Abstract
Novel 3-acetyl-2-aminothiophenes were prepared from cyanoacetone and 1,4‑dithianyl-2,5‑diols using a modified Gewald reaction. The syntheses of the corresponding acetamides, as well as that of 3‑acetyl-2‑amino-5‑nitrothiophene – an interesting building-block for thiophene azo dyes – are reported. Detailed spectroscopic investigations (1H‑NMR, 13C-NMR, MS, IR) of the obtained compounds are presented.
Keywords: Gewald reaction, Thiophene synthesis, Cyclization, Condensation
Introduction
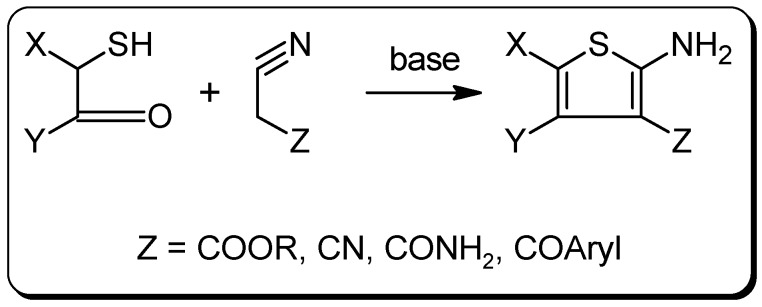
Various substituted and fused 2‑aminothiophenes have attracted considerable attention as structural motifs in numerous pharmaceuticals and dyes, whereof the tranquillizer brotizolam (Lendormin®), containing a thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepine moiety, represents the best-known of such derivatives [1,2,3]. Although several thiophene ring syntheses are known in the literature [1] for the synthesis of thiophen-2-amines, the subsequent introduction of the amino group into an existing thiophene moiety is difficult and thus remains a challenging task. In contrast, during the classical Gewald thiophene synthesis – the reaction of an activated nitrile with an α‑mercaptoaldehyde or with an α‑mercaptoketone – an amino function is introduced right at the 2 position and therefore this reaction proves to be second to none for the construction of an 2-aminothiophene moiety (Scheme 1) [4].
Scheme 1.
The classical Gewald thiophene synthesis.
Activated nitriles used so far in the Gewald reaction are cyanoacetic acid esters, malonodinitrile, cyanoacetohydrazides and benzoylacetonitrile, which lead to the corresponding 3‑alkoxycarbonyl-, 3‑cyano-, 3‑carbamoyl-, and 3‑benzoylthiophen-2‑amines, respectively [5]. To the best of our knowledge, however, the use of cyanoacetone for this purpose has not been reported yet. In this regard we now report an extension to the reaction's scope for the synthesis of 3-acetyl-2‑aminothiophenes that are foreseen to be interesting building blocks.
Results and Discussion
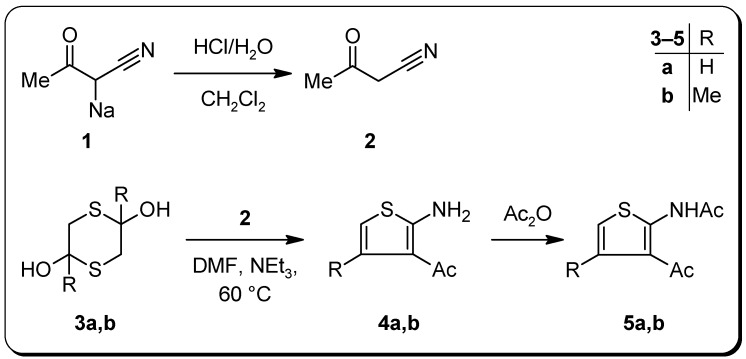
In a modified Gewald reaction the labile cyanoacetone (2) – easily accessible from its commercially available sodium salt 1 – was cyclized with the appropriate α-mercaptoaldehyde dimers 3 using triethylamine in DMF at 60 °C to afford the novel 3‑acetyl-2 aminothiophenes 4, moderately stable at room temperature. Subsequent heating with acetic anhydride gave the stable acetamides 5 (Scheme 2).
Scheme 2.
Synthesis of the title compounds 4 and the corresponding acetamides 5.
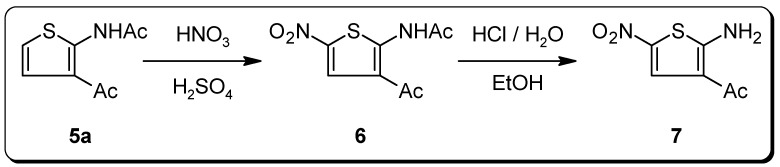
One potential practical application of acetamides 5 was realized in the synthesis of the azo-dye building-block 7. It is well-known that related 3‑alkoxycarbonyl and 3‑cyanothiophenyl-2-amines containing an unsubstituted 5 position in the thiophene ring are labile compounds which undergo unwanted dimerization upon attempted diazotization [6]. However, this problem can be circumvented by 'blocking' this activated 5-position with a nitro group [7]. According to this strategy, compound 5a was transformed into the corresponding 5-nitro derivative 6, which after acidic hydrolysis gave the nitrothiophenamine 7. This deeply yellow colored compound has already been used in azo-couplings but neither its synthesis nor any analytical data have been reported so far [8].
Scheme 3.
Preparation of the thiophene azo-dye building block 7.
Conclusions
A variation of the Gewald thiophene synthesis was developed to yield novel 3‑acetyl-2‑aminothiophenes. Nevertheless, the possibility of adapting this new procedure to other 3‑oxoalkanenitriles and other 1,4‑dithianes of type 3 that are not yet commercially available requires further investigation.
Experimental Section
General
Melting points were determined on a Reichert–Kofler hot-stage microscope and are uncorrected. Mass spectra were obtained on a Shimadzu QP 1000 instrument (EI, 70 eV). IR spectra were recorded on a Perkin-Elmer FTIR 1605 spectrophotometer. Elemental analyses were performed at the Microanalytical Laboratory at the University of Vienna. 1H- and 13C-NMR spectra were recorded on a Varian UnityPlus 300 spectrometer (299.95 MHz for 1H, 75.43 MHz for 13C) at 28 °C. The centre of the solvent signal was used as an internal standard which was related to TMS with δ = 7.26 ppm (1H in CDCl3), δ = 2.49 ppm (1H in DMSO-d6), δ = 77.0 ppm (13C in CDCl3), and δ = 39.5 ppm (13C in DMSO-d6). All reagents and solvents were purchased from Sigma-Aldrich, except compounds 1, 3a, and 3b, which were obtained from Merck, Fluka, and Oakwood Products, respectively.
3-Oxobutanenitrile (2) [9]
To a solution of cyanoacetone sodium salt (1, 5.25 g, 50 mmol) in water (150 mL) was added CH2Cl2 (100 mL). Whilst stirring, the mixture was adjusted to pH 1 with conc. HCl. The organic layer was separated, the aqueous layer was extracted once again with CH2Cl2 (100 mL) and the combined organic layers were dried over anhydrous Na2SO4. While keeping the bath temperature below 30 °C, the solvent was removed under reduced pressure to afford 2 (3.60 g, 87%) as a colorless and unstable oil (pure according to 1H-NMR), which was used immediately and without further purification in the next reaction step. 1H-NMR [10] (CDCl3): δ = 2.34 (s, 3H, CH3), 3.48 (s, 2H, CH2); 13C-NMR (CDCl3): δ = 29.2 (CH3, 1J = 128.9 Hz), 32.7 (CH2, 1J = 128.9 Hz, 2J(CH2,CH3) = 1.8 Hz), 113.7 (CN, 2J(CN,CH2) = 9.7 Hz), 195.0 (CO, 2J(CO,CH2) = 6.4 Hz, 2J(CO,CH3) = 6.4 Hz).
1-(2-Amino-3-thienyl)ethanone (4a)
Triethylamine (1 g, 10 mmol) was added with stirring to a solution of crude 2 (3.60 g, 43 mmol) and dithiane 3a (3.35 g, 22 mmol) in DMF (10 mL), whereupon the temperature of the solution rose slightly. After 15 min, the solution was heated to 60 °C for 3 h. Then, the solvent was removed under reduced pressure and H2O (50 mL), diethyl ether (50 mL), and glacial acetic acid (ca. 1–3 mL) were added to the oily residue until the organic layer became clear. After separation of the ethereal layer and further extraction of the aqueous layer with diethyl ether (50 mL), the combined organic layers were washed subsequently with 5% aqueous NaHCO3 and H2O, dried over anhydrous Na2SO4 and evaporated under reduced pressure to afford 4a (3.98 g, 65%) as a yellow, slowly darkening oil, which was pure according to TLC and 1H-NMR. B.p.: 148 °C (7 mbar); IR (KBr): 3382, 3283, 1616, 1589 cm-1; 1H-NMR (CDCl3): δ = 2.38 (s, 1H, CH3), 6.12 (d, 3J(H5,H4) = 5.8 Hz, 1H, H5), 6.84 (s, 2H, NH2), 6.91 (d, 3J(H5,H4) = 5.8 Hz, 1H, H4); 13C-NMR (CDCl3): δ = 28.1 (CH3, 1J = 127.1 Hz), 106.3 (C5, 1J = 190.5 Hz, 2J(C5,H4) = 5.6 Hz), 115.8 (C3, 3J(C3,H5) = 8.6 Hz, 3J(C3,H4) = 6.0 Hz, 3J(C3,CH3) = 1.4 Hz), 125.9 (C4, 1J = 166.6 Hz, 2J(C4,H5) = 3.9 Hz), 164.2 (C2, 3J(C2,H4) = 10.2 Hz, 3J(C2,H5) = 6.5 Hz), 193.8 (CO, 2J(CO,CH3) = 5.8 Hz); MS m/z (%) = 141 (M+, 66), 126 (100), 98 (17); HRMS m/z calcd. for C6H7NOS: 141.0248. Found: 141.0251.
1-(2-Amino-4-methyl-3-thienyl)ethanone (4b)
Triethylamine (1 g, 10 mmol) was added with stirring to a solution of crude 2 (2.08 g, 25 mmol) and dithiane 3b (2.25 g, 12.5 mmol) in DMF (10 mL) and the mixture was heated to 60 °C for 5 h. Then, the solvent was removed under reduced pressure and the semi-solid residue was recrystallized from cyclohexane–CH2Cl2 to afford 1.63 g (41%) of 4b as yellowish to brownish crystals. M.p.: 145–148 °C; IR (KBr): 3332, 3228, 3127, 1602, 1575 cm–1; 1H-NMR (CDCl3): δ = 2.34 (d, 4J(CH3,H5) = 1.2 Hz, 3H, CH3), 2.44 (s, 3H, COCH3), 5.97 (q, 4J(H5,CH3) = 1.2 Hz, 1H, H5), 7.00 (s, 2H, NH2); 13C‑NMR (CDCl3): δ = 19.6 (CH3, 1J = 127.8 Hz, 3J(CH3,H5) = 4.0 Hz), 30.6 (COCH3, 1J = 127.2 Hz), 103.2 (C5, 1J = 187.7 Hz, 3J(C5,CH3) = 7.1 Hz), 116.0 (C3), 134.7 (C4, 2J(C4,CH3) = 6.3 Hz, 2J(C4,H5) = 3.4 Hz), 166.3 (C2, 3J(C2,H5) = 6.8 Hz), 194.5 (CO, 2J(CO,CH3) = 5.8 Hz); MS m/z (%) = 155 (M+, 84), 140 (100); Anal. calcd. for C7H9NOS ∙ 0.1 H2O: C, 53.54; H, 5.91; N, 8.92. Found: C, 53.52; H, 5.57; N, 8.67.
N-(3-Acetyl-2-thienyl)acetamide (5a)
Thiophenamine 4a (1.41 g, 10 mmol) and excess acetic anhydride (5 mL) were refluxed for 15 min. Then, H2O (ca. 10 mL) was added and the mixture was heated again for a further 5 min. Upon cooling overnight, the product crystallized as slightly yellowish needles. Concentration of the mother liquor gave additional crystals. In total 1.74 g (95%) of 5a were obtained. M.p.: 92–94 °C; IR (KBr): 1683, 1635 cm–1; 1H-NMR (CDCl3): δ = 2.29 (s, 3H, NCOCH3), 2.52 (s, 3H, COCH3), 6.72 (d, 3J(H5,H4) = 5.8 Hz, 1H, H5), 7.18 (d, 3J(H4,H5) = 5.8 Hz, 1H, H4), 11.88 (s, 1H, NH); 13C-NMR (CDCl3): δ = 23.5 (NCOCH3, 1J = 129.0 Hz), 28.7 (COCH3, 1J = 127.7 Hz), 116.0 (C5, 1J = 187.0 Hz, 2J(C5,H4) = 5.0 Hz), 120.8 (C3), 124.1 (C4, 1J = 168.3 Hz, 2J(C4,H5) = 3.6 Hz), 149.4 (C2), 167.7 (NCO, 2J(NCO,NH) = 6.4 Hz, 2J(NCO,NCOCH3) = 4.6 Hz), 195.8 (CO, 2J(CO,COCH3) = 5.8 Hz); MS m/z (%) = 183 (M+, 18), 141 (68), 126 (100); Anal. calcd. for C8H9NO2S: C, 52.44; H, 4.95; N, 7.64. Found: C, 52.37; H, 4.74; N, 7.54.
N-(3-Acetyl-4-methyl-2-thienyl)acetamide (5b)
Applying a procedure similar to that used for the synthesis of acetamide 5a, 0.91 g (92%) of 5b were obtained as almost colorless needles starting from 4b (0.78 g, 5 mmol) and excess acetic anhydride (3 mL). M.p.: 128–132 °C; IR (KBr): 1676, 1619 cm–1; 1H-NMR (CDCl3): δ = 2.26 (s, 3H, NCOCH3), 2.45 (d, 3J(CH3,H5) = 1.2 Hz, 3H, CH3), 2.54 (s, 3H, COCH3), 6.38 (q, 3J(H5,CH3) = 1.2 Hz, 1H, H5), 12.32 (s, 1H, NH); 13C-NMR (CDCl3): δ = 19.1 (CH3, 1J = 128.0 Hz, 3J(CH3,H5) = 4.0 Hz), 23.8 (NCOCH3, 1J = 128.9 Hz), 31.3 (COCH3, 1J = 127.9 Hz), 113.9 (C5, 1J = 184.2 Hz, 3J(C5,CH3) = 6.6 Hz), 120.8 (C3), 133.2 (C4, 2J(C4,CH3) = 6.2 Hz, 2J(C4,H5) = 3.2 Hz), 151.7 (C2), 167.9 (NCO), 196.9 (CO); MS m/z (%) = 197 (M+, 32), 155 (66), 140 (100), 43 (71); Anal. calcd. for C9H11NO2S: C, 54.80; H, 5.62; N, 7.10. Found: C, 54.52; H, 5.36; N, 7.19.
N-(3-Acetyl-5-nitro-2-thienyl)acetamide (6)
Acetamide 5b (1.37 g, 7.5 mmol) was added carefully over a period of 30 min at 0 °C to a well stirred mixture of HNO3 (65%, 2.5 mL, d = 1.4 g/mL, 36 mmol) and H2SO4 (98%, 2.5 mL, d = 1.8 g/mL, 46 mmol). After the addition was complete, the deeply colored reaction mixture was stirred for a further 10 min and was then poured cautiously into excess ice-water. The precipitate formed was filtered off, washed with H2O, and dried under reduced pressure to afford 2.07 g (91%) of 6 (pure according to 1H‑NMR). An analytically pure sample was obtained by recrystallization from MeOH–DMF. M.p.: 178–179 °C; IR (KBr): 3182, 3079, 1683, 1655 cm–1; 1H-NMR (DMSO-d6): δ = 2.36 (s, 3H, NCOCH3), 2.57 (s, 3H, COCH3), 8.55 (s, 1H, H4), 11.94 (s, 1H, NH); 13C-NMR (DMSO-d6): δ = 23.1 (NCOCH3, 1J = 129.7 Hz), 28.6 (COCH3, 1J = 128.3 Hz), 119.7 (C3, 2J(C3,H4) = 3.6 Hz, 3J(C3,NCOCH3) = 1.4 Hz), 129.2 (C4, 1J = 177.6 Hz), 139.8 (C2), 151.4 (C5, 2J(C5,H4) = 11.9 Hz), 170.2 (NCO, 2J(NCO,NCOCH3) = 6.7 Hz), 195.6 (CO, 2J(CO,COCH3) = 6.1 Hz, 3J(CO,H4) = 1.8 Hz); MS m/z (%) = 228 (M+, 4), 186 (20), 69 (42), 43 (100); Anal. calcd. for C8H8N2O4S: C, 42.10; H, 3.53; N, 12.27. Found: C, 42.11; H, 3.66; N, 12.16.
1-(2-Amino-5-nitro-3-thienyl)ethanone (7)
A mixture of nitroacetamide 6 (1.14 g, 5 mmol), EtOH (5 mL), and conc. HCl (15 mL) was refluxed overnight (18 h). Removal of EtOH under reduced pressure and refrigeration of the reaction mixture for 48 h produced a dark precipitate. The solid was filtered off, washed with water, and dried under reduced pressure. The residue was treated three times with hot acetone (10 mL) and filtered. The combined acetone phases were concentrated to almost dryness to afford pure 7 (0.66 g, 70%) as a dark yellow powder. M.p.: 222–224 °C; 1H-NMR (DMSO-d6): δ = 2.40 (s, 3H, CH3), 8.28 (s, 1H, H4), 9.11 (s, 2H, NH2); 13C-NMR (DMSO-d6): δ = 27.80 (CH3, 1J = 127.7 Hz), 114.5 (C3, 2J(C3,H4) = 3.8 Hz, 3J(C3,CH3) = 1.5 Hz), 129.7 (C5), 133.4 (C4, 1J = 174.6 Hz), 168.3 (C2, 3J(C2,H4) = 12.1 Hz), 194.2 (CO, 2J(CO,CH3) = 5.8 Hz, 3J(CO,H4) = 1.8 Hz); MS m/z (%) = 186 (M+, 100), 69 (58); Anal. calcd. for C6H6N2O3S: C, 38.70; H, 3.25; N, 15.05. Found: C, 38.95; H, 3.33; N, 14.80.
Footnotes
Sample Availability: Available from the authors.
References
- 1.Schatz J. Product class 10: thiophenes, thiophene 1,1-dioxides, and thiophene 1-oxides. Sci. Synth. 2002;9:287–422. [Chem. Abstr.2002, 139, 230513] [Google Scholar]
- 2.Langley M. S., Clissold S. P. Brotizolam. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy as an hypnotic. Drugs. 1988;35:104–122. doi: 10.2165/00003495-198835020-00002. [DOI] [PubMed] [Google Scholar]
- 3.Sekar N. Thiophene and thiazole based dyestuffs. Colourage. 1994;41:38–42. [Google Scholar]
- 4.Gewald K. Heterocycles from CH-acidic nitriles. VII. 2 Aminothiophene from α-oxo mercaptans and methylene-active nitriles. Chem. Ber. 1965;98:3571–3577. doi: 10.1002/cber.19650981120. [DOI] [Google Scholar]
- 5.Sabnis R. W., Rangnekar D. W., Sonawane N. D. 2 Aminothiophenes by the Gewald reaction. J. Heterocycl. Chem. 1999;36:333–345. [Google Scholar]
- 6.Corral C., Lasso A., Lissavetzky J., Sanchez Alvarez-Insua A., Valdeolmillos A. M. The behavior of vicinal alkyl aminothiophenecarboxylates in the Sandmeyer and Schiemann reactions. Heterocycles. 1985;23:1431–1435. [Google Scholar]
- 7.Hallas G., Towns A. D. Dyes derived from aminothiophenes. Part 4: Synthesis of some nitro-substituted thiophene-based azo disperse dyes. Dyes Pigm. 1997;33:319–336. doi: 10.1016/S0143-7208(96)00052-6. [DOI] [Google Scholar]
- 8.Leaver A. T. Thiophene azo dyes, their mixtures and their use. WO 9704030 Al. Int. Patent Appl. 1997 [Chem. Abstr.1997, 126, 226511]
- 9.Troschütz R., Grün L. Synthese von basisch substituierten 5H-Pyrimido[4,5-c]-2-benzazepinen. Arch. Pharm. (Weinheim, Ger.) 1993;326:857–964. doi: 10.1002/ardp.19933261104. [DOI] [Google Scholar]
- 10.Perez J. D., Yranzo G. I., Wunderlin D. A. Kinetic evidence for the intermediacy of 1-azirines in the gas-phase thermal isomerization of 3H-isoxazoles to α-carbonylacetonitrile derivatives. J. Org. Chem. 1982;47:982–984. [Google Scholar]