Abstract
Esterifications of carboxylic acids with equimolar amount of alcohols could be efficiently catalyzed by ZrOCl2·8H2O. Acrylate esters were obtained in good yields under solvent-free conditions at ambient temperature. The esterification of other carboxylic acids with alcohols also proceeded at ambient temperature or at 50 oC to afford esters in high yields. If the esterification was performed in toluene under azeotropic reflux conditions to remove water, both the catalytic activity of ZrOCl2·8H2O and the rate of esterification could be increased greatly. Furthermore, in the present catalytic system, the esters could be easily separated from the reaction mixtures and the catalyst could be easily recovered and reused.
Keywords: Esterification, acrylic acid, carboxylic acid, alcohol, zirconium (IV) dichloride oxide hydrate
Introduction
Esters are the important class of organic compounds, which are usually prepared by the esterification of carboxylic acids with alcohols catalyzed by H2SO4, TsOH [1,2] and other catalysts [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18]. Because the esterification is a reversible reaction, in order to obtain a high yield of ester, an excess amount of one of the reactants is normally required, and/or removal of H2O by azeotropic distillation during the reaction is usually performed. However, the former is neither an atom economical nor environmentally benign process, while the latter is not suitable for the esterification of carboxylic acids which have low boiling points, and polymerization can easily occur at the higher temperatures used under the acidic reaction conditions in the cases of particularly susceptible substrates such as acrylic acid, α-methylacrylic acid, etc. Consequently, development of an efficient catalytic system for esterification using 1:1 mixtures of carboxylic acids and alcohols at ambient or low temperature is still an important and interesting objective in organic synthesis.
Since some inorganic salts display Lewis acid properties and they are cheap and easily separated from the organic products, they have become interesting candidates of choice as catalysts. Very recently, a direct esterification of carboxylic acids with equimolar amounts of alcohols catalyzed by inorganic salts has been reported [19,20].
The aim of our research described here was to develop an efficient catalytic procedure for the direct esterification of carboxylic acids with alcohols under solvent-free conditions with the following requirements: (1) esters should be obtained in high yields by the esterification of equimolar amounts of carboxylic acids and alcohols; (2) esterification should proceed at ambient or low temperature and the catalyst system should be suitable for the esterification of highly reactive carboxylic acids such as acrylic acid; (3) the esters should be easy to isolate and purify, and the catalyst should be recyclable. After screening a number of inorganic salts, we have found that ZrOCl2·8H2O worked as an efficient catalyst to catalyze esterification while possessing the necessary properties to meet the requirements as described above [21].
Results and Discussion
Esterification of acrylic acid with alcohols
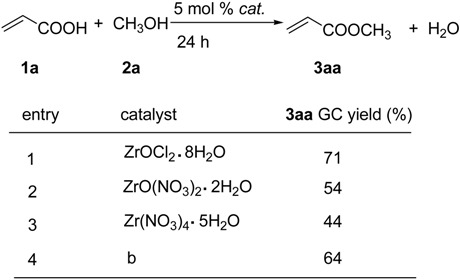
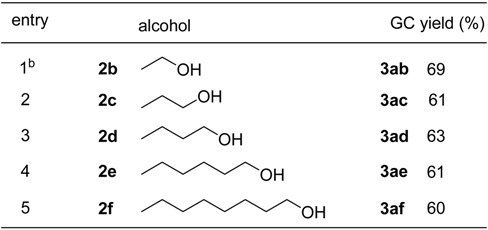
Table 1 and Table 2 summarize the results of the esterification of acrylic acid (1a) with equimolar amount of alcohols catalyzed by zirconium salts. This was carried out by simply stirring 1a, an equimolar amount of alcohol and 5 mol% of the zirconium salt under solvent-free conditions at ambient temperature or at 50 oC for 24h.
Table 1.
Zirconium compounds-catalyzed esterification of acrylic acid 1a with methanol 2aa
(a) The reactions were carried out using 5 mmol of 1a, 5 mmol of 2a and 0.025 mmol of ZrOCl2·8H2O at r.t. for 24h. (b) The reused catalyst.
Table 2.
ZrOCl2·8H2O-catalyzed esterification of acrylic acid (1a) with aliphatic alcoholsa
a The reactions were carried out using 5 mmol of 1a, 5 mmol of alcohol and 0.025 mmol of ZrOCl2·8H2O at 50 oC for 24h. (b) At r.t. for 24h.
Table 1 shows the catalytic activity of the different zirconium salts tested in the reaction of 1a with equimolar amounts of methanol (2a) at ambient temperature. Among the chosen zirconium compounds, ZrOCl2·8H2O (entry 1) was very efficient in this esterification, affording methyl acrylate (3aa) in 71% yield (GC) without any removal of water, while ZrO(NO3)2·2H2O and Zr(NO3)4·5H2O showed somewhat lower catalytic activities to give 3aa in moderate yields (entries 2 and 3).
It should be noted that in all these cases, upon completion of the esterification, the reaction mixture became two phases at ambient temperature and the catalyst and esters were easily separated by simply decanting. GC analyses of the two phases revealed that the organic phase (the upper layer) was the product 3aa, containing small amounts of the starting materials 1a and 2a. The aqueous phase (the lower layer) was a mixture of H2O, 1a, 2a and trace amounts of 3aa. 1H- and 13C-NMR of the reaction mixture confirmed that under the present reaction conditions, polymerization of 1a did not occur.
After removal of the organic phase, we have examined the catalytic activity of catalyst in the aqueous phase. Thus, 5.0 mmol of 1a and 5.0 mmol of 2a were directly added to the aqueous phase, and the resulting mixture was then stirred. After 24h, 3aa was formed in 64% GC yield (entry 4). If we removed the volatiles and H2O from the aqueous phase (80 oC, under vacuum), a solid residue could be obtained. The solid residue showed the almost same catalytic activity as the fresh ZrOCl2·8H2O did to afford 3aa in 69% GC yield. These results indicated that ZrOCl2·8H2O was a reusable catalyst.
Furthermore, the reactions of 1a with other aliphatic alcohols at ambient temperature or at 50 oC without removal of water were also studied. As shown in Table 2, the yield of esterification of 1a with ethanol (2b) at ambient temperature gave ethyl acrylate (3ab) in 69% GC yield (entry 1). The result was similar to that observed when 2a used. It was found that there was a slight decrease in the yields with an increase of the carbon number of the alcohols (C3 ~ C8), and the corresponding esters were obtained in a range of 60 ~ 63% yields (entries 2 ~ 5). These results indicated that the yields of the esterification with C3 ~ C8 alcohols at 50 oC without removal of water were not greatly dependent on the carbon number of alcohols.
Esterification of other carboxylic acids with alcohols
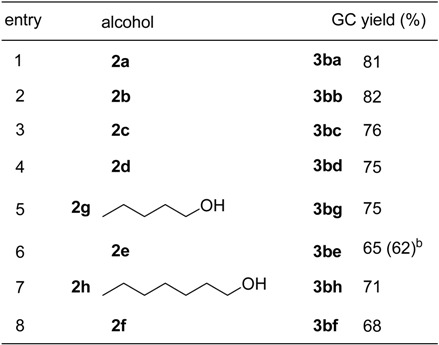
To assess the scope of ZrOCl2·8H2O-catalyzed esterification, the reactions of various other carboxylic acids with several alcohols have been investigated. Table 3 summarizes the results of the esterification of propionic acid (1b) with equimolar amount of alcohols at ambient temperature for 24 h. As can be seen from this data, ZrOCl2·8H2O showed highly catalytic activity for the esterification of 1b with various alcohols. Reactions of 1b with 2a or 2b afford 3ba and 3bb in 81% and 82% GC yields, respectively (entries 1 and 2). Similarly efficient esterifications took place using other alcohols, though the yields were slight decreased (entries 3 – 8, Table 3).
Table 3.
ZrOCl2·8H2O-catalyzed esterification of propionic acid (1b) with alcohols a
a The reactions were carried out using 5 mmol of acid, 5 mmol of alcohol and 0.025 mmol of ZrOCl2·8H2O at r.t. for 24h; b The yield in parenthesis was obtained by using the reused catalyst.
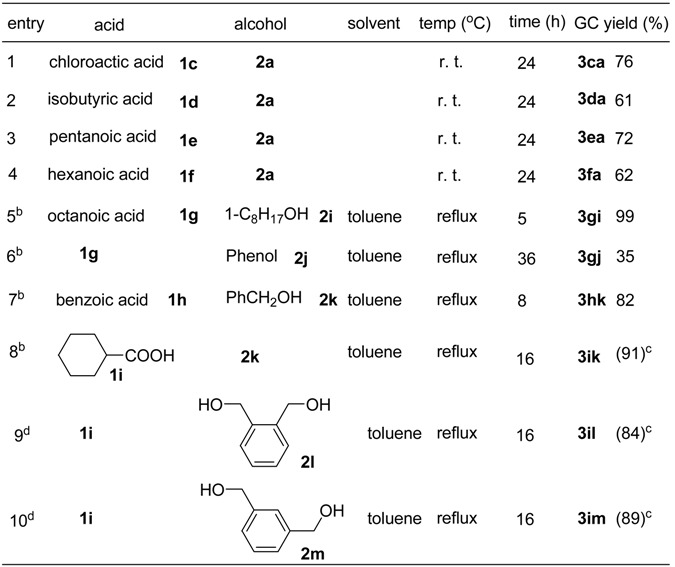
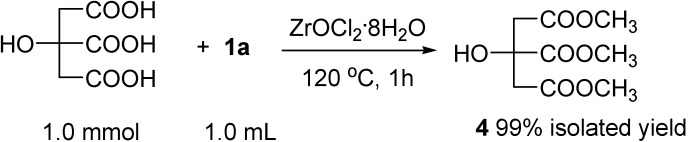
Table 4 shows the ZrOCl2·8H2O-catalyzed esterification of several different acids with equimolar amounts of a variety of alcohols at ambient temperature without removal of water or under azeotropic reflux conditions. As 1a and 1b did, the reactions of chloroacetic acid (1c), isobutyric acid (1d), pentanoic acid (1e) and hexanoic acid (1f) with 2a at ambient temperature gave the corresponding esters in good yields (entries 1 ~ 4). If we carried out the esterification in toluene under azeotropic reflux conditions to remove water, the rate of reaction could be greatly increased. For example, in the presence of 0.5 mol% of ZrOCl2·8H2O, the reaction of octanoic acid (1g) with 1-C8H17OH (2i) in toluene under azeotropic reflux for 5 h afforded the corresponding ester 3gi in 99% GC yield (entry 5). Although the rate of esterification of phenol (2j) was slower than with aliphatic alcohols, the esterification of 1g with 2j also proceeded with the azeotropic removal of water technique to give the expected ester 3gj in 35% GC yield after 36 h (entry 6). Under the same conditions, the ZrOCl2·8H2O-catalyzed the esterification of benzoic acid (1h) with benzyl alcohol (2k) gave 3hk in 82% yield (entry 7). In addition, the esterification of cyclohexanecarboxylic acid (1i) with 2k produced 3ik in high isolated yield (entry 8), and the dehydration of 1i with 0.5 equivalent of a diol, such as 1,2-benzenedimethanol (2l) or 1,3-benzenedimethanol (2m) proceeded completely to afford the expected esters in good isolated yields (entries 9 and 10). Furthermore, we examined the esterification of citric acid with an excess of 1a at 120 oC for 1h. As shown Scheme 1, ester 4 was isolated in almost quantitative yield.
Table 4.
ZrOCl2·8H2O-catalyzed esterification of carboxylic acids with alcohols a
a The reactions were carried out using 5 mmol of acid, 5 mmol of alcohol and 0.025 mmol of ZrOCl2·8H2O; b 3.0 mL of toluene was used; c Isolated yield; d 1i:diol = 2:1.
Scheme 1.
Conclusions
In summary, we have investigated the ZrOCl2·8H2O-catalyzed esterification of carboxylic acids with equimolar amount of alcohols at ambient temperature, at 50 oC or under azeotropic reflux conditions. It has been found that ZrOCl2·8H2O showed high catalytic activities in the esterification of acrylic acid and other aliphatic acids with alcohols at ambient temperature or at 50 oC without removal of water, although both the catalytic activity of ZrOCl2·8H2O and the rate of esterification could be increased greatly under azeotropic reflux conditions in toluene. In addition, in the present catalytic system, the esters could be easily separated from the reaction mixture, and catalyst could be easily recovered and reused. The present procedures offer some merits from the viewpoint of green chemistry.
Experimental Section
General
All the organic reagents and zirconium salts are commercially available and were used without further purification. GC analyses of organic compounds were performed on an Agilent Technologies 1790 GC instrument with a TC-WAX 25 m capillary column. GC-MS was performed on a Hewlett Packard 5890 Series II GC/MS spectrometer with a PEG-25M column. 1H-NMR (300 MHz) and 13C-NMR (75 MHz) spectra were recorded on a Bruker DPX-300 spectrometer.
Typical procedure for esterification of propionic acid (1b) with 1-butanol (2d) at ambient temperature (Table 3, entry 4):
A mixture of propionic acid (1b, 5 mmol), 1-butanol (2d, 5 mmol) and ZrOCl2·8H2O (0.025 mmol) was stirred at ambient temperature in a 5-mL round-bottomed glass tube under an air atmosphere for 24 h. Upon completion the reaction, diethyl ether (15 mL) was added to the reaction mixture, and the mixture was washed with water and dried with anhydrous magnesium sulfate. After removal of drying agent, the filtrate was concentrated to ca. 3.0 mL and analyzed by gas chromatography after addition of an appropriate amount of mesitylene as internal standard. 3bd was formed in 75% GC yield.
Typical procedure for esterification of octanoic acid (1g) with 1-octanol (2i) in toluene under azeotropic reflux conditions (Table 4, entry 5):
A mixture of octanoic acid (1g, 5.0 mmol), 1-octanol (2i, 5.0 mmol) and ZrOCl2·8H2O (0.025 mmol) in toluene (3.0 mL) was heated to reflux in a 25-mL round bottom flask equipped with distillation condenser and a water knockout vessel containing a quartz cotton plug and anhydrous magnesium sulfate to remove water. The consumption of reactants was monitored by GC. The reaction mixture was cooled after heating for 8 h, and diluted by addition of diethyl ether (15 mL). The ether solution was subjected to gas chromatographic analysis after addition of an appropriate amount of mesitylene as internal standard. 3gi was isolated in 92% yield (purity: 97%) by distillation under reduced pressure, the GC yield was found in 99%.
Esters 3il and 3im are new compounds, and they were characterized by 1H-, 13C-NMR, elemental analysis and GC-MS. 3ik and 4 are known compounds and their structures were confirmed by 1H-, 13C-NMR and GC-MS. The other esters are all known compounds and their identities were confirmed by GC and GC-MS comparison with the authentic samples. GC yields of esters were determined by addition of the appropriate internal standards.
Selected spectral data
3ik: 1H-NMR (CDCl3) δ 7.31~7.35 (m, 5H), 5.10 (s, 2H), 2.34 (m, 1H), 1.22~1.95 (m, 10H); 13C-NMR (CDCl3) δ 175.9, 136.3, 128.5, 128.1, 128.0, 65.9, 43.2, 29.0, 25.7, 25.4; GCMS m/z (% rel. inten.): 218 (M+, 16), 111 (24), 91 (100), 83 (59), 55 (34).
3il: 1H-NMR (CDCl3) δ 7.33~7.42 (m, 4H), 5.18 (s, 4H), 2.37 (m, 1H), 1.22~1.94 (m, 10H); 13C-NMR (CDCl3) δ 175.7, 134.7, 129.5, 128.5, 63.6, 43.2, 29.0, 25.7, 25.4; GCMS m/z (% rel. inten.): 230 (10), 148 (9), 111 (82), 83 (100), 55 (21); Anal Calcd for C22H30O4: C,73.71; H8.44. Found C, 73.82; H, 8.41.
3im: 1H-NMR (CDCl3) δ 7.26~7.38 (m, 4H), 5.11 (s, 4H), 2.36 (m, 1H), 1.18~1.95 (m, 10H); 13C-NMR (CDCl3) δ 175.8, 136.7, 128.7, 127.6, 127.3, 65.6, 43.2, 29.0, 25.7, 25.4; GCMS m/z (% rel. inten.) 230 (7), 111 (99), 83 (100), 44 (20); Anal Calcd for C22H30O4: C, 73.71; H, 8.44. Found C, 73.81; H, 8.44.
4: 1H-NMR (CDCl3) δ 4.14 (sb, 1H), 3.84 (s, 3H), 3.70 (s, 6H), 2.91 (d, 2H, AB, J = 15Hz), 2.82 (d, 2H, AB, J = 15Hz); 13C-NMR (CDCl3) δ 173.9, 170.3, 73.3, 53.3, 52.1, 43.1; m/z (% rel. inten.): 175 (15), 143 (100), 111 (4), 101 (57), 59 (13), 43 (7).
Acknowledgements
This project (20573061) was supported by National Natural Science Foundation of China.
Footnotes
Sample availability: Available from the authors.
References and Notes
- 1.Larock R. C. Comprehensive Organic Transformations. VCH; New York: 1989. [Google Scholar]
- 2.March J. Advanced Organic Chemistry: reactions, mechanisms, and structure. 4th ed. Wiley-Interscience; New York: 1992. [Google Scholar]
- 3.Dupont P., Védrine J. C., Paumard E., Hecquet G., Lefebvre F. Heteropolyacids supported on activated carbon as catalysts for the esterification of acrylic acid by butanol. Appl. Catal. A: Gen. 1995;129:217–227. [Google Scholar]
- 4.Storck S., Maier W. F., Salvado I. M. M., Ferreira J. M. F., Guhl D., Souverijns W., Martens J. A. Amorphous Sn/Si mixed oxides, mild solid Lewis acid catalysts for esterification and etherification reactions. J. Catal. 1997;172:414–426. doi: 10.1006/jcat.1997.1847. [DOI] [Google Scholar]
- 5.Segawa K., Ozawa T. Two-dimensional composite zirconium phosphonates: preparation and catalytic activities. J. Mol. Catal. A: Chem. 1999;141:249–255. [Google Scholar]
- 6.Chen X., Xu Z., Okuhara T. Liquid phase esterification of acrylic acid with 1-butanol catalyzed by solid acid catalysts. Appl. Catal. A: Gen. 1999;180:261–269. [Google Scholar]
- 7.Wakasugi K., Misaki T., Yamada K., Tanabe Y. Diphenylammonium triflate (DPAT): efficient catalyst for esterification of carboxylic acids and for transesterification of carboxylic esters with nearly equimolar amount of alcohols. Tetrahedron Lett. 2000;41:5249–5252. doi: 10.1016/S0040-4039(00)00821-2. [DOI] [Google Scholar]
- 8.Pizzio L., Vázquez P., Cáceres C., Blanco M. Tungstophosphoric and molybdophosphoric acids supported on zirconium as esterification catalysts. Catal. Lett. 2001;77:233–239. doi: 10.1023/A:1013218307792. [DOI] [Google Scholar]
- 9.Wakasugi K., Nakamura A., Tanabe Y. Me2NSO2Cl and N,N-dimethylamines: a novel and efficient agent for esterification, amidation between carboxylic acids, and equimolar amounts of alcohols and amines. Tetrahedron Lett. 2001;42:7427–7430. doi: 10.1016/S0040-4039(01)01444-7. [DOI] [Google Scholar]
- 10.Dyke C. A., Bryson T. A. Esterification of carboxylic acids with boron trichloride. Tetrahedron Lett. 2001;42:3959–3961. doi: 10.1016/S0040-4039(01)00602-5. [DOI] [Google Scholar]
- 11.Deng Y., Shi F., Beng J., Qiao K. Ionic liquid as a green catalytic reaction medium for esterifications. J. Mol. Catal. A: Chem. 2001;165:33–41. [Google Scholar]
- 12.Palaniappan S., Ram M. S. Esterification of carboxylic acids with alcohols catalyzed by polyaniline salts. Green Chem. 2002;4:53–55. doi: 10.1039/b109891h. [DOI] [Google Scholar]
- 13.Ramalinga K., Vijayalakshmi P., Kaimal T. N. B. A mild and efficient method for esterification and transesterification catalyzed by iodine. Tetrahedron Lett. 2002;43:879–882. doi: 10.1016/S0040-4039(01)02235-3. [DOI] [Google Scholar]
- 14.Zhu H.-P., Yang F., Tang J., He M.-Y. Bronsted acidic ionic liquid 1-methylimidazolium tetrafluoroborate: a green catalyst and recyclable medium for esterification. Green Chem. 2003;5:38–39. doi: 10.1039/b209248b. [DOI] [Google Scholar]
- 15.Ram M. S., Palaniappan S. Benzoyl peroxide oxidation route to polyaniline salts and its use as catalyst in the esterification reactions. J. Mol. Catal. A: Chem. 2003;201:289–296. [Google Scholar]
- 16.Kawabata T., Mizugaki T., Ebitani K., Kaneda K. Highly efficient esterification of carboxylic acids with alcohols by montmorillonite-enwrapped titanium as a heterogeneous acid catalyst. Tetrahedron Lett. 2003;44:9205–9208. doi: 10.1016/j.tetlet.2003.10.024. [DOI] [Google Scholar]
- 17.Srinivas K. V. N. S., Das B. A highly convenient, efficient and selective process for preparation of esters and amides from carboxylic acids using Fe3+-K-10 montmorillonite clay. J. Org. Chem. 2003;68:1165–1167. doi: 10.1021/jo0204202. [DOI] [PubMed] [Google Scholar]
- 18.Hao X., Yoshida A., Nishikido J. Recyclable and selective Lewis acid catalysts for transesterifications and direct esterifications in a fluorous biphase system: tin(IV) and hafnium(IV) bis(perfluorooctanesulfonyl)amide complexes. Tetrahedron Lett. 2004;45:781–785. doi: 10.1016/j.tetlet.2003.11.035. [DOI] [Google Scholar]
- 19.Ishihara K., Ohara S., Yamamoto H. Direct condensation of carboxylic acids with alcohols catalyzed by hafnium(IV) salts. Science. 2000;290:1140–1142. doi: 10.1126/science.290.5494.1140. [DOI] [PubMed] [Google Scholar]
- 20.Ishihara K., Nakayama M., Ohara S., Yamamoto H. Direct ester condensation from a 1:1 mixture of carboxylic acids and alcohols catalyzed by hafnium(IV) or zirconium(IV) salts. Tetrahedron. 2002;58:8179–8188. and references cited therein. [Google Scholar]
- 21.The catalytic activity of ZrOCl2·8H2O has been briefly examined in the esterification of 4-phenyl-butyric acid with benzyl alcohol in toluene under azeotropic reflux to give the corresponding ester in 53 % yield [20].