Abstract
The brain is an important organ that controls all sensory and motor actions, memory, and emotions. Each anatomical and physiological modulation in various brain centers, results in psychological, behavioral, and sensory-motor changes. Alcohol and addictive drugs such as opioids and amphetamines have been shown to exert a great impact on brain, specifically on the hippocampus. Emerging evidence has indicated that altered hippocampal neurogenesis is associated with the pathophysiology of neuropsychological disorders including addiction. The addictive drugs impair neurogenesis and undermine the function of neural stem/progenitor cells in hippocampus. This feature was claimed to be one of the underlying mechanisms of behavioral changes in patients with addiction. As the impairment of stem cells’ function has been proven to be the underlying cause of pathologic neuroadaptations in the brain, the administration of stem cell populations has shown promising results for re-modulating of neuronal status in the brain and especially in the hippocampus. Among the different types of stem cells, bone marrow derived mesenchymal stem cells are the most proper candidates for stem cell therapies. In this review article, the recent studies on the effects of addictive drugs on brain neurogenesis, and also the promising potential effects of stem cells in curing addiction related hippocampal damages are discussed.
Key Words: Addiction, hippocampus, neurogenesis, neural stem/progenitor cells, mesenchymal stem cells
Addiction is defined as a chronic disease with obligation to take drugs or alcohol, no control on restraining intake, and having negative emotional feeling during withdrawal period. Addiction does not just affect the addict’s life, but also it has a huge burden on the society and economy. It was revealed that the addictive agents have a great anatomical and physiological impact on the brain centers, resulting in psychological, behavioral, and sensory-motor changes(1). It was demonstrated that addictive substances affect dopaminergic pathways which connect the ventral tegmental area to the prefrontal cortex via limbic system in particular in the nucleus accumbens, amygdala, ventral pallidum, and hippocampus (2).
Brain has the ability to produce new neural stem/ progenitor cells (NSPCs) during adulthood. Hippocampus might be the most plastic region of the brain, where granular cells in the dentate gyrus are born in adulthood. The precursors of these cells are placed in the subgranular zone (SGZ), the tissue between hilus and granule cell layer (3, 4). The sensible characteristic of adult-born neurons in the hippocampus is their specific electrophysiological capability for extreme changes required in early stages of maturation. This property is pivotal for formation of memories and further physiological actions (5). The SGZ provides a proper niche for proliferation and differentiation of stem cells in dentate gyrus (6). Astrocytes as important cellular components of SGZ, play an active role in proliferation and neuronal fate commitment of NSPCs (7) through release of molecular signals such as Wnt (8), Ephrin B2 (9), and sonic hedgehog (Shh) (10,11). Moreover, they have been shown to play essential roles in neural cell survival, immune responding, and modulation and metabolism of neurotransmitters (12). Therefore, each stimulant that can affect NSPCs or their niche in the hippocampus could make a vast modification in the memory and behavior. Bulk of studies have found the alterations in adult neurogenesis of hippocampus in neuropsycho-logical disorders such as depression (13), schizophrenia (14), bipolar disease (15), and addiction (16). A large amount of evidence indicates the changes in adult neurogenesis of dentate gyrus in abusing drugs such as opioids (17), amphetamines (18), and alcohol (19).
Addictive drugs and alcohol can regulate NSPCs by a variety of mechanisms. Some of these mechanisms are shared among them (16). For example, they regulate adult neurogenesis by modulating cell cycling pathways (20), and G protein-coupled receptor (GPCR) signaling cascades (21). Moreover, molecules involved in supporting or inhibiting neurogenesis including brain-derived neurotrophic factor (BDNF), interleukin 1 beta (IL1β) or vascular endothelial growth factor (VEGF), could be influenced by some addictive drugs (22). Additionally, they can exacerbate mitochondrial function and invoke oxidative stress (23). There is an evidence that 4-hydroxynonenal (HNE), an aldehydic product of membrane lipid peroxidation, is a key mediator of neuronal apoptosis induced by oxidative stress (24).
Some signaling molecules and pathways such as the mitogen-activated protein kinase (MAPK) signaling pathway, cell cycle regulatory molecules, and microRNAs (miRNAs) which may function independently or act in conjunction with one another have been identified to play important roles in these modulations (25).
Although neurons are the principal targets of drug addiction, it has recently been shown that nearly all drugs of abuse also affect glial cells (26). Astrocytes as the most abundant glial cells in brain (27) are well characterized for their role in the clearance of neurotransmitters, such as glutamate, from the synaptic cleft. Synaptic clearance of glutamate occurs primarily through the glutamate transporter 1 (GLT-1), expressed exclusively on astrocytes (28).
Several lines of evidence indicate that ethanol and other drugs of abuse downregulate the expression of GLT-1, leading to excessive accumulation of glutamate in synaptic cleft. Excess glutamate massively stimulates N-methyl-D-aspartate receptors (NMDARs). Massive stimulation of NMDARs leads to an excessive cellular influx of ions, particularly calcium, causing the activation of proteases, phospholipases, and endonucleases that end in cellular death (29). This form of neuronal death caused by hyperactivity of excitatory amino acids, mainly glutamate, is named excitotoxicity. It is one of the other mechanisms that have been proposed for alcohol and drug induced brain damages (30). Drug relapse observed for alcohol and other drugs is causally associated with the existence of high levels of extracellular glutamate (31).
Addictive drugs also activate microglia and
astrocytes via toll-like receptor 4 (TLR4), leading to the release of pro-inflammatory cytokines, and reactive oxygen species which in turn, promote neuronal death in hippocampus and other brain regions (32).
In the first part of this review, the effects of drug and alcohol abuse on neurogenesis are discussed, then we provide an overview of the promising effects of bone marrow derived mesenchymal stem cells (BM-MSCs) for the treatment of addiction related hippocampal damages.
Drug associated alterations in neurogenesis in hippocampus
The principal centers that are directing the feelings and are affected by addictive drugs are hippocampus and medial prefrontal cortex. It has been indicated that the behaviors of drug seeking and relapsing to drug abuse are mediated by these centers (33, 34).
Animal studies showed that self-administration of drugs attenuates neurogenesis in dentate gyrus (1, 35-43). The examples of these results include the decrease in proliferation and differentiation of NSPCs in dentate gyrus following nicotine self-administration (1), and attenuation in proliferation of NSPCs after heroin (38) and cocaine self-administration (41, 43). Access to cocaine augments the differentiation of dentate NSPCs, but does not affect their survival (39). Acute cocaine exposure was shown to cause a significant increase in oxidative stress in human NSPCs, which was followed by drastic apoptotic effects (44). Further studies demonstrated that although the proliferating cells in both SGZ and SVZ of rats decreased after 3 weeks of cocaine self-administration, the effects were reversed by 4 weeks of withdrawal (39).
Methamphetamines as another drug have non-linear effect on the dentate gyrus stem cells. In the case of daily self-administration of methamphetamine, the survival, proliferation, and differentiation of progenitors are decreasing. However, intermittent access raises proliferation and differentiation, but this type of increase in the population of immature neuronal cells does not alter survival and neurogenesis of hippocampal progenitors, perhaps because of opposing effects on proliferation of late progenitors and differentiation of post-mitotic neurons (36). Methamphetamine delays cell cycle progression from G0/G1-to-S phase. This effect could be due to the down-regulation of cyclin E, and to the decrease of epidermal growth factor receptor (EGFR) and ERK1/2 phosphorylation which are involved in cell proliferation progression (45). Several studies using animal models have shown the involvement of oxidative stress and excitotoxicity in the neurotoxicity produced by methamphetamines (46). Neurogenesis in the dentate gyrus decreased markedly in amphetamine-treated rats following four weeks of withdrawal from amphetamine (47).
Neuroinflammation is also associated with the chronic use of addictive drugs including cocaine, opiates, marijuana, and methamphetamine (48).
It is vital to indicate that all above studies show a correlation between daily drug intake and alteration in neurogenesis. As the amount of drug intake increases, the pathologic effects are more on the dentate gyrus neurogenesis.
Alcohol-associated alterations in the neurog-enesis in hippocampus
Alcohol abuse often leads to the alcohol use disorder (AUD) that has great deteriorating impacts on the brain. Excessive use of alcohol results in progressive neurodegeneration in brain that also accelerates AUD (49). Alcohol abuse causes general changes in white and gray matters in the central nervous system (50-52); nevertheless, some brain centers are more affected by alcohol abuse. Ethanol neurotoxicity greatly disturbs hippocampus and frontal cortex (52, 53). The altered integrity of hippocampus in alcoholics leads to abnormal cognitive functions and psychopathological actions (54, 55), which further results in AUD development (55, 56). Studies on human and animal models generated valuable information about the effects of alcohol abuse on the brain. Examples of alcohol effects on the brain structure and function include cell loss in corticolimbic regions (57, 58), decrease of the complexity of dendritic branching (59), and alterations in dendritic spine structure (60, 61). Moreover, alcohol self-administration in animal models resulted in decrease in survival, proliferation and differentiation of NSPCs in dentate gyrus in the hippocampus (37, 62, 63). Hippocampus is the most pathologically affected area of brain by chronic ethanol intake. Ethanol can significantly alter the expression of genes involved in neural differentiation pathways including axon guidance, hedgehog signaling, TGF-b signaling,; cell adhesion molecules, and Wnt signaling in differentiating human neuronal stem cells (64). Cumulative evidence indicates that ethanol activates microglia and astrocytes via TLR4, which can be evidenced by specific morphological changes including increased length and thickness of primary processes (65). This activation promotes the release of pro-inflammatory cytokines that in turn, promote neuronal death in the hippocampus and other brain regions (32).
Withdrawal from ethanol exposure enhanced cell proliferation in the hippocampus, resulting in initial microglial proliferation followed by the production of immature neurons and eventual neurogenesis (66, 67). The mechanisms underlying ethanol withdrawal-induced aberrant neurogenesis in the dentate gyrus are not yet completely elucidated.
The role of stem cells in treatment of addiction related hippocampal damages
What we summarized in above topics are brief description of the effects of drugs and alcohol in addicted patients on adult neurogenesis. The NSPCs are introduced as the main affected cell population in addiction. In this part, the potential of BM-MSCs for treating injuries to the brain with focus on addiction-derived alterations is discussed.
Stem cells are primary cells with self-renewal and differentiation ability (68). The most famous multipotent stem cells are MSCs which are recognized by their ability to differentiate into adipocytes, chondrocytes and osteocytes, and their plastic adherence (69). Available results from clinical studies support the overall safety of cell therapy using MSCs (70). The other extreme valuable characteristic of MSCs is the immunomodulatory effect of these cells. This feature is so beneficial in disorders with inflammatory components (71). The MSCs are also non-immunogenic; therefore, they can be easily obtained from allogeneic sources as they are not provoking lymphoproliferative responses (72, 73).
MSCs have been applied in neurological degenerative disorders such as Parkinson’s disease (74), Alzheimers disease (75), amyotrophic lateral sclerosis (76), and traumatic and ischemic brain injuries (77, 78). BM-MSCs have been differentiated into neuron-like and glial cells both in vivo and in vitro (80-82). There is an evidence for crossing the blood barrier of adult rat brain by human adipose-derived mesenchymal stromal cells (82). The BM-MSCs have also the potential to pass through blood brain barrier in hypoxic-ischemic encephalopathy animal model (83).
A considerable body of evidence has revealed the potential of BM-MSCs secretome to modulate neuronal survival and differentiation. This effect is attributed to BM-MSCs secretion of BDNF, GDNF (glial derived neurotrophic factor), and basic fibroblast growth factor (bFGF). These neuro-regenerative effects were accompanied by the improvement in animals’ memory and motor behavior (84). It was revealed that MSCs increase hippocampal neurogenesis and neuronal differentiation by enhancing the Wnt signaling pathway (85). Stromal cell-derived factor 1 (SDF)-1α as another cytokine released from BM-MSCs is associated with neural protection through anti-apoptotic based mechanisms (86). Additionally, the secretion of BDNF in vivo by BM-MSCs, was correlated with the activation of endogenous stem cells (87). Secretion of these factors by MSCs not only protects neurons from further degeneration and enhances neurogenesis, but also acts as immune response modulator. The overall expression of pro-inflammatory cytokines, such as IL-1β, IL-2, IL-12, tumor necrosis factor alpha (TNF-α), and interferon γ (INF γ) decreased after MSC transplantation. It has been shown that MSCs and their released cytokines and growth factors protect hippocampal neurons from oxidative stress and synapse damage induced by amyloid-β oligomers (88). Conditioned medium from MSCs also protect CNS neurons against glutamate excitotoxicity by inhibiting glutamate receptor expression and function (89).
Besides soluble growth factors and cytokines, MSCs also secrete microvesicles and exosomes containing mRNAs and/or miRNAs, which are believed to mediate cell-to-cell communication (90). Exosomes secreted by BMSCs in vitro not only mediate communication with neurons and astrocytes, but also regulate neurite outgrowth by transfering miRNA (miR-133b) into neural cells (91).
MSCs have shown therapeutic effects on the brain pathologies. A crucial finding in mental illnesses like bipolar disorder, major depression, and schizophrenia is the disturbance of neurotrophic factors and immunomodulatory systems in the brain. The pro- inflammatory cytokines such as TNF-α, IL-6, and IL- 2 are increasing and BDNF is decreasing in such disorders (92-94). Furthermore, as we specified above, there are alterations in anatomy and neurogenesis of hippocampus in psychological disorders and in individuals with addiction to drugs and alcohol (95-97). There are few studies with the aim of evaluating the effects of MSCs in psychiatric models. Application of MSCs in an animal model of depression has led to the improvement in hippocampal neurogenesis and depressive behaviors (98). In addition, intra-hippocampal injection of MSCs improved neurogenesis with no behavioral changes in rats, which indicates the safety of MSCs transplantation in brain (99). The secretion of neurotrophic factors from MSCs and the immunomodulatory function of these cells could be the possible regenerative mechanism of these cells on neurogenesis (100, 101).
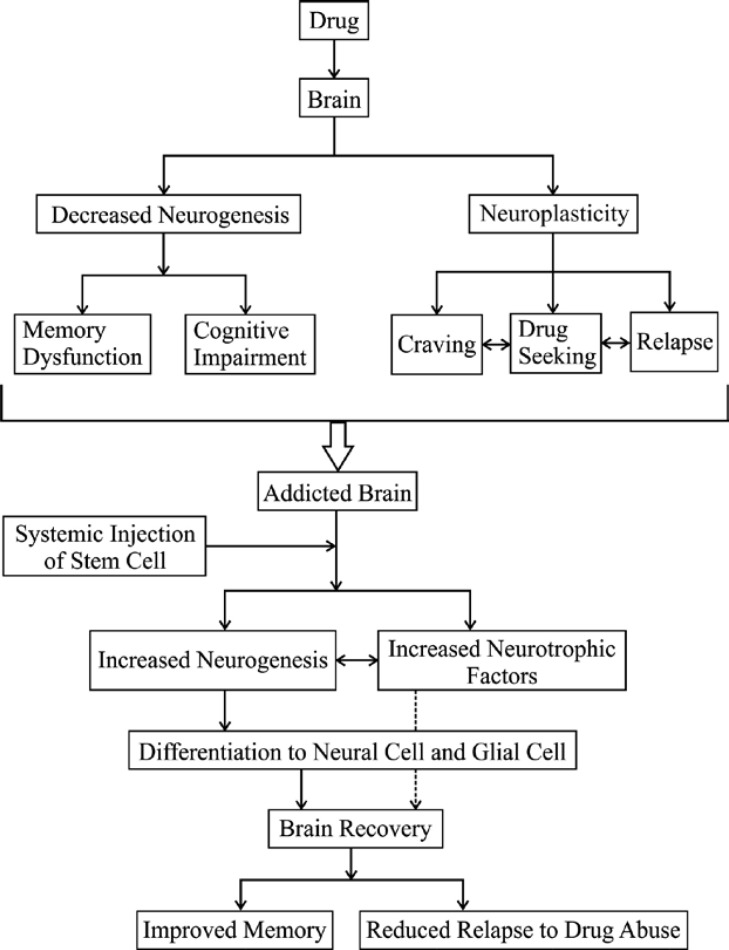
A brief description of the effects of drug on brain and the benefits of stem cell injection on addicted brains is represented in figure 1.
Fig. 1.
Schematic representation of drug effects on brain, and the benefits of stem cell injection
Yang et al. transplanted labeled BM-MSCs into the hippocampus of alcohol-associated dementia animal model. Evaluation of the behavior and hippocampus structure of the injected rats revealed that their learning and memory function were enhanced, alcohol-induced hippocampal injures were inhibited in histological examinations, the number of apoptotic neural cells was decreased, and the activity of total superoxide dismutase was increased in the hippocampus. Transplantation of BM-MSCs also increased the level of BDNF protein (102).
In a study by Israel et al, alcohol drinking rats were injected with human BM-MSCs and adipose tissue-derived MSCs intra- cerebro- ventricularly (ICV). The results showed that injected MSCs survived and became attached to cerebral ventricles. Transplanted MSCs reduced 24-h alcohol intake and also blocked alcohol relapse-like drinking induced in the alcohol deprivation effect condition (103). Ezquer et al. showed that administration of a single dose of human BM-MSC-spheroids, whether ICV or intravenously, greatly reduced neuro-inflammation, and inhibited chronic alcohol intake and relapse-like drinking. Administration of BM-MSC-spheroids also markedly increased the levels of the GLT-1, leading to inhibition of relapse. It was also revealed that human BM-MSC-spheroid administration in alcoholic rats fully normalized astrocyte activation, and decreased MCP1 expression in the hippocampus, suggesting a potent anti-inflammatory effect of BM-MSC-spheroids. Furthermore, oxidative stress was normalized by MSC- spheroid administration (104).
Therapeutic promises of BM‐MSCs have been overshadowed by concerns regarding their limited homing potential or migration to non-target sites (105, 106). Although, extensive investigations have provided significant potential for enhancing targeted stem/progenitor cell homing (107-110) there are some limitations that make it difficult to apply these findings in clinics, especially in neurodegenerative disorders.
Conclusion
Several studies indicated the damaging effects of drug and/or alcohol abuse on the brain neuroanatomy and function. Experiments have revealed that addiction leads to impairment in adult neurogenesis in behavioral centers of brain including hippocampus and medial prefrontal cortex. Numerous experiments on animal models have shown the effects of addictive drugs such as morphine, cocaine, methamphetamines, and alcohol on the proliferation, survival and differentiation of progenitor cells in the hippocampus. Animal studies showed promising results after hippocampal transplantation of BM-MSCs in psychological disorders e.g. depression and alcohol abuse. More studies on the stem cell therapy of psychological defects related to addiction are required.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Abrous DN, Adriani W, Montaron MF, et al. Nicotine self-administration impairs hippocampal plasticity. J Neurosci. 2002;22:3656–62. doi: 10.1523/JNEUROSCI.22-09-03656.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koob GF, Simon EJ. The Neurobiology of Addiction: Where We Have Been and Where We Are Going. J Drug Issues. 2009;39:115–32. doi: 10.1177/002204260903900110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rietze R, Poulin P, Weiss S. Mitotically active cells that generate neurons and astrocytes are present in multiple regions of the adult mouse hippocampus. J Comp Neurol. 2000;424:397–408. [PubMed] [Google Scholar]
- 4.Liu S, Wang J, Zhu D, et al. Generation of functional inhibitory neurons in the adult rat hippocampus. J Neurosci. 2003;23:732–6. doi: 10.1523/JNEUROSCI.23-03-00732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snyder JS, Kee N, Wojtowicz JM. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol. 2001;85:2423–31. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- 6.Mercier F, Kitasako JT, Hatton GI. Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J Comp Neurol. 2002;451:170–88. doi: 10.1002/cne.10342. [DOI] [PubMed] [Google Scholar]
- 7.Falk S, Gotz M. Glial control of neurogenesis. Curr Opin Neurobiol. 2017;47:188–95. doi: 10.1016/j.conb.2017.10.025. [DOI] [PubMed] [Google Scholar]
- 8.Lie DC, Colamarino SA, Song HJ, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–5. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 9.Ashton RS, Conway A, Pangarkar C, et al. Astrocytes regulate adult hippocampal neurogenesis through ephrin-B signaling. Nat Neurosci. 2012;15:1399. doi: 10.1038/nn.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao PJ, Petralia RS, Mattson MP. Sonic Hedgehog Signaling and Hippocampal Neuroplasticity. Trends Neurosci. 2016;39:840–50. doi: 10.1016/j.tins.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiao J, Chen DF. Induction of neurogenesis in nonconventional neurogenic regions of the adult central nervous system by niche astrocyte-produced signals. Stem Cells. 2008;26:1221–30. doi: 10.1634/stemcells.2007-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miguel-Hidalgo JJ. The Role of Glial Cells in Drug Abuse. Curr Drug Abuse Rev. 2009;2:76–82. doi: 10.2174/1874473710902010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H, Warner-Schmidt J, Varela S, et al. Norbin ablation results in defective adult hippocampal neurogenesis and depressive-like behavior in mice. Proc Natl Acad Sci U S A. 2015;112:9745–50. doi: 10.1073/pnas.1510291112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ouchi Y, Banno Y, Shimizu Y, et al. Reduced adult hippocampal neurogenesis and working memory deficits in the Dgcr8-deficient mouse model of 22q112 deletion-associated schizophrenia can be rescued by IGF2. J Neurosci. 2013;33:9408–19. doi: 10.1523/JNEUROSCI.2700-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takamura N, Nakagawa S, Masuda T, et al. The effect of dopamine on adult hippocampal neurogenesis. Prog Neuropsychopharmacol Biol Psychiatry. 2014;50:116–24. doi: 10.1016/j.pnpbp.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Chambers RA. Adult hippocampal neurogenesis in the pathogenesis of addiction and dual diagnosis disorders. Drug Alcohol Depend. 2013;130:1–12. doi: 10.1016/j.drugalcdep.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Loh HH, Law PY. Effect of Opioid on Adult Hippocampal Neurogenesis. ScientificWorldJournal. 2016;2016:2601264. doi: 10.1155/2016/2601264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Cabrerizo R, Garcia-Fuster MJ. Comparative effects of amphetamine-like psychostimulants on rat hippocampal cell genesis at different developmental ages. Neurotoxicology. 2016;56:29–39. doi: 10.1016/j.neuro.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 19.McClain JA, Morris SA, Marshall SA, et al. Ectopic hippocampal neurogenesis in adolescent male rats following alcohol dependence. Addict Biol. 2014;19:687–99. doi: 10.1111/adb.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arguello AA, Harburg GC, Schonborn JR, et al. Time course of morphine's effects on adult hippocampal subgranular zone reveals preferential inhibition of cells in S phase of the cell cycle and a subpopulation of immature neurons. Neuroscience. 2008;157:70–9. doi: 10.1016/j.neuroscience.2008.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson KA, Lovinger DM. Presynaptic G Protein-Coupled Receptors: Gatekeepers of Addiction? Front Cell Neurosci. 2016;10:264. doi: 10.3389/fncel.2016.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arguello AA, Fischer SJ, Schonborn JR, et al. Effect of chronic morphine on the dentate gyrus neurogenic microenvironment. Neuroscience. 2009;159:1003–10. doi: 10.1016/j.neuroscience.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deavall DG, Martin EA, Horner JM, et al. Drug-induced oxidative stress and toxicity. J Toxicol. 2012;2012:645460. doi: 10.1155/2012/645460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kruman I, Bruce-Keller AJ, Bredesen D, et al. Evidence that 4-hydroxynonenal mediates oxidative stress-induced neuronal apoptosis. J Neurosci. 1997;17:5089–100. doi: 10.1523/JNEUROSCI.17-13-05089.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu C, Loh HH, Law PY. Effects of addictive drugs on adult neural stem/progenitor cells. Cell Mol Life Sci. 2016;73:327–48. doi: 10.1007/s00018-015-2067-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bachtell RK, Jones JD, Heinzerling KG, et al. Glial and neuroinflammatory targets for treating substance use disorders. Drug Alcohol Depend. 2017;180:156–70. doi: 10.1016/j.drugalcdep.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–40. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, Gozen O, Watkins A, et al. Presynaptic regulation of astroglial excitatory neurotransmitter transporter GLT1. Neuron. 2009;61:880–94. doi: 10.1016/j.neuron.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Velasco M, Rojas-Quintero J, Chávez-Castillo M. Excitotoxicity: an organized crime at the cellular level. Journal of Neurology and Neuroscience. 2017;8:193. [Google Scholar]
- 30.Dong XX, Wang Y, Qin ZH. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol Sin. 2009;30:379–87. doi: 10.1038/aps.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crews FT, Walter TJ, Coleman LG Jr, et al. Toll-like receptor signaling and stages of addiction. Psychopharmacology (Berl) 2017;234:1483–98. doi: 10.1007/s00213-017-4560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ward RJ, Colivicchi MA, Allen R, et al. Neuro-inflammation induced in the hippocampus of 'binge drinking' rats may be mediated by elevated extracellular glutamate content. J Neurochem. 2009;111:1119–28. doi: 10.1111/j.1471-4159.2009.06389.x. [DOI] [PubMed] [Google Scholar]
- 33.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaham Y, Shalev U, Lu L, et al. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 35.Eisch AJ, Harburg GC. Opiates, psychostimulants, and adult hippocampal neurogenesis: Insights for addiction and stem cell biology. Hippocampus. 2006;16:271–86. doi: 10.1002/hipo.20161. [DOI] [PubMed] [Google Scholar]
- 36.Mandyam CD, Wee S, Crawford EF, et al. Varied access to intravenous methamphetamine self-administration differentially alters adult hippocampal neurogenesis. Biol Psychiatry. 2008;64:958–65. doi: 10.1016/j.biopsych.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richardson HN, Chan SH, Crawford EF, et al. Permanent impairment of birth and survival of cortical and hippocampal proliferating cells following excessive drinking during alcohol dependence. Neurobiol Dis. 2009;36:1–10. doi: 10.1016/j.nbd.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eisch AJ, Barrot M, Schad CA, et al. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci U S A. 2000;97:7579–84. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noonan MA, Choi KH, Self DW, et al. Withdrawal from cocaine self-administration normalizes deficits in proliferation and enhances maturity of adult-generated hippocampal neurons. J Neurosci. 2008;28:2516–26. doi: 10.1523/JNEUROSCI.4661-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Catlow BJ, Badanich KA, Sponaugle AE, et al. Effects of MDMA ("ecstasy") during adolescence on place conditioning and hippocampal neurogenesis. Eur J Pharmacol. 2010;628:96–103. doi: 10.1016/j.ejphar.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 41.Brown TE, Lee BR, Ryu V, et al. Reducing hippocampal cell proliferation in the adult rat does not prevent the acquisition of cocaine-induced conditioned place preference. Neurosci Lett. 2010;481:41–6. doi: 10.1016/j.neulet.2010.06.048. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Fuster MJ, Perez JA, Clinton SM, et al. Impact of cocaine on adult hippocampal neurogenesis in an animal model of differential propensity to drug abuse. Eur J Neurosci. 2010;31:79–89. doi: 10.1111/j.1460-9568.2009.07045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sudai E, Croitoru O, Shaldubina A, et al. High cocaine dosage decreases neurogenesis in the hippocampus and impairs working memory. Addict Biol. 2011;16:251–60. doi: 10.1111/j.1369-1600.2010.00241.x. [DOI] [PubMed] [Google Scholar]
- 44.Cunha-Oliveira T, Rego AC, Oliveira CR. Cellular and molecular mechanisms involved in the neurotoxicity of opioid and psychostimulant drugs. Brain Res Rev. 2008;58:192–208. doi: 10.1016/j.brainresrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 45.Baptista S, Lasgi C, Benstaali C, et al. Methamphetamine decreases dentate gyrus stem cell self-renewal and shifts the differentiation towards neuronal fate. Stem Cell Res. 2014;13:329–41. doi: 10.1016/j.scr.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 46.McDonnell-Dowling K, Kelly JP. The Role of Oxidative Stress in Methamphetamine-induced Toxicity and Sources of Variation in the Design of Animal Studies. Curr Neuropharmacol. 2017;15:300–14. doi: 10.2174/1570159X14666160428110329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barr JL, Renner KJ, Forster GL. Withdrawal from chronic amphetamine produces persistent anxiety-like behavior but temporally-limited reductions in monoamines and neurogenesis in the adult rat dentate gyrus. Neuropharmacology. 2010;59:395–405. doi: 10.1016/j.neuropharm.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crews FT. Immune function genes, genetics, and the neurobiology of addiction. Alcohol Res. 2012;34:355–61. [PMC free article] [PubMed] [Google Scholar]
- 49.Morris SA, Eaves DW, Smith AR, et al. Alcohol inhibition of neurogenesis: a mechanism of hippocampal neurodegeneration in an adolescent alcohol abuse model. Hippocampus. 2010;20:596–607. doi: 10.1002/hipo.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pfefferbaum A, Sullivan EV, Hedehus M, et al. In vivo detection and functional correlates of white matter microstructural disruption in chronic alcoholism. Alcohol Clin Exp Res. 2000;24:1214–21. [PubMed] [Google Scholar]
- 51.Pfefferbaum A, Lim KO, Zipursky RB, et al. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcohol Clin Exp Res. 1992;16:1078–89. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- 52.Pfefferbaum A, Sullivan EV, Mathalon DH, et al. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res. 1997;21:521–9. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- 53.Sullivan EV, Marsh L, Mathalon DH, et al. Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcohol Clin Exp Res. 1995;19:110–22. doi: 10.1111/j.1530-0277.1995.tb01478.x. [DOI] [PubMed] [Google Scholar]
- 54.Laakso MP, Vaurio O, Savolainen L, et al. A volumetric MRI study of the hippocampus in type 1 and 2 alcoholism. Behav Brain Res. 2000;109:177–86. doi: 10.1016/s0166-4328(99)00172-2. [DOI] [PubMed] [Google Scholar]
- 55.Clark DB, Thatcher DL, Tapert SF. Alcohol, psychological dysregulation, and adolescent brain development. Alcohol Clin Exp Res. 2008;32:375–85. doi: 10.1111/j.1530-0277.2007.00601.x. [DOI] [PubMed] [Google Scholar]
- 56.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–63. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- 57.Crews FT, Braun CJ, Hoplight B, et al. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24:1712–23. [PubMed] [Google Scholar]
- 58.Lukoyanov NV, Brandao F, Cadete-Leite A, et al. Synaptic reorganization in the hippocampal formation of alcohol-fed rats may compensate for functional deficits related to neuronal loss. Alcohol. 2000;20:139–48. doi: 10.1016/s0741-8329(99)00069-5. [DOI] [PubMed] [Google Scholar]
- 59.He J, Nixon K, Shetty AK, et al. Chronic alcohol exposure reduces hippocampal neurogenesis and dendritic growth of newborn neurons. Eur J Neurosci. 2005;21:2711–20. doi: 10.1111/j.1460-9568.2005.04120.x. [DOI] [PubMed] [Google Scholar]
- 60.Chandler LJ, Carpenter-Hyland E, Hendricson AW, et al. Structural and functional modifications in glutamateric synapses following prolonged ethanol exposure. Alcohol Clin Exp Res. 2006;30:368–76. doi: 10.1097/01.ALC.0000167959.84516.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carpenter-Hyland EP, Woodward JJ, Chandler LJ. Chronic ethanol induces synaptic but not extrasynaptic targeting of NMDA receptors. J Neurosci. 2004;24:7859–68. doi: 10.1523/JNEUROSCI.1902-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taffe MA, Kotzebue RW, Crean RD, et al. Long-lasting reduction in hippocampal neurogenesis by alcohol consumption in adolescent nonhuman primates. Proc Natl Acad Sci U S A. 2010;107:11104–9. doi: 10.1073/pnas.0912810107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nixon K. Alcohol and adult neurogenesis: roles in neurodegeneration and recovery in chronic alcoholism. Hippocampus. 2006;16:287–95. doi: 10.1002/hipo.20162. [DOI] [PubMed] [Google Scholar]
- 64.Vangipuram SD, Lyman WD. Ethanol affects differentiation-related pathways and suppresses Wnt signaling protein expression in human neural stem cells. Alcohol Clin Exp Res. 2012;36:788–97. doi: 10.1111/j.1530-0277.2011.01682.x. [DOI] [PubMed] [Google Scholar]
- 65.Beynon SB, Walker FR. Microglial activation in the injured and healthy brain: what are we really talking about? Practical and theoretical issues associated with the measurement of changes in microglial morphology. Neuroscience. 2012;225:162–71. doi: 10.1016/j.neuroscience.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 66.Recinto P, Samant AR, Chavez G, et al. Levels of neural progenitors in the hippocampus predict memory impairment and relapse to drug seeking as a function of excessive methamphetamine self-administration. Neuropsychopharmacology. 2012;37:1275–87. doi: 10.1038/npp.2011.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mandyam CD. The Interplay between the Hippocampus and Amygdala in Regulating Aberrant Hippocampal Neurogenesis during Protracted Abstinence from Alcohol Dependence. Front Psychiatry. 2013;4:61. doi: 10.3389/fpsyt.2013.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kalra K, Tomar P. Stem Cell: Basics, Classification and Applications. American Journal of Phytomedicine and Clinical Therapeutics. 2014;2:919–30. [Google Scholar]
- 69.Kobolak J, Dinnyes A, Memic A, et al. Mesenchymal stem cells: Identification, phenotypic characterization, biological properties and potential for regenerative medicine through biomaterial micro-engineering of their niche. Methods. 2016;99:62–8. doi: 10.1016/j.ymeth.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 70.Lee JS, Hong JM, Moon GJ, et al. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28:1099–106. doi: 10.1002/stem.430. [DOI] [PubMed] [Google Scholar]
- 71.Torrente Y, Polli E. Mesenchymal stem cell transplantation for neurodegenerative diseases. Cell Transplant . 2008;17:1103–13. doi: 10.3727/096368908787236576. [DOI] [PubMed] [Google Scholar]
- 72.Mahmud N, Pang W, Cobbs C, et al. Studies of the route of administration and role of conditioning with radiation on unrelated allogeneic mismatched mesenchymal stem cell engraftment in a nonhuman primate model. Exp Hematol. 2004;32:494–501. doi: 10.1016/j.exphem.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 73.Momin EN, Mohyeldin A, Zaidi HA, et al. Mesenchymal stem cells: new approaches for the treatment of neurological diseases. Curr Stem Cell Res Ther. 2010;5:326–44. doi: 10.2174/157488810793351631. [DOI] [PubMed] [Google Scholar]
- 74.Chao YX, He BP, Tay SS. Mesenchymal stem cell transplantation attenuates blood brain barrier damage and neuroinflammation and protects dopaminergic neurons against MPTP toxicity in the substantia nigra in a model of Parkinson's disease. J Neuroimmunol. 2009;216:39–50. doi: 10.1016/j.jneuroim.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 75.Bae JS, Jin HK, Lee JK, et al. Bone marrow-derived mesenchymal stem cells contribute to the reduction of amyloid-beta deposits and the improvement of synaptic transmission in a mouse model of pre-dementia Alzheimer's disease. Curr Alzheimer Res. 2013;10:524–31. [PubMed] [Google Scholar]
- 76.Bai L, Lennon DP, Caplan AI, et al. Hepatocyte growth factor mediates mesenchymal stem cell-induced recovery in multiple sclerosis models. Nat Neurosci. 2012;15:862–70. doi: 10.1038/nn.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang ZX, Guan LX, Zhang K, et al. A combined procedure to deliver autologous mesenchymal stromal cells to patients with traumatic brain injury. Cytotherapy. 2008;10:134–9. doi: 10.1080/14653240701883061. [DOI] [PubMed] [Google Scholar]
- 78.Li Y, Chen J, Zhang CL, et al. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49:407–17. doi: 10.1002/glia.20126. [DOI] [PubMed] [Google Scholar]
- 79.Woodbury D, Schwarz EJ, Prockop DJ, et al. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–70. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 80.Suzuki H, Taguchi T, Tanaka H, et al. Neurospheres induced from bone marrow stromal cells are multipotent for differentiation into neuron, astrocyte, and oligodendrocyte phenotypes. Biochem Biophys Res Commun. 2004;322:918–22. doi: 10.1016/j.bbrc.2004.07.201. [DOI] [PubMed] [Google Scholar]
- 81.Mori T, Kiyono T, Imabayashi H, et al. Combination of hTERT and bmi-1, E6, or E7 induces prolongation of the life span of bone marrow stromal cells from an elderly donor without affecting their neurogenic potential. Mol Cell Biol. 2005;25:5183–95. doi: 10.1128/MCB.25.12.5183-5195.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Haddad-Mashadrizeh A, Bahrami AR, Matin MM, et al. Evidence for crossing the blood barrier of adult rat brain by human adipose-derived mesenchymal stromal cells during a 6-month period of post-transplantation. Cytotherapy. 2013;15:951–60. doi: 10.1016/j.jcyt.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 83.Guan XQ, Yu JL, Li LQ, et al. [Study on mesenchymal stem cells entering the brain through the blood-brain barrier] Zhonghua Er Ke Za Zhi. 2004;42:920–3. [PubMed] [Google Scholar]
- 84.Salgado AJ, Sousa JC, Costa BM, et al. Mesenchymal stem cells secretome as a modulator of the neurogenic niche: basic insights and therapeutic opportunities. Front Cell Neurosci. 2015;9:249. doi: 10.3389/fncel.2015.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oh SH, Kim HN, Park HJ, et al. Mesenchymal Stem Cells Increase Hippocampal Neurogenesis and Neuronal Differentiation by Enhancing the Wnt Signaling Pathway in an Alzheimer's Disease Model. Cell Transplant. 2015;24:1097–109. doi: 10.3727/096368914X679237. [DOI] [PubMed] [Google Scholar]
- 86.Wang F, Yasuhara T, Shingo T, et al. Intravenous administration of mesenchymal stem cells exerts therapeutic effects on parkinsonian model of rats: focusing on neuroprotective effects of stromal cell-derived factor-1alpha. BMC Neurosci. 2010;11:52. doi: 10.1186/1471-2202-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cova L, Armentero MT, Zennaro E, et al. Multiple neurogenic and neurorescue effects of human mesenchymal stem cell after transplantation in an experimental model of Parkinson's disease. Brain Res. 2010;1311:12–27. doi: 10.1016/j.brainres.2009.11.041. [DOI] [PubMed] [Google Scholar]
- 88.de Godoy MA, Saraiva LM, de Carvalho LRP, et al. Mesenchymal stem cells and cell-derived extracellular vesicles protect hippocampal neurons from oxidative stress and synapse damage induced by amyloid-beta oligomers. J Biol Chem. 2018;293:1957–75. doi: 10.1074/jbc.M117.807180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Voulgari-Kokota A, Fairless R, Karamita M, et al. Mesenchymal stem cells protect CNS neurons against glutamate excitotoxicity by inhibiting glutamate receptor expression and function. Exp Neurol. 2012;236:161–70. doi: 10.1016/j.expneurol.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 90.Baglio SR, Pegtel DM, Baldini N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol. 2012;3:359. doi: 10.3389/fphys.2012.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xin H, Li Y, Buller B, et al. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells. 2012;30:1556–64. doi: 10.1002/stem.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Asevedo E, Gadelha A, Noto C, et al. Impact of peripheral levels of chemokines, BDNF and oxidative markers on cognition in individuals with schizophrenia. J Psychiatr Res . 2013;47:1376–82. doi: 10.1016/j.jpsychires.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 93.Kapczinski F, Dal-Pizzol F, Teixeira AL, et al. Peripheral biomarkers and illness activity in bipolar disorder. J Psychiatr Res. 2011;45:156–61. doi: 10.1016/j.jpsychires.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 94.Kunz M, Cereser KM, Goi PD, et al. Serum levels of IL-6, IL-10 and TNF-alpha in patients with bipolar disorder and schizophrenia: differences in pro- and anti-inflammatory balance. Rev Bras Psiquiatr. 2011;33:268–74. doi: 10.1590/s1516-44462011000300010. [DOI] [PubMed] [Google Scholar]
- 95.Caletti E, Paoli RA, Fiorentini A, et al. Neuropsychology, social cognition and global functioning among bipolar, schizophrenic patients and healthy controls: preliminary data. Front Hum Neurosci. 2013;7:661. doi: 10.3389/fnhum.2013.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Torrent C, Martinez-Aran A, del Mar Bonnin C, et al. Long-term outcome of cognitive impairment in bipolar disorder. J Clin Psychiatry. 2012;73:e899–905. doi: 10.4088/JCP.11m07471. [DOI] [PubMed] [Google Scholar]
- 97.Trivedi MH, Greer TL. Cognitive dysfunction in unipolar depression: implications for treatment. J Affect Disord. 2014;152-154:19–27. doi: 10.1016/j.jad.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 98.Lin YT, Chern Y, Shen CK, et al. Human mesenchymal stem cells prolong survival and ameliorate motor deficit through trophic support in Huntington's disease mouse models. PLoS One. 2011;6:e22924. doi: 10.1371/journal.pone.0022924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Coquery N, Blesch A, Stroh A, et al. Intrahippocampal transplantation of mesenchymal stromal cells promotes neuroplasticity. Cytotherapy. 2012;14:1041–53. doi: 10.3109/14653249.2012.694418. [DOI] [PubMed] [Google Scholar]
- 100.Li Y, Chen J, Chen XG, et al. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002;59:514–23. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- 101.Yoo SW, Kim SS, Lee SY, et al. Mesenchymal stem cells promote proliferation of endogenous neural stem cells and survival of newborn cells in a rat stroke model. Exp Mol Med. 2008;40:387–97. doi: 10.3858/emm.2008.40.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang HY, Wu XM, Liu Y, et al. Transplantation of bone marrow mesenchymal stem cells promotes learning and memory functional recovery and reduces hippocampal damage in rats with alcohol-associated dementia. Transplantation. 2015;99:492–9. doi: 10.1097/TP.0000000000000535. [DOI] [PubMed] [Google Scholar]
- 103.Israel Y, Ezquer F, Quintanilla ME, et al. Intracerebral Stem Cell Administration Inhibits Relapse-like Alcohol Drinking in Rats. Alcohol Alcohol. 2017;52:1–4. doi: 10.1093/alcalc/agw068. [DOI] [PubMed] [Google Scholar]
- 104.Ezquer F, Morales P, Quintanilla ME. Intravenous administration of anti-inflammatory mesenchymal stem cell spheroids reduces chronic alcohol intake and abolishes binge-drinking. Sci Rep. 2018;8:4325. doi: 10.1038/s41598-018-22750-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.LaBarge MA, Blau HM. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell. 2002;111:589–601. doi: 10.1016/s0092-8674(02)01078-4. [DOI] [PubMed] [Google Scholar]
- 106.Barbash IM, Chouraqui P, Baron J, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108:863–8. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 107.Mirahmadi M, Ahmadiankia N, Naderi-Meshkin H, et al. Hypoxia and laser enhance expression of SDF-1 in muscles cells. Cell Mol Biol (Noisy-le-grand) 2016;62:31–7. [PubMed] [Google Scholar]
- 108.Bidkhori HR, Ahmadiankia N, Matin MM, et al. Chemically primed bone-marrow derived mesenchymal stem cells show enhanced expression of chemokine receptors contributed to their migration capability. Iran J Basic Med Sci. 2016;19:14–9. [PMC free article] [PubMed] [Google Scholar]
- 109.Naderi-Meshkin H, Matin MM, Heirani-Tabasi A, et al. Injectable hydrogel delivery plus preconditioning of mesenchymal stem cells: exploitation of SDF-1/CXCR4 axis toward enhancing the efficacy of stem cells' homing. Cell Biol Int. 2016;40:730–41. doi: 10.1002/cbin.10474. [DOI] [PubMed] [Google Scholar]
- 110.Naderi-Meshkin H, Bahrami AR, Bidkhori HR, et al. Strategies to improve homing of mesenchymal stem cells for greater efficacy in stem cell therapy. Cell Biol Int. 2015;39:23–34. doi: 10.1002/cbin.10378. [DOI] [PubMed] [Google Scholar]