Abstract
Nine known and one new ent-kaurene diterpenoid were isolated from the acetone extract of Sideritis stricta Boiss & Heldr. The new compound, identified as ent-1β-hydroxy-7α-acetyl-15β,16β-epoxykaurane (1) by IR, 1D and 2D NMR techniques and mass spectra, was isolated along with sideroxol (2), 7-acetyl sideroxol (3), 7-epicandicandiol (4), linearol (5), ent-7α,15β,18-trihydroxy-kaur-16-ene (6), ent-7α-acetyl,15,18-dihydroxy-kaur-16-ene (7), foliol (8), sideridiol (9) and siderol (10). The antibacterial and antifungal activities of these compounds and the whole crude acetone extract were evaluated against E. coli, S. aureus, K. pneumeonia and C. albicans.
Keywords: Kaurene, Diterpenoids, Sideritis stricta, Lamiaceae, Antibacterial Activity
Introduction
There are 46 Sideritis flora species in Turkey, of which 36 species and 10 subspecies are endemic [1]. Sideritis species have been used in folk medicine in Turkey and Europe for their antinflammatory, antirheumatic, digestive and antimicrobial properties [2,3]. Sideritis species contain mainly kaurene diterpenoids, but they rarely have labdane, pimarane or atisene diterpenoids. In this study, one new and nine known ent-kaurene diterpenoids were isolated from Sideritis stricta and the antibacterial and antifungal activities of these compounds against E. coli, S. aureus, K. pneumeonia and C. albicans was evaluated.
Results and Discussion
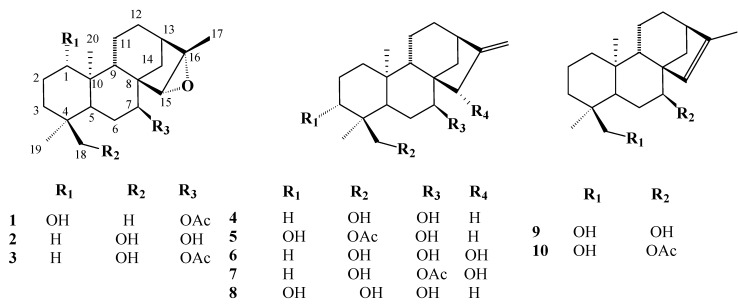
A new ent-kaurane, identified as ent-1β-hydroxy-7α-acetyl-15β,16β-epoxykaurane (1) was isolated, along with nine known ent-kaurenes, from the whole plant extract of S. stricta. The known kaurene diterpenes were identified as sideroxol (2) [4,5], 7-acetyl sideroxol (3) [4] 7-epicandicandiol (4), [6,7,8,9] linearol (5), [6,7,8,9,10] ent-7α,15β,18-trihydroxy-kaur-16-ene (6) [10,11], ent-7α-acetyl,15,18-dihydroxy-kaur-16-ene (7) [10,11], foliol (8) [12], sideridiol (9) [13,14] and siderol (10) [15], respectively (Figure 1). All the compounds were identified based on IR, 1H- and 13C-NMR and mass spectroscopic techniques. The structures of known compounds were confirmed by comparison to literature data.
Figure 1.
The IR spectrum of compound 1 showed the presence of an acetyl group, with absorption bands at 1720 and 1280 cm-1. An epoxy group at 1085 cm-1 and a hydroxyl group at 3400 cm-1 were also observed. In the HRMS spectrum, compound 1 gave a molecular ion peak at m/z 362.2560, corresponding to a molecular composition of C22H34O4. In the 1H-NMR spectrum four signals (s, 3H) for four methyl groups were observed at δ 0.78, 0.82, 1.08, and 1.44 ppm. In addition, there was an acetyl methyl signal at δ 2.08, which was corroborated with a signal at δ 4.86 appearing as a narrow triplet (J = 2.5 Hz) and attributed to the C-7 α proton. The presence of a hydroxyl group at C-1 was observed as a dd (J=10 and 5 Hz) and the corresponding C-1 carbon signal was observed at 80.3 ppm. These chemical shifts and the doublet of doublets are characteristic signals for the α position of C-1 [16]. The presence of a singlet at δ 2.98 was indicative of a characteristic H-15β-epoxy proton, as observed in similar kaurane diterpenes [17]. The APT 13C-NMR spectrum revealed 22 carbon signals, consisting of five methyls, six methylenes, six methines and five quaternary carbon atoms. A methine carbon at δ 74.1 was assigned to C-7, while the one at δ 80.3 was assigned to C-1. Another methine carbon at δ 62.4 was attributed to the epoxy methine carbon (C-15), while the quaternary carbon of this epoxy group was observed at δ 77.9. The assignments of protonated carbon signals were carried out by a HMQC experiment. Thus, the structure of this diterpenoid 1 was elucidated as ent-1β-hydroxy-7α-acetyl-15β,16β-epoxykaurane, which has now been isolated for the first time from Nature.
Biological activity
The acetone extract of S. stricta and the pure compounds 1-10 were tested against standard bacterial and fungal strains (Table 1). The MIC values indicated that they showed very little activity against the bacterial and fungal species tested, compared to gentamycin and flucanozole.
Table 1.
Antibacterial and antifungal activity of acetone extract of S. stricta and kaurene diterpenoids.
| Tested material | E. coli | S. aureus | K. pneumonia | C. albicans |
|---|---|---|---|---|
| S. stricta extract | 300 | 600 | 300 | NA |
| 1 | NA | 600 | NA | NA |
| 2 | NA | NA | NA | NA |
| 3 | NA | 600 | 600 | NA |
| 4 | 200 | 200 | NA | NA |
| 5 | 600 | 600 | 600 | NA |
| 8 | 600 | 600 | NT | NA |
| 9 | 300 | 600 | 600 | NA |
| 10 | NA | NT | 300 | NA |
| Gentamycin* | 0.97 | 0.48 | 0.48 | NT |
a MIC values are given as mg/L, NA: Non-Active; NT: Not tested
* Gentamycin and Flucanozole were used as positive controls and results were given as μg/mL.
Conclusions
We have reported the isolation from S. stricta of several known diterpenoids and a new ent-kaurane diterpenoid, identified as ent-1β-hydroxy-7α-acetyl-15β,16β-epoxykaurane (1). The antimicrobial activity of the crude acetone extract of the studied plant and the pure compounds is reported. Neither the extract nor any of the individual kaurane diterpenoids showed good activity against E. coli, S. aureus, K. pneumonia and C. albicans.
Experimental
General
1H- and 13C-NMR spectra were obtained in CDCl3 at 500 and 125 MHz, respectively, using a Bruker Avance 500 NMR. IR and mass spectra were recorded with a IR: Perkin-Elmer 980 (in CHCl3) and a VG ZabSpec High Resolution Mass Spectrometer. Silicagel 60 was used for column chromatography and Kieselgel 60F254 precoated plates (E. Merck) for prep. TLC. All the solvents were purchased from Merck.
Plant material
Sideritis stricta Boiss. & Heldr. was collected in July 2004 from Termesos National Park (Antalya Province, Turkey). The plant was identified by Prof Dr. G. Tümen (Balıkesir University), and a voucher specimen (TD 1485) was deposited at the Herbarium of the Faculty of Pharmacy, Anadolu University.
Extraction and isolation
The powdered whole plant (1.5 kg) was extracted with acetone to give a crude extract (54 g). A portion of this extract (25 g) was fractionated on a silica gel column. Elution was started with hexane and continued with gradients of chloroform, acetone and then methanol to give ent-1β-hydroxy-7α-acetyl-15β,16β-epoxykaurane (23 mg, 1), sideroxol (54 mg, 2), 7-acetylsideroxol (102 mg, 3), 7-epi-candicandiol (178 mg, 4), linearol (210 mg, 5), ent-7α, 15β, 18-trihydroxy-kaur-16-ene (32 mg, 6), ent-7α-acetyl-15,18-dihydroxy-kaur-16-ene (17 mg, 7), foliol (48 mg, 8), sideridiol (205 mg, 9) and siderol (183 mg, 10). Purification of the new compound 1 was carried out by preparative TLC, using CHCl3-acetone (9:1) as eluent. IR 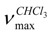 ent-1β-hydroxy-7α-acetyl-15β,16β-epoxykaurane (1) IR
ent-1β-hydroxy-7α-acetyl-15β,16β-epoxykaurane (1) IR 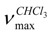 cm-1 : 3400, 1725 and 1270 (C=O), 1050 (C-O); 1H-NMR δ: 4.86 (1H, t, J=2.5, H-7), 3.32 (1H, dd, J=10 and 5 Hz, H-1), 2.97 (1H, s, H-15), 2.09 (3H,s, OAc), 2.98 (1H, s, H-15), 2.08 (3H, s, OAc),1.44 (3H, s, Me-17), 1.08 (3H, s, Me-20), 0.78 (3H,s, Me-18) 0.82 (3H,s, Me-19); 13C-NMR δ: 80.3 (C-1), 29.2 (C-2), 34.8 (C-3), 37.6 (C-4), 34.8 (C-5), 26.3 (C-6), 74.1 (C-7), 48.3 (C-8), 45.9 (C-9), 38.9 (C-10), 17.3 (C-11), 27.4 (C-12), 39.3 (C-13), 31.2 (C-14), 62.4 (C-15), 77.9 (C-16), 17.9 (C-17), 18.6 (C-18), 17.5 (C-19), 15.3 (C-20), 21.1 (OCOCH3), 178.6 (OCO-CH3); EIMS (rel.int.) m/z: 362.2 [M]+ (10), 344 [M-OH]+ (28) 302.2 [M-COOCH3]+(23), 288.2 (45), 254.1 (85), 225.1 (30), 201.1 (50), 131.0 (60), 120.0 (50), 108.9 (82), 95.1 (65), 80.0 (23), 69.0 (97); HRMS: 362.2560 (calcd for C22H34O4 362.2457).
cm-1 : 3400, 1725 and 1270 (C=O), 1050 (C-O); 1H-NMR δ: 4.86 (1H, t, J=2.5, H-7), 3.32 (1H, dd, J=10 and 5 Hz, H-1), 2.97 (1H, s, H-15), 2.09 (3H,s, OAc), 2.98 (1H, s, H-15), 2.08 (3H, s, OAc),1.44 (3H, s, Me-17), 1.08 (3H, s, Me-20), 0.78 (3H,s, Me-18) 0.82 (3H,s, Me-19); 13C-NMR δ: 80.3 (C-1), 29.2 (C-2), 34.8 (C-3), 37.6 (C-4), 34.8 (C-5), 26.3 (C-6), 74.1 (C-7), 48.3 (C-8), 45.9 (C-9), 38.9 (C-10), 17.3 (C-11), 27.4 (C-12), 39.3 (C-13), 31.2 (C-14), 62.4 (C-15), 77.9 (C-16), 17.9 (C-17), 18.6 (C-18), 17.5 (C-19), 15.3 (C-20), 21.1 (OCOCH3), 178.6 (OCO-CH3); EIMS (rel.int.) m/z: 362.2 [M]+ (10), 344 [M-OH]+ (28) 302.2 [M-COOCH3]+(23), 288.2 (45), 254.1 (85), 225.1 (30), 201.1 (50), 131.0 (60), 120.0 (50), 108.9 (82), 95.1 (65), 80.0 (23), 69.0 (97); HRMS: 362.2560 (calcd for C22H34O4 362.2457).
Antibacterial and antifungal activity
The acetone extract of S. stricta and the individual compounds 1, 2, 3, 4, 5, 8, 9, and 10 were tested against standard bacterial strains such as E. coli ATCC 29995, S. aureus ATCC 6538P, K. pneumonia CCM 2318, and the yeast C. albicans ATCC 10239. The agar diffusion method was used to determine the inhibition zones of the tested compounds and acetone extract of S. stricta against these standard bacterial strains. The acetone extract of the species and the pure compounds with inhibition zones larger than 7 mm were selected for determination of quantitative antimicrobial activity expressed as minimum inhibition concentrations (MIC) [18]. The broth microdilution method was applied for this purpose [18,19,20].
Acknowledgements
The author thanks TÜBİTAK for supporting this study as a part of the project TBAG-2319 (103T067).
Footnotes
Sample Availability: Available from the author.
References
- 1.Mill M.R. In: Flora of Turkey and the East Aegean Islands. Davis P.H., editor. Vol.7. University Press; Edinburgh: 1982. pp. 192–193. [Google Scholar]
- 2.Baytop T. Therapy with Medicinal Plants in Turkey (Past and Present), 2. Nobel Tıp Kitabevleri; Istanbul: 1984. [Google Scholar]
- 3.Yeşilada E., Ezer N. Essential oil composition of four Turkish species of Sideritis. Phytochemistry. 1996;41:203–205. doi: 10.1016/0031-9422(95)00601-X. [DOI] [Google Scholar]
- 4.Kilic T., Yildiz Y.K., Topçu G., Gören A.C., Ay M., Bodige S., Watson W.H. X-ray analysis of sideroxol from Sideritis leptoclada. J. Chem. Cryst. 2005;35:647–650. doi: 10.1007/s10870-005-6163-z. [DOI] [Google Scholar]
- 5.Venturella P., Bellino A., Piozzi F. Diterpenes from Sideritis theezans. Phytochemistry. 1975;14:1451–1452. doi: 10.1016/S0031-9422(00)98662-X. [DOI] [Google Scholar]
- 6.Gonzalez A.G., Fraga B.M., Hernandez M.G., Hanson J.R. The 13C-NMR Spectra of Some ent-18-hydroxykaur-16-enes. Phytochemistry. 1981;20:846–847. doi: 10.1016/0031-9422(81)85195-3. [DOI] [Google Scholar]
- 7.Aljancic I., Macura S., Juranic S., Andjelkovic N., Randjelovic N., Milosavljevic S. Diterpenes from Achillea clypeolata. Phytochemistry. 1996;43:169–172. [Google Scholar]
- 8.Başer K.H.C., Bondi M.L., Bruno M., Kırımer N., Piozzi F., Tümen G., Vasollo N. An ent-kaurene from Sideritis Huber-Morathi. Phytochemistry. 1996;43:1293–1296. doi: 10.1016/S0031-9422(96)00371-8. [DOI] [Google Scholar]
- 9.Fraga B.M., Hernandez M.G., Fernandez C., Arteaga J.M. Diterpenes from Sideritis dendrochahorra and S. cystosiphon. Phytochemistry. 1987;26:775–777. doi: 10.1016/S0031-9422(00)84784-6. [DOI] [Google Scholar]
- 10.Topçu G., Gören A.C., Kılıç T., Yıldız Y.K., Tümen G. Diterpenes from Sideritis sipylea and S. dichotoma. Turk. J. Chem. 2002;26:189–194. [Google Scholar]
- 11.Venturella P., Bellino A. Eubotriol and Eupol, New Diterpenoids from Sideritis euboea. Experientia. 1977;33:1270–1271. doi: 10.1007/BF01920125. [DOI] [Google Scholar]
- 12.Cabrera E., Garcia-Granados A., Buruaga A.S.D., Buruaga J.M.S. Diterpenoids from Sideritis hirsuta ssp. Nivalis. Phytochemistry. 1983;22:2779–2781. doi: 10.1016/S0031-9422(00)97695-7. [DOI] [Google Scholar]
- 13.Fraga B.M., Hernandez M.G., Santana J.M.H., Artega J.M. Diterpenes from Sideritis ferrensis. Phytochemistry. 1991;30:913–915. doi: 10.1016/0031-9422(91)85278-8. [DOI] [Google Scholar]
- 14.Venturella P., Bellino A., Marino M.L. New Diterpenes from Sideritis sicula. Phytochemistry. 1978;17:811–812. [Google Scholar]
- 15.Queseda T.G.D., Rodrigez B., Valverde S. Diterpenenes from Sideritis lagascana and sideritis valverd. Phytochemistry. 1974;13:2008–2009. [Google Scholar]
- 16.Fraga B.M., Hernandez M.G., Diaz C.E. On the ent kaurene diterpenes from Sideritis athoa. Nat. Prod. Res. 2003;17:141–144. doi: 10.1080/1478641031000103722. [DOI] [PubMed] [Google Scholar]
- 17.Topçu G., Gören A.C., Kılıç T., Yıldız Y.K., Tümen G. Diterpenes from Sideritis trojana. Nat. Prod. Lett. 2002;16:33–37. doi: 10.1080/1057563029001/4827. [DOI] [PubMed] [Google Scholar]
- 18.Goren A.C., Topçu G., Bilsel G., Bilsel M., Wilkinson J.M., Cavanagh H.M. Analysis of essential oil of Satureja thymbra by hydrodistillation, thermal desorber and headspace GC/MS techniques and its antimicrobial activity. Nat. Prod. Res. 2004;18:189–195. doi: 10.1080/14786410310001608145. [DOI] [PubMed] [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards . Standard methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically. Vol. 10. Villanova, PA: 1990. NCCLS Approved Standard M7-A2. No. 8. [Google Scholar]
- 20.Goren A. C., Bilsel G., Bilsel M., Demir H., Kocabas E.E. Analysis of Essential Oil of Coridothymus capitatus (L.) and Its Antibacterial and Antifungal Activity. Z. Naturforsch C. 2003;58:687–690. doi: 10.1515/znc-2003-9-1016. [DOI] [PubMed] [Google Scholar]