986, 911 cm-1; 1H-NMR: δ = 7.40-7.27 (5H, m, -Ph), 6.01 (1H, dd, J1= 17.2 Hz, J2= 11.0 Hz, H-1’), 5.00 (1H, d, J= 11.0 Hz, H-2’cis), 4.99 (1H, d, J= 17.2 Hz, H-2’trans), 4.67 (1H, d, J= 11.7 Hz, O-CH2-Ph, HA), 4.48 (1H, d, J= 11.7 Hz, O-CH2-Ph, HB), 3.21 (1H, dd, J1=11.0 Hz, J2= 4.2 Hz, H-3), 2.21-1.51 (4H, m, H-4 and H-5), 1.27, 1.24 and 1.18 (3H each, s, H-7, H-8 and H-9); 13C-NMR: δ = 19.2 (C-7), 19.5 (C-4), 27.7 (C-9), 29.7 (C-8), 30.2 (C-5), 68.8 (C-6), 71.1 (O-CH2-Ph), 73.4 (C-2), 79.9 (C-3), 108.3 (C-2’), 125.1 (Ph), 126.0 (Ph), 136.7 (Phipso), 144.0 (C-1’); HRMS-ESI: [M+Na]+ calcd for C17H24O2Na, 283.1668; found, 283.1657.
5-(benzyloxy)-tetrahydro-2,6,6-trimethyl-2H-pyran-2-carbaldehyde (16)
A catalytic amount of OsO4 (0.1 mL of a 2.5 % w/v solution in t-butanol) and N-methyl-morpholine-N-oxide (183 mg, 1.34 mmol) were added to a stirred solution of 15 (100 mg, 0.38 mmol) in THF/H2O (2:1, 1 mL). The reaction mixture was stirred at room temperature for 20 hours and then quenched by addition of saturated aqueous Na2SO3 solution. After extracting with EtOAc (3 x 50 mL), the organic layers were combined and washed successively with saturated aqueous Na2SO3 solution, H2O, brine and then dried over anhydrous Na2SO4. After filtering and evaporating the solvents, the crude product was dissolved in 5:1 THF/H2O (3 mL), then NaIO4 (182 mg, 0.85 mmol) was added and the reaction mixture was stirred at room temperature for 20 minutes. It was diluted with H2O (10 mL), extracted with Et2O (3 x 50 mL) and the organic layers combined and washed with H2O and brine. After drying over anhydrous Na2SO4, filtering and evaporating the solvents, 86 mg (0.33 mmol, 84%) of aldehyde 16 were obtained. IR (film): 2975, 2929, 2867, 2789, 1728, 1454, 1361, 1257, 1097, 1009 cm-1; 1H-NMR: δ = 9.60 (1H, s, H-9), 7.34-7.26 (5H, m, -Ph), 4.63 (1H, d, J= 12 Hz, O-CH2-Ph, HA), 4.43 (1H, d, J= 12 Hz, O-CH2-Ph, HB), 3.21-3.15 (1H, m, H-3), 2.41-1.35 (4H, m, H-4 and H-5), 1.31, 1.12 and 1.09 (3H each, s, H-7, H-8 and H-9); 13C-NMR: δ = 21.0 (C-10), 21.7 (C-4), 24.3 (C-7), 29.3 (C-8), 29.8 (C-5), 71.2 (O-CH2-Ph), 76.5 (C-2), 78.3 (C-6), 81.0 (C-3), 127.7 (Ph), 127.7 (Ph), 128.5 (Ph), 138.9 (Phipso), 205.0 (C-9).
5-(benzyloxy)-6-hydroxy-6-methylheptan-2-one (17)
To a stirred solution of aldehyde 16 (38 mg, 0.14 mmol) in CH3CN (2.5 mL) at room temperature, aq. Na2EDTA (1.5 mL of 0.4 mM solution) was added. Then, a mixture of Oxone® (450 mg, 0.72 mmol KHSO5) and NaHCO3 (190 mg, 2.25 mmol) was added in small portions over 30 minutes. The reaction mixture was stirred for 12 hours and then diluted with H2O (10 mL) and extracted with EtOAc (3 x 15 mL). The organic layers were combined, washed with brine and dried over anhydrous Na2SO4. After filtering and evaporating the solvents, the crude was purified by flash chromatography (7:3 hexane/Et2O) to afford 18 mg (0.07 mmol, 50%) of 17. IR (film): 3452, 2926, 2855, 1715, 1458, 1364, 1260, 1096, 1030 cm-1; 1H-NMR: δ = 7.33-7.31 (5H, m, -Ph), 4.62 (2H, s, O-CH2-Ph), 3.24 (1H, dd, J1= 8.2 Hz, J2= 3.4 Hz, H-2), 2.5-2.48 (2H, m, H-4), 2.08 (3H, s, H-6), 1.87-1.66 (2H, m, H-3), 1.23 (6H, s, H-7 and H-8); 13C-NMR: δ = 22.9 (C-3), 24.6 (C-8), 24.6 (C-7), 27.2 (C-6), 40.4 (C-4), 73.8 (C-1), 74.9 (O-CH2-Ph), 85.7 (C-2), 128.0 (Ph), 128.8 (Ph), 138.6 (Phipso), 209.0 (C-5); HRMS-ESI: [M+Na]+ calcd for C15H22O3Na, 273.1461; found, 273.1451.
Acknowledgements
The authors wish to thank the Junta de Castilla y León (SA045A05), the Spanish MEC (CTQ2005-06813/BQU) and F. S. E. for financial support. M.G.N thanks the Spanish MEC for a FPU doctoral fellowship.
Footnotes
Sample Availability: Samples of compounds 2-6 and 13-17 are available from the authors.
References and Notes
- 1. Urones J. G., Díez D., Marcos I. S., Basabe P., Garrido N. M., Escarcena R., Lithgow A. M., Dominguez M. F., Sánchez J. M. Synlett. 1995:855–856. Díez D., Marcos I. S., Basabe P., Romero R. E., Moro R. F., Lumeras W., Rodríguez L., Urones J. G. Synthesis. 2001:1013–1022. Díez D., Moro R. F., Lumeras W., Rodríguez L., Marcos I. S., Basabe P., Escarcena R., Urones J. G. Synlett. 2001:1335–1337. Díez D., Moro R. F., Lumeras W., Rodríguez L., Marcos I. S., Basabe P., Escarcena R., Urones J. G. Synthesis. 2002:175–184. Díez D., Nuñez M.G., Moro R. F., Lumeras W., Marcos I. S., Basabe P., Urones J. G. Synlett. 2005:939–941.
- 2. Elliott M. C. J. Chem. Soc., Perkin Trans. 1. 2002:2301–2340. doi: 10.1039/b201515n. Elliott M. C., Williams E. J. Chem. Soc., Perkin Trans. 1. 2001:2303–2323. doi: 10.1039/b007290g. Elliott M. C. J. Chem. Soc., Perkin Trans. 1. 2000:1291–1318. doi: 10.1039/a903885j.
- 3.Armstrong A., Chung H. Tetrahedron Lett. 2006;47:1617–1619. [Google Scholar]
- 4.Reviews: Frohn M., Shi Y. Synthesis. 2000:1979–2000. doi: 10.1055/s-2000-8715. Armstrong A. Angew. Chem., Int. Ed. 2004;43:1460–1462. doi: 10.1002/anie.200301716. Shi Y. Acc. Chem. Res. 2004;37:488–496. doi: 10.1021/ar030063x. Yang D. Acc. Chem. Res. 2004;37:497–505. doi: 10.1021/ar030065h. Tsuchiya T., Armstrong A. Tetrahedron. 2006;62:257–263. doi: 10.1016/j.tet.2005.08.112. Armstrong A., Hayter B. R. Chem. Commun. 1998:621–622. doi: 10.1039/a708695d. Armstrong A., Hayter B. R., Moss W. O., Reeves J. R., Wailes J. S. Tetrahedron: Asymmetry. 2000;11:2057–2061. doi: 10.1016/S0957-4166(00)00170-1. Armstrong A., Moss W. O., Reeves J. R. Tetrahedron: Asymmetry. 2001;12:2779–2781. doi: 10.1016/S0957-4166(01)00495-5. Armstrong A., Ahmed G., Dominguez-Fernandez B., Hayter B. R., Wailes J. S. J. Org. Chem. 2002;67:8610–8617. doi: 10.1021/jo026322y. Shing T. K. M., Leung G. Y. C., Yeung K. W. Tetrahedron Lett. 2003;44:9225–9228. Shing T. K. M., Leung Y. C., Yeung K. W. Tetrahedron. 2003;59:2159–2168. doi: 10.1016/S0040-4020(03)00145-5. Shing T. K. M., Leung G. Y. C. Tetrahedron. 2002;58:7545–7552. doi: 10.1016/S0040-4020(02)00577-X. Stearman C. J., Behar V. Tetrahedron Lett. 2002;43:1943–1946. doi: 10.1016/S0040-4039(02)00173-9. Matsumoto K., Tomioka K. Tetrahedron Lett. 2002;43:631–633. doi: 10.1016/S0040-4039(01)02204-3. Klein S., Roberts S. M. J. Chem. Soc., Perkin Trans. 1. 2002:2686–2691. doi: 10.1039/b205946k. Denmark S. E., Matsuhashi H. J. Org. Chem. 2002;67:3479–3486. doi: 10.1021/jo020050h. Bortolini O., Fantin G., Fogagnolo M., Forlani R., Maietti S., Pedrini P. J. Org. Chem. 2002;67:5802–5806. doi: 10.1021/jo020146b. Tian H. Q., She X. G., Shu L. H., Yu H. W., Shi Y. J. Am. Chem. Soc. 2000;122:11551–11552. Tian H. Q., She X. G., Xu J. X., Shi Y. Org. Lett. 2001;3:1929–1931. doi: 10.1021/ol010066e. Tian H. Q., She X. G., Yu H. W., Shu L. H., Shi Y. J. Org. Chem. 2002;67:2435–2446. doi: 10.1021/jo010838k. Shu L. H., Wang P. Z., Gan Y. H., Shi Y. Org. Lett. 2003;5:293–296. doi: 10.1021/ol020229e. Shu L. H., Shi Y. Tetrahedron Lett. 2004;45:8115–8117. doi: 10.1016/j.tetlet.2004.08.124. Hickey M., Goeddel D., Crane Z., Shi Y. Proc. Natl. Acad. Sci. U.S.A. 2004;101:5794–5798. doi: 10.1073/pnas.0307548101. Armstrong A., Dominguez-Fernandez B., Tsuchiya T. Tetrahedron. 2006;62:6614–6620. doi: 10.1016/j.tet.2005.12.073.
- 5. Bez G., Zhao C-G. Tetrahedron Lett. 2003;44:7403–7406. doi: 10.1016/j.tetlet.2003.08.040. Travis B. R., Sivakumar M., Hollist G. O., Borhan B. Org. Lett. 2003;5:1031–1034. doi: 10.1021/ol0340078. Webb K. S., Ruszkay S. J. Tetrahedron. 1998;54:401–410. doi: 10.1016/S0040-4020(97)10299-X.
- 6.Ley S. V., Norman J., Griffith W. P., Marsden S. P. Synthesis. 1994:639–666. [Google Scholar]
- 7.Wang Z. X., Tu Y., Fhron M., Zhang J. R., Shi Y. J. Am. Chem. Soc. 1997;119:11224–11235. doi: 10.1021/ja972272g. [DOI] [Google Scholar]
- 8.Yang D., Wong M. K., Yip Y. C. J. Org. Chem. 1995;60:3887–3889. doi: 10.1021/jo00117a046. [DOI] [Google Scholar]
- 9.Clive D. J., Ardelean E-S. J. Org. Chem. 2001;66:4841–4844. doi: 10.1021/jo010206y. [DOI] [PubMed] [Google Scholar]
- 10.Measured by chiral HPLC (ChiralPak AD-H, 25 cm x 10 mm, 99:1 hexane/iPrOH), retention times minor enantiomer (4.6 min) and major enantiomer (4.9 min).
- 11.Reviews: Renz M., Meunier B. Eur. J. Org. Chem. 1999:737–750. doi: 10.1002/(SICI)1099-0690(199904)1999:4<737::AID-EJOC737>3.0.CO;2-B. ten Brink G.J., Arends I.W.C.E., Sheldon R. A. Chem. Rev. 2004;104:4105–4123. doi: 10.1021/cr030011l. Krow G. R. Org. React. 1993;43:251–798. Crudden C. M., Chen A. C., Calhoun L. A. Angew. Chem., Int. Ed. 2004;43:2851–2855. doi: 10.1002/1521-3773(20000818)39:16<2851::aid-anie2851>3.0.co;2-y. Krasutsky P. A., Kolomytsin I. V. Arkivoc. 2005:151–171. doi: 10.3998/ark.5550190.0006.412. Alvarez-Idaboy J. R., Reyes L., Cruz J. Org. Lett. 2006;8:1763–1765. doi: 10.1021/ol060261z. Frison J-C., Palazzi C., Bolm C. Tetrahedron. 2006;62:6700–6706. doi: 10.1016/j.tet.2005.12.080. Chang M-Y., Kung Y-H., Chen S-T. Tetrahedron Lett. 2006;47:4865–4870. doi: 10.1016/j.tetlet.2006.05.027.
- 12.Dave V., Warnhoff E. W. J. Org. Chem. 1983;48:2590–2598. doi: 10.1021/jo00163a034. Also see ref. [11c]. [DOI] [Google Scholar]
- 13.Urones J. G., Díez D., Marcos I. S., Basabe P., Lithgow A. M., Moro R. F., Garrido N. M., Escarcena R. Tetrahedron. 1995;51:3691–3704. doi: 10.1016/0040-4020(95)00084-L. [DOI] [Google Scholar]
- 14.Greene T.W., Wuts P.G.M. Protective Groups in Organic Chemistry. 3rd ed. John Wiley & Sons; New York: 1999. [Google Scholar]
- 15.Hubbs J. L., Heathcock C. H. J. Am. Chem. Soc. 2003;125:12836–12843. doi: 10.1021/ja030316h. [DOI] [PubMed] [Google Scholar]