Abstract
Aims
Antithrombotic treatment plays a key role in stroke prevention, but their direct effects on the composition of carotid artery atherosclerotic plaques are unknown. To investigate the association of antithrombotic treatment with carotid artery plaque composition, with a specific focus on an intraplaque haemorrhage (IPH).
Methods and results
From the population-based Rotterdam Study, 1740 participants with carotid atherosclerosis on ultrasound (mean age 72.9 years, 46.0 women) underwent magnetic resonance imaging of the carotid arteries to assess plaque composition. Information on the use of oral anticoagulants [vitamin K antagonists (VKA)] and antiplatelet agents (salicylates), including duration of use and dosage, was obtained from pharmacy records for all participants. We used logistic regression models to assess the association between the use of anticoagulants and antiplatelet agents, and the different plaque components adjusting for confounders. Current and past use of VKA [adjusted odds ratio (OR): 1.88, 95% confidence interval (CI): 0.74–4.75 and OR 1.89, 95% CI: 0.91–3.93] and antiplatelet agents (OR: 1.22, 95% CI: 0.91–1.62), and (OR: 1.23, 95% CI: 0.86–1.75) showed positive trend with a higher presence of IPH. Also, a longer duration of use was associated with a higher frequency of IPH (OR: 3.15, 95% CI: 1.23–8.05) for the use of VKA, and longer duration of the use for antiplatelet agents showed a positive trend (OR: 1.21, 95% CI: 0.88–1.67). We also found that higher levels of international normalized ratio above 2.97 for VKA (OR: 1.48, 95% CI: 1.03–2.15) and higher daily defined dosage than 1.0 for antiplatelet agents (OR: 1.50, 95% CI: 1.21–1.87) were related to a higher frequency of IPH. We found no association with lipid core or calcification.
Conclusions
The use of antithrombotic treatment relates to a higher frequency of IPH in carotid atherosclerotic plaques.
Keywords: Carotid artery, Vitamin K antagonists, Antiplatelet agents, Intraplaque haemorrhage, Atherosclerosis, Epidemiology
Introduction
Atherosclerotic disease in the carotid artery is considered a key risk factor for ischaemic stroke. In recent years, considerable efforts have been put in the development of strategies to prevent the occurrence of both new and recurrent ischaemic strokes.1–5 An important cornerstone of these strategies is the prescription of oral anticoagulants and antiplatelet agents, given their beneficial effect on lowering the risk of cardiovascular events, including strokes.6 Despite this benefit of oral anticoagulants and antiplatelet agents,7 their effects on the development or changes in already existing atherosclerotic carotid plaque are unknown.8 Emerging technological development in magnetic resonance imaging (MRI) enables a detailed characterization of carotid plaque components like intraplaque haemorrhage (IPH), lipid core and calcification,8–10 which play an important role in future thromboembolic events.11–14 Recently, in a relatively small sample of symptomatic patients, it was highlighted that antithrombotic treatment may exert a potentially harmful effect on the composition of carotid atherosclerotic plaques.8 Specifically, the use of antiplatelet agents was related to a higher presence of IPH, which is a plaque component that is known to be more prevalent in plaques that are prone to rupture.8 However, several important questions on this topic remain as previous studies were in symptomatic patients with advanced atherosclerosis. First, the influence of using vitamin K antagonists (VKA), which is also frequently prescribed for prevention of cardiovascular events of cardiac origin like as atrial fibrillation, on the composition of carotid atherosclerosis remains unclear. Second, the duration and dosage of use of VKA or antiplatelet agents may substantially affect the carotid plaque composition but has not been studied before. Therefore, we investigated in a large sample of subjects with subclinical atherosclerosis from the Rotterdam Study, the associations between oral antithrombotic treatment and carotid plaque composition, with a special focus on IPH.
Methods
Study population
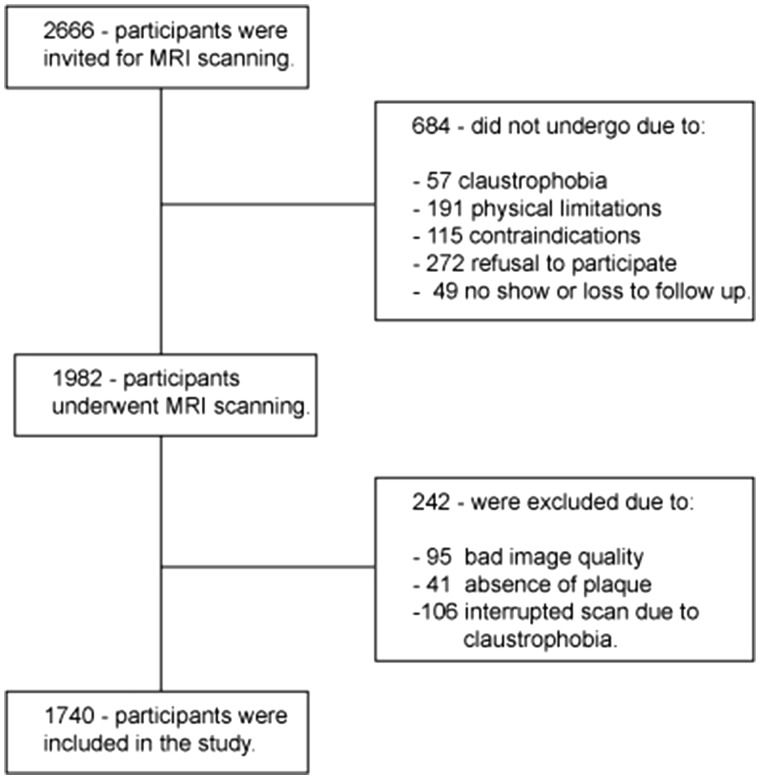
This study is embedded within a prospective population-based cohort, The Rotterdam Study.15 Between the year 2007 and 2012 participants with carotid atherosclerosis were invited to undergo an MRI scan of the carotid arteries. Previously, participants were selected on the basis of a carotid artery ultrasound examination (intima-media thickness ≥2.5 mm in one or both carotid arteries) performed in all participants of the Rotterdam Study. From the 2666 invited participants, 684 did not undergo MRI scan, and 1982 did (74%). From them, 242 participants were excluded and 1740 were included in this study (Figure 1). The Rotterdam Study has been approved by the Medical Ethical Committee of the Erasmus MC and by the Dutch Ministry of Health, Welfare and Sports, implementing the ‘Wet Bevolkings Onderzoek: ERGO (Population Screening Act: Rotterdam Study)’. All participants provided written informed consent to participate in the study and to obtain information from their treating physicians.
Figure 1.
Flowchart of the study population.
Carotid scanning and analysis of plaque components
A 1.5 Tesla scanner (GE Healthcare, Milwaukee, WI, USA) with a dedicated bilateral phase-array surface coil (Machnet, Eelde, the Netherlands) was used to perform bilateral imaging of the carotid artery, with a standardized scanning protocol, which required an approximate total scanning time of 30 min. The protocol included four sequences in axial plane: a proton density weighted (PDw)-fast spin echo (FSE)-black blood (BB) sequence (in-plane resolution 130/160 × 130/128 = 0.8 × 1 cm); a PDw-FSE-BB with an increased in-plane resolution (in-plane resolution 130/224 × 130/160 = 0.5 × 0.8 cm); a PDw-echo planar imaging (EPI) sequence (in-plane resolution 130/160 × 70/160 = 0.8 × 0.4 cm); a T2-weighted-EPI sequence (in-plane resolution 130/160 × 70/160 = 0.8 × 0.4 cm) and two three-dimensional (3D) sequences: a 3D-T1-weighted (T1w)-gradient echo sequence (in-plane resolution 180/192 × 180/180 = 0.9 × 1 cm), and a 3 D phased-contrast magnetic resonance angiography (3D-PC-MRA) (in-plane resolution 180/256 × 180/128 = 0.7 × 1.4 cm).16 More details of the scanning protocol, reading procedure, and reproducibility are described in detail elsewhere.17 The images of the carotid were evaluated for the presence of three different plaque components: IPH, lipid core, and calcification. Calcification was defined as the presence of a hypointense region in the plaque on all sequences.16,18,19 Intraplaque haemorrhage was defined as the presence of a hyperintense region in the atherosclerotic plaque on 3D-T1w-GRE.20,21 Lipid core presence was defined as a hypointense region, not classified as IPH or calcification, in the plaque on PDw-FSE or PDw-EPI and T2w-EPI images or a region of relative signal intensity drop in the T2w-EPI images compared with the PDw-EPI images.18,19,22 Two independent MRI readers, with 3 years of experience, not being aware of any of the clinical characteristics of the participants, including the medication recorded subjects as positive for the presence of any plaque component if the component was identified in one or both carotid arteries. Testing the intra-subject variability, 40 participants underwent a second MRI scan (average time between scans 15 ± 9 days).17 For an interobserver reproducibility analysis, MRI examinations were selected randomly (n = 50) and read by a second independent observer with three years of experience. Further, using Cohens’ Kappa statistics interobserver and an intra-scan agreement was calculated. The intra-subject agreement was good for all measurements. The Kappa value for the presence of IPH was 0.95 (95% CI 0.88–0.99); for lipid core was 0.85 (95% CI 0.74–0.96) and for calcification was 0.91 (95% CI 0.82–0.99).17 The interobserver agreement was good for all measurements. The Kappa value for IPH was 0.86 (95% CI 0.72–0.99); for lipid core presence 0.86 (95% CI 0.72–0.99) and for calcification 0.94 (95% CI 0.86–0.99).17
Assessment of antithrombotic treatment
Dispensing information for the VKA and antiplatelet agents [acetylsalicylic acid (ATC code B01AC06) and carbasalate calcium (ATC code B01AC08)] was obtained using a computerized platform linking the study database and the pharmacies in the study area. All prescriptions for VKA and antiplatelet agents from 1 January 1991 to 26 October 2012, included the product name of the drug, the anatomical therapeutic chemical code (ATC code), the amount dispensed, the prescribed dose regimen, and the date of dispensing.23,24 The average measured international normalized ratios (INRs)25 were obtained for VKA use and daily defined dosage (DDD) was obtained for antiplatelet agents use.26 Among the antithrombotic users, we distinguished the main therapeutic indications such as atrial fibrillation, recent coronary or cerebrovascular events.
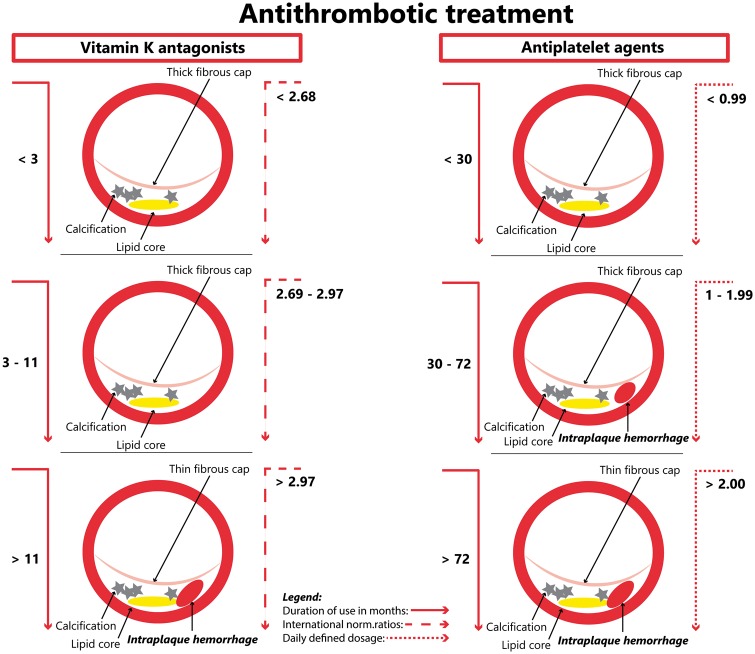
At the time of the MRI, all subjects were classified into one of the following mutually exclusive categories: ‘current user’ if the subject was an active user at time of MRI; ‘past user’ if the subject discontinued the use before the MRI scan date, or ‘never user’ if subject never used any of these drugs. We used tertiles for the duration of use to classify the use of VKA and antiplatelet agents. This resulted in the VKA duration of use ≤3 months; duration of use 3–11 months, duration of use >11 months, and for the antiplatelet agents’ duration of use ≤30 months; duration of use 30–72 months, duration of use >72 months since the end of the last prescription episode. To facilitate direct dose-dependent relation between drugs from the same therapeutic drug group, we used the INRs25 for the VKA use and a daily dose of antiplatelet agents expressed in DDD.26 We created three categories of measured INR and DDD and compared them separately with never use. The INRs categories were set based on tertiles and antiplatelet DDD categories were set based on the median. Only five subjects, previously treated with VKA due to atrial fibrillation and with the recent coronary syndrome, were in concomitant use of dual therapy at the time of MRI and none in past use category. Considering the prolonged effect of anticoagulation medication, we have not reallocated any subject from past use into current use categories among VKA users as a minimum discontinuation period was 22 days but we reclassified three subjects in past use category, among antiplatelet agent users, to current use category since the minimum discontinuation period was 3–5 days.25,27
Other risk factors in the Rotterdam Study
Information on the other cardiovascular risk factors as relevant measurements was obtained by interview, physical examination, and blood sampling.15 Among the other measures smoking status was categorized into never, the past, and current smoking, diabetes mellitus was defined as fasting blood glucose >6.9 mmol/L, nonfasting glucose >11.0 mmol/L, or use of glucose-lowering medication, systolic and diastolic blood pressure was measured using a random-zero sphygmomanometer on the right arm and two measurements were performed and the average of the two was used in the analyses. Body mass index was calculated based on weight in kilograms divided by height in metres squared. Serum total cholesterol and high-density lipoprotein levels were measured using standard laboratory techniques. We were able to consider the use of antihypertensive medication and statin use, which were obtained from pharmacy records.15
Statistical analysis
We used the means [standard deviations (SDs)], medians [interquartile ranges (IQRs)] and percentages to describe the distribution of continuous and categorical variables, respectively. To investigate the association between antithrombotic treatment and plaque components, IPH, lipid core, and calcification, a three-step statistical analysis approach was used. Initially, we prepared three models, using logistic regression, to assess the association of antithrombotic treatment (never, current, past) with the presence of each component in one or both carotid arteries. In the first model, we adjusted these analyses for age and sex and in second model we adjusted for other risk factors. Additionally, in model three for VKA users, we adjusted for the INRs levels. Further, as a second step, we examined whether the duration of antithrombotic treatment was associated with any of the plaque components. Third, we assessed whether the INRs levels for VKA and DDDs for antiplatelet agents were associated with each plaque component. Finally, we performed sensitivity analyses and stratified analyses. First, to address confounding by indication, we restricted analyses among subjects without known cardiovascular diseases (CVD), only by excluding the participants with known CVD history and performed the propensity-score matching for untreated and treated participants. Cardiovascular diseases were defined as known and verified the history of stroke, myocardial infarction or coronary heart disease. Second, we performed stratified analyses for sex and age below and above 70 years of age, to investigate whether associations are different between sex and age groups. Antithrombotic treatment is usually prescribed to subjects at risk for or with a history of CVD.
All analyses were carried out using IBM SPSS Statistical package version 21 (Chicago, IL, USA).
Results
The study population characteristics are provided in Table 1. The mean age of the population was 72.9 years (SD: 9.1 years) and 46.0% were women. At the time of MRI, a total of 6.8% of the participants was using VKA treatment and 29.9% used antiplatelet (salicylates) treatment. The median VKA use was 11 months (IQR 3–43 months), and median antiplatelet agents use was 72 months (IQR 30–123 months). The IPH was more frequently found in the users of antithrombotic treatment compared to never-users (Table 2).
Table 1.
Baseline characteristics of the study population (n = 1740)
| Age (years) | 72.9 ± 9.1 |
| Women (%) | 46.0 |
| Smoking, current (%) | 31.5 |
| Diabetes mellitus (%) | 14.4 |
| Systolic blood pressure (mmHg) | 145 ± 21 |
| Diastolic blood pressure (mmHg) | 80 ± 11 |
| BMI (kg/m2) | 27.3 ± 3.5 |
| Total cholesterol (mmol/L) | 5.6 ± 1.0 |
| HDL cholesterol (mmol/L) | 1.4 ± 0.3 |
| Use of antihypertensive medication (%) | 39.3 |
| Use of statins (%) | 29.0 |
| Vitamin K antagonists (%) | |
| Current | 6.8 |
| Past | 9.0 |
| Antiplatelet agents use (%) | |
| Current | 29.9 |
| Past | 11.9 |
| Wall thickness (mm) | 3.2 ± 0.6 |
| Degree of stenosis (%) | 14.4 (0.0–26.8) |
| History of stroke (%) | 6.3 |
| History of coronary heart disease (%) | 11.4 |
Values are presented as means (standard deviations) or median (interquartile ranges) for continuous variables and percentages for dichotomous or categorical variables.
BMI, body mass index; CHD, coronary heart disease; HDL, high-density lipoprotein; IPH, intraplaque haemorrhage.
Table 2.
Presence of the components in the carotid artery plaque according to antithrombotic treatment
| IPH | Lipid core | Calcification | |
|---|---|---|---|
| Vitamin K antagonists | |||
| Never use | 31.9 | 43.6 | 82.0 |
| Current use | 50.4 | 47.9 | 87.4 |
| Past use | 48.1 | 44.9 | 81.4 |
| Antiplatelet agents | |||
| Never use | 28.6 | 43.8 | 78.7 |
| Current use | 42.5 | 44.4 | 87.9 |
| Past use | 44.4 | 44.0 | 86.0 |
Values are presented as percentages.
IPH, intraplaque haemorrhage.
Antithrombotic therapy and carotid plaque composition
Table 3 summarizes the associations of oral VKA and antiplatelet agents use with the three plaque components. Although not statistically significant, we found a trend that current and past use of VKA [adjusted odds ratio (OR): 1.88, 95% confidence interval (CI): 0.74–4.75 and OR 1.89, 95% CI: 0.91–3.93, respectively] (Table 3) and current and past use of antiplatelet agents (OR: 1.22, 95% CI: 0.91–1.62 and OR: 1.23, 95% CI: 0.86–1.75, respectively) related to a higher presence of IPH (Table 3). We found no associations between antithrombotic treatment with a lipid core or calcification.
Table 3.
Association between antithrombotic treatment and carotid artery plaque composition
| IPH, OR (95% CI) | Lipid core, OR (95% CI) | Calcification, OR (95% CI) | |
|---|---|---|---|
| Vitamin K antagonists | |||
| Model 1 | |||
| Never use | Ref | Ref | Ref |
| Current use | 1.48 (1.00–2.19) | 1.01 (0.69–1.49) | 0.96 (0.54–1.71) |
| Past use | 1.55 (1.10–2.19) | 0.97 (0.69–1.36) | 0.66 (0.42–1.03) |
| Model 2 | |||
| Never use | Ref | Ref | Ref |
| Current use | 1.34 (0.87–2.06) | 1.06 (0.70–1.59) | 0.88 (0.48–1.61) |
| Past use | 1.46 (1.01–2.12) | 0.99 (0.70–1.41) | 0.59 (0.37–0.94) |
| Model 3 | |||
| Never use | Ref | Ref | Ref |
| Current use | 1.88 (0.74–4.75) | 0.87 (0.36–2.11) | 1.09 (0.33–3.53) |
| Past use | 1.89 (0.91–3.93) | 0.85 (0.42–1.73) | 0.70 (0.28–1.77) |
| Antiplatelet agents use | |||
| Model 1 | |||
| Never use | Ref | Ref | Ref |
| Current use | 1.46 (1.16–1.84) | 0.92 (0.74–1.15) | 1.51 (1.10–2.07) |
| Past use | 1.57 (1.14–2.17) | 0.94 (0.69–1.29) | 1.19 (0.77–1.84) |
| Model 2 | |||
| Never use | Ref | Ref | Ref |
| Current use | 1.22 (0.91–1.62) | 1.00 (0.77–1.30) | 1.07 (0.74–1.54) |
| Past use | 1.23 (0.86–1.75) | 0.91 (0.66–1.27) | 1.03 (0.65–1.61) |
Model 1 – adjusted for age, sex. Model 2 – model 1 + smoking, diabetes mellitus, systolic blood pressure, diastolic blood pressure, body mass index, total cholesterol, high-density lipoprotein, antihypertensive medication use, statin use, and carotid wall thickness. Model 3 – model 2+ average INR.
CI, confidence interval; INR, international normalized ratio; IPH, intraplaque haemorrhage; OR, odds ratios.
Duration of use and dosage of antithrombotic therapy
The use of VKA for 3 months or less was not associated with the presence of IPH whereas the use of VKA for more than 3 months was significantly related to the presence of IPH. This relation became more prominent when we additionally adjusted for INRs levels (adjusted OR: 3.15, 95% CI: 1.23–8.05) (Table 4). The use of antiplatelet agents for less than or more than 30 months was not related to the presence of IPH (adjusted OR: 1.21, 95% CI: 0.88–1.67) (Table 4).
Table 4.
Association between duration of use of antithrombotic treatment and intraplaque haemorrhage in the carotid artery
| IPH |
|||
|---|---|---|---|
| Model 1, OR (95% CI) | Model 2, OR (95% CI) | Model 3, OR (95% CI) | |
| Vitamin K antagonists | |||
| Never use duration | Ref | Ref | Ref |
| ≤3 months | 0.81 (0.48–1.37) | 0.76 (0.43–1.35) | 1.21 (0.51–2.86) |
| 3–11 months | 1.63 (0.99–2.69) | 1.62 (0.96–2.76) | 2.54 (1.11–5.82) |
| >11 months | 1.95 (1.35–2.82) | 1.74 (1.16–2.60) | 3.15 (1.23–8.05) |
| Antiplatelet agents use | |||
| Never use duration | Ref | Ref | |
| ≤30 months | 1.30 (0.92–1.83) | 1.14 (0.78–1.66) | |
| 30–72 months | 1.54 (1.10–2.16) | 1.32 (0.91–1.92) | |
| >72 months | 1.57 (1.21–2.04) | 1.21 (0.88–1.67) | |
Model 1 – adjusted for age, sex. Model 2 – model 1 + smoking, diabetes mellitus, systolic blood pressure, diastolic blood pressure, body mass index, total cholesterol, high-density lipoprotein, antihypertensive medication use, statin use and carotid wall thickness. Model 3 – model 2+ average INR.
CI, confidence interval; INR, international normalized ratio; IPH, intraplaque haemorrhage; OR, odds ratios.
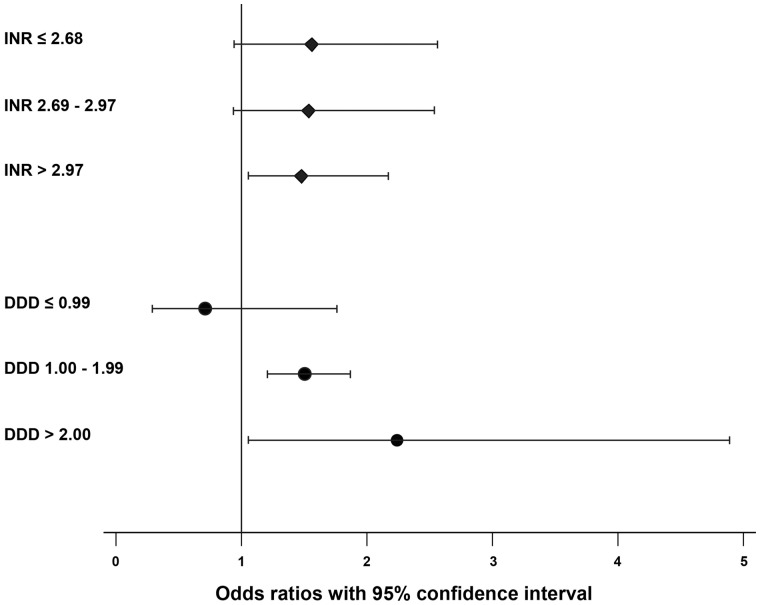
Furthermore, when considering the effect of INRs and DDDs, the dose-response relation was found in both groups. Among oral vitamin K antagonist users, INRs levels higher than 2.97 were significantly associated with the presence of IPH, whereas among the antiplatelet users DDDs levels higher than 1.0 were significantly associated with the presence of IPH (Table 5 and Figure 2). When restricting our analyses only to subjects without a history of CVD, we found a prominent positive trend with IPH, among current and past users of VKA, and a positive trend with IPH among current and past users of antiplatelet agents (Supplementary material online, Table S1). Further, propensity-score matched analyses yielded similar results (Supplementary material online, Table S2).
Table 5.
Association of antithrombotic treatment with intraplaque haemorrhage, according to international normalized ratios for vitamin K antagonists and daily defined dosage for antiplatelet agents
| IPH, OR (95% CI) | |
|---|---|
| Vitamin K antagonists | |
| INR | |
| ≤2.68 | 1.56 (0.94–2.56) |
| 2.69–2.97 | 1.54 (0.93–2.54) |
| >2.97 | 1.48 (1.03–2.15) |
| Antiplatelet agents | |
| DDD | |
| ≤0.99 | 0.71 (0.29–1.76) |
| 1.00–1.99 | 1.50 (1.21–1.87) |
| >2.00 | 2.24 (1.03–4.87) |
Values are adjusted for age and sex.
CI, confidence interval; INR, international normalized ratio; IPH, intraplaque haemorrhage; OR, odds ratios.
Figure 2.
Association of antithrombotic treatment with intraplaque haemorrhage, according to international normalized ratios for vitamin K antagonists and daily defined dosage for antiplatelet agents. Values on the y-axis represent the odds ratios and 95% confidence interval. The values are adjusted for age and sex. The P-trend in both groups <0.001.
Take home figure.
Duration and dosage of use of antithrombotic treatment play important role in the presence of intraplaque haemorrhage (IPH) in the atherosclerotic plaque of the carotid artery.
Additionally, investigating for age differences, we found a more prominent association of VKA current use with IPH in the younger age group ≤70 for both drug groups (Supplementary material online, Table S3). Moreover, when investigating the sex differences, we found a prominent trend between VKA use and IPH in females compared to males and similar trend between antiplatelet agents use and IPH for both sexes (Supplementary material online, Table S4).
Discussion
In this large population-based sample of individuals with subclinical carotid atherosclerosis, we observed that current and past use of antithrombotic treatment is associated with IPH in the carotid artery plaques. Moreover, we found that longer duration of use and higher dosages of antithrombotic treatment were related to a higher frequency of IPH.
The association of antithrombotic treatment with IPH in atherosclerotic plaques has been studied before, but only in high risk, symptomatic patients. In these studies, it was found that the use of antithrombotic treatment related to the presence of IPH.8,28,29 Moreover, a histopathological study on carotid endarterectomy specimens demonstrated an effect of antithrombotic treatment on the presence of IPH, in particular of VKA.28 Apart from this, the earlier study also found that before surgical intervention the users of antiplatelet agents, suffered a higher presence of multiple haemorrhages in plaques (68% compared with 17%) compared with patients who did not use.29 Finally, a recent report from the PARISK-study highlighted an association of antiplatelet agents with IPH.8 Our results further elucidate the effect of antithrombotic treatment on IPH in carotid atherosclerosis on multiple levels and corroborate findings of studies with symptomatic high-risk patients. First, we demonstrated that already in the subclinical phase of carotid atherosclerosis there is a prominent association of antithrombotic treatment. Second, duration of use is important with regard to the presence of IPH. Third, independently of other risk factors higher levels of INRs and DDDs influence on the presence of IPH.
One of the main mechanisms underlying the relation of antithrombotic treatment with a higher presence of IPH may be the leakage of neovessels (neovascularization) in the plaque under influence of antithrombotic treatment.8 Histological observations suggest that IPH arises from the adventitia,30 as inward sprouting neovessels towards the plaque and neovessels are considered immature and highly susceptible to leakage.31 In this context, we hypothesize that antithrombotic treatment may predispose persons to extended leakage from neovessels, due to their antithrombotic effects. Additionally, our findings may be explained through prior histopathological evidence, which suggests that oral VKA relate to haemorrhage from the neovessels within the plaque whereas antiplatelet agents increase densities of these neovessels.32,33 Similarly, a capillary bleeding in humans has been found in patients using coumarin-type anticoagulation.34 On the other hand, animal studies exhibited that capillary dilation and permeability, or capillary bleeding, increase when using coumarin-type anticoagulants.35–37 Although VKA inhibit the vitamin K conversion cycle and salicylates inhibit the platelet aggregation and induce vasodilation, both drugs may contribute similarly with their effects in regard to IPH formation.25,27
These findings seem paradoxical to current knowledge as the goal of antithrombotic treatment is to prevent cardiovascular events. Of note is that the beneficial effect of antithrombotic treatment7 is well documented under medical conditions such as ischaemic cardiovascular or cerebrovascular diseases.6,38,39 Yet, our findings emphasize that antithrombotic treatment should be used with care and in low dosage7 and not prescribed unless the clinical benefits outweigh the risks. With regard to the clinical effects of antithrombotic treatment, a meta-analysis of two large trials (International stroke trial and Chinese acute stroke trial), revealed that antithrombotic treatment reduced the risk of ischaemic stroke only in the 6–12 weeks directly after the stroke, but established no beneficial effect after this period.40 Due to the observational nature of the study, the findings should be regarded as hypothesis generating and further confirmation is required.
The major strengths of the current study include the long follow-up time, the duration and dosage of exposure to antithrombotic treatment for all study participants, and the standardized MRI-based assessment of carotid atherosclerotic plaques. This is the first population-based study to investigate the association of antithrombotic treatment with plaque components in the carotid artery within a relatively large study sample. Moreover, the MRI enabled to characterize in great detail each specific component of the carotid plaque. Nonetheless, some limitations of our study should also be taken into account. First, we did not have data on the use of new oral anticoagulants (NOAC’s). Second, as in many observational studies investigating the effect of specific medication, we should acknowledge the issue of confounding-by-indication given that antithrombotic treatment is more often prescribed to persons with an increased risk of CVD. However, sensitivity analysis did not show prominent differences in the results. Third, although salicylates are prescribed by doctors, such drugs are available ‘over the counter’, and used for other therapeutic indications for e.g. like analgesics. Fourth, the cross-sectional design limits our ability to draw causal inferences between antithrombotic treatment and IPH.
Conclusion
The use of antithrombotic treatment relates to a higher frequency of IPH in carotid atherosclerotic plaques. Further studies are warranted to replicate this finding in a longitudinal design.
Supplementary Material
Acknowledgements
The dedication, commitment, and contribution of the inhabitants, general practitioners, and pharmacists of the Ommoord district to the Rotterdam Study are gratefully acknowledged.
Funding
The Rotterdam Study is supported by the Erasmus MC and Erasmus University Rotterdam; the Netherlands Organization for Scientific Research (NWO); the Netherlands Organization for Health Research and Development (ZonMw); the Research Institute for Diseases in the Elderly (RIDE); the Netherlands Genomics Initiative (NGI); the Ministry of Education, Culture and Science, the Ministry of Health, Welfare and Sports; the European Commission (DG XII); and the Municipality of Rotterdam.
Conflict of interest: O.H.F. works in ErasmusAGE, a centre for aging research across the life course funded by Nestlé Nutrition (Nestec Ltd); Metagenics Inc.; and AXA. The other authors report no potential conflicts of interest.
Footnotes
See page 3377 for the editorial comment on this article (doi: 10.1093/eurheartj/ehy583)
References
- 1. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P; Authors/Task Force Members, Document Reviewers. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS: the Task Force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Endorsed by the European Stroke Organisation (ESO). Eur Heart J 2016;37:2893–2962.27567408 [Google Scholar]
- 2. Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvanne M, Scholte Op Reimer WJ, Vrints C, Wood D, Zamorano JL, Zannad F; European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG) . European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J 2012;223:1–701. [DOI] [PubMed] [Google Scholar]
- 3. Meschia JF, Bushnell C, Boden-Albala B, Braun LT, Bravata DM, Chaturvedi S, Creager MA, Eckel RH, Elkind MS, Fornage M, Goldstein LB, Greenberg SM, Horvath SE, Iadecola C, Jauch EC, Moore WS, Wilson JA. ; American Heart Association StrokeCouncil; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Functional Genomics and Translational Biology; Council on Hypertension . Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:3754–3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, Fang MC, Fisher M, Furie KL, Heck DV, Johnston SC, Kasner SE, Kittner SJ, Mitchell PH, Rich MW, Richardson D, Schwamm LH, Wilson JA; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease . Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:2160–2236. [DOI] [PubMed] [Google Scholar]
- 5. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron EG, Budts W, Carerj S, Casselman F, Coca A, De CR, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GY, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K.. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 6. Anand SS, Yusuf S.. Oral anticoagulant therapy in patients with coronary artery disease: a meta-analysis. JAMA 1999;282:2058–2067. [DOI] [PubMed] [Google Scholar]
- 7. Aboyans V, Ricco JB, Bartelink MEL, Bjorck M, Brodmann M, Cohnert T, Collet JP, Czerny M, De Carlo M, Debus S, Espinola-Klein C, Kahan T, Kownator S, Mazzolai L, Naylor AR, Roffi M, Rother J, Sprynger M, Tendera M, Tepe G, Venermo M, Vlachopoulos C, Desormais I; ESC Scientific Document Group. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Endorsed by: the European Stroke Organization (ESO). The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J 2018;39:763–816. [DOI] [PubMed] [Google Scholar]
- 8. Liem MI, Schreuder FH, van Dijk AC, de Rotte AA, Truijman MT, Daemen MJ, van der Steen AF, Hendrikse J, Nederveen AJ, van der Lugt A, Kooi ME, Nederkoorn PJ.. Use of antiplatelet agents is associated with intraplaque hemorrhage on carotid magnetic resonance imaging: the plaque at risk study. Stroke 2015;46:3411–3415. [DOI] [PubMed] [Google Scholar]
- 9. Gupta A, Baradaran H, Schweitzer AD, Kamel H, Pandya A, Delgado D, Dunning A, Mushlin AI, Sanelli PC.. Carotid plaque MRI and stroke risk: a systematic review and meta-analysis. Stroke 2013;44:3071–3077. [DOI] [PubMed] [Google Scholar]
- 10. Saam T, Hetterich H, Hoffmann V, Yuan C, Dichgans M, Poppert H, Koeppel T, Hoffmann U, Reiser MF, Bamberg F.. Meta-analysis and systematic review of the predictive value of carotid plaque hemorrhage on cerebrovascular events by magnetic resonance imaging. J Am Coll Cardiol 2013;62:1081–1091. [DOI] [PubMed] [Google Scholar]
- 11. El Aidi H, Mani V, Weinshelbaum KB, Aguiar SH, Taniguchi H, Postley JE, Samber DD, Cohen EI, Stern J, van der Geest RJ, Reiber JH, Woodward M, Fuster V, Gidding SS, Fayad ZA.. Cross-sectional, prospective study of MRI reproducibility in the assessment of plaque burden of the carotid arteries and aorta. Nat Clin Pract Cardiovasc Med 2009;6:219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yuan C, Beach KW, Smith LH Jr, Hatsukami TS.. Measurement of atherosclerotic carotid plaque size in vivo using high resolution magnetic resonance imaging. Circulation 1998;98:2666–2671. [DOI] [PubMed] [Google Scholar]
- 13. Spagnoli LG, Mauriello A, Sangiorgi G, Fratoni S, Bonanno E, Schwartz RS, Piepgras DG, Pistolese R, Ippoliti A, Holmes DR Jr.. Extracranial thrombotically active carotid plaque as a risk factor for ischemic stroke. JAMA 2004;292:1845–1852. [DOI] [PubMed] [Google Scholar]
- 14. Nicolaides AN, Kakkos SK, Kyriacou E, Griffin M, Sabetai M, Thomas DJ, Tegos T, Geroulakos G, Labropoulos N, Dore CJ, Morris TP, Naylor R, Abbott AL; Asymptomatic Carotid Stenosis and Risk of Stroke (ACSRS) Study Group . Asymptomatic internal carotid artery stenosis and cerebrovascular risk stratification. J Vasc Surg 2010;52:1486–1496.e1–5. [DOI] [PubMed] [Google Scholar]
- 15. Hofman A, Brusselle GG, Darwish Murad S, van Duijn CM, Franco OH, Goedegebure A, Ikram MA, Klaver CC, Nijsten TE, Peeters RP, Stricker BH, Tiemeier HW, Uitterlinden AG, Vernooij MW.. The Rotterdam Study: 2016 objectives and design update. Eur J Epidemiol 2015;30:661–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mujaj B, Lorza AM, van Engelen A, de Bruijne M, Franco OH, van der Lugt A, Vernooij MW, Bos D.. Comparison of CT and CMR for detection and quantification of carotid artery calcification: the Rotterdam Study. J Cardiovasc Magn Reson 2017;19:28.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van den Bouwhuijsen QJ, Vernooij MW, Hofman A, Krestin GP, van der Lugt A, Witteman JC.. Determinants of magnetic resonance imaging detected carotid plaque components: the Rotterdam Study. Eur Heart J 2012;33:221–229. [DOI] [PubMed] [Google Scholar]
- 18. Cappendijk VC, Cleutjens KB, Kessels AG, Heeneman S, Schurink GW, Welten RJ, Mess WH, Daemen MJ, van Engelshoven JM, Kooi ME.. Assessment of human atherosclerotic carotid plaque components with multisequence MR imaging: initial experience. Radiology 2005;234:487–492. [DOI] [PubMed] [Google Scholar]
- 19. Saam T, Ferguson MS, Yarnykh VL, Takaya N, Xu D, Polissar NL, Hatsukami TS, Yuan C.. Quantitative evaluation of carotid plaque composition by in vivo MRI. Arterioscler Thromb Vasc Biol 2005;25:234–239. [DOI] [PubMed] [Google Scholar]
- 20. Bitar R, Moody AR, Leung G, Symons S, Crisp S, Butany J, Rowsell C, Kiss A, Nelson A, Maggisano R.. In vivo 3D high-spatial-resolution MR imaging of intraplaque hemorrhage. Radiology 2008;249:259–267. [DOI] [PubMed] [Google Scholar]
- 21. Moody AR. Magnetic resonance direct thrombus imaging. J Thromb Haemost 2003;1:1403–1409. [DOI] [PubMed] [Google Scholar]
- 22. Yuan C, Mitsumori LM, Ferguson MS, Polissar NL, Echelard D, Ortiz G, Small R, Davies JW, Kerwin WS, Hatsukami TS.. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation 2001;104:2051–2056. [DOI] [PubMed] [Google Scholar]
- 23. Akoudad S, Darweesh SK, Leening MJ, Koudstaal PJ, Hofman A, van der Lugt A, Stricker BH, Ikram MA, Vernooij MW.. Use of coumarin anticoagulants and cerebral microbleeds in the general population. Stroke 2014;45:3436–3439. [DOI] [PubMed] [Google Scholar]
- 24. Vernooij MW, Haag MD, van der Lugt A, Hofman A, Krestin GP, Stricker BH, Breteler MM.. Use of antithrombotic drugs and the presence of cerebral microbleeds: the Rotterdam Scan Study. Arch Neurol 2009;66:714–720. [DOI] [PubMed] [Google Scholar]
- 25. Hirsh J, Fuster V, Ansell J, Halperin JL, American Heart A, American College Of Cardiology F.. American Heart Association/American College of Cardiology Foundation guide to warfarin therapy. Circulation 2003; 107:1692–1711. [DOI] [PubMed] [Google Scholar]
- 26. de Keyser CE, de Lima FV, de Jong FH, Hofman A, de Rijke YB, Uitterlinden AG, Visser LE, Stricker BH.. Use of statins is associated with lower serum total and non-sex hormone-binding globulin-bound testosterone levels in male participants of the Rotterdam Study. Eur J Endocrinol 2015; 173:155–165. [DOI] [PubMed] [Google Scholar]
- 27. Awtry EH, Loscalzo J.. Aspirin. Circulation 2000; 101:1206–1218. [DOI] [PubMed] [Google Scholar]
- 28. Derksen WJ, Peeters W, Tersteeg C, de Vries JP, de Kleijn DP, Moll FL, van der Wal AC, Pasterkamp G, Vink A.. Age and coumarin-type anticoagulation are associated with the occurrence of intraplaque hemorrhage, while statins are associated less with intraplaque hemorrhage: a large histopathological study in carotid and femoral plaques. Atherosclerosis 2011; 214:139–143. [DOI] [PubMed] [Google Scholar]
- 29. AbuRahma AF, Boland JP, Robinson P, Decanio R.. Antiplatelet therapy and carotid plaque hemorrhage and its clinical implications. J Cardiovasc Surg (Torino) 1990;31:66–70. [PubMed] [Google Scholar]
- 30. Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, Wrenn SP, Narula J.. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol 2005;25:2054–2061. [DOI] [PubMed] [Google Scholar]
- 31. Michel J-B, Martin-Ventura JL, Nicoletti A, Ho-Tin-Noé B.. Pathology of human plaque vulnerability: mechanisms and consequences of intraplaque haemorrhages. Atherosclerosis 2014; 234:311–319. [DOI] [PubMed] [Google Scholar]
- 32. Kolodgie FD, Gold HK, Burke AP, Fowler DR, Kruth HS, Weber DK, Farb A, Guerrero LJ, Hayase M, Kutys R, Narula J, Finn AV, Virmani R.. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med 2003;349:2316–2325. [DOI] [PubMed] [Google Scholar]
- 33. Li X, Vink A, Niessen HW, Kers J, de Boer OJ, Ploegmakers HJ, Tijssen JG, de Winter RJ, van der Wal AC.. Total burden of intraplaque hemorrhage in coronary arteries relates to the use of coumarin-type anticoagulants but not platelet aggregation inhibitors. Virchows Arch 2014;465:723–729. [DOI] [PubMed] [Google Scholar]
- 34. Leithauser B, Mrowietz C, Hiebl B, Pindur G, Jung F.. Capillary bleeding under oral anticoagulation. Clin Hemorheol Microcirc 2009;43:167–171. [DOI] [PubMed] [Google Scholar]
- 35. Fuchs U. [ Submicroscopic changes of the rat lung following the administration of a coumarin derivative] Submikroskopische Veranderungen der Rattenlunge nach Verabreichung eines Cumarinderivates. Frankf Z Pathol 1965;74:555–564. [PubMed] [Google Scholar]
- 36. Pratesi F, Spinelli P, Caramelli L, Tesi M, Dabizzi RP.. Ultrastructure of the cerebral capillaries in experimental ischaemia and pharmacological action on it. Bibl Anat 1969;10:174–183. [PubMed] [Google Scholar]
- 37. Kahn RA, Johnson SA, DeGraff AF.. Effects of sodium warfarin on capillary ultrastructure. Am J Pathol 1971;65:149–156. [PMC free article] [PubMed] [Google Scholar]
- 38. Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. EAFT (European Atrial Fibrillation Trial) Study Group. Lancet (London, England) 1993;342:1255–1262. [PubMed] [Google Scholar]
- 39. Group ES, Halkes PH, van Gijn J, Kappelle LJ, Koudstaal PJ, Algra A.. Medium intensity oral anticoagulants versus aspirin after cerebral ischaemia of arterial origin (ESPRIT): a randomised controlled trial. Lancet Neurol 2007;6:115–124. [DOI] [PubMed] [Google Scholar]
- 40. Rothwell PM, Algra A, Chen Z, Diener HC, Norrving B, Mehta Z.. Effects of aspirin on risk and severity of early recurrent stroke after transient ischaemic attack and ischaemic stroke: time-course analysis of randomised trials. Lancet (London, England) 2016;388:365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.