Abstract
Although microwave chemistry and its applications have undergone rapid growth over the last decade, the technology is not yet employed routinely in all synthetic laboratories. A significant obstacle to implementation concerns the empirical work required to adapt established conditions into alternatives employing higher temperatures. We now have established predictive tools to translate reaction conditions from conventional heated (ambient pressure) to elevated temperature (ambient or elevated pressure). We have studied 45 reactions (including published literature examples) and made in excess of 200 estimations for specific yield or conversion, with a high degree of accuracy. Linear regression analysis of estimated vs. experimental conversion or yield was 0.90 (first iteration) and 0.98 (second iteration).
Keywords: Chemical Reaction Optimization, Translation of Reaction Conditions and Chemical Reaction Prediction
Introduction
Literature data for more than 2 million organic chemical reactions show that approximately 70% are carried out below 100°C and that the majority of reaction times are 1-4 hours [1]. Traditional methods for organic synthesis mostly involve glass reaction vessels operated at atmospheric pressure or below. Routine use of such equipment for the past one and a half centuries accounts for the bias toward modest temperatures and lengthy times for reactions.
Our work toward the development of cleaner processes for preparative organic chemistry has produced new thermolytic technologies and methods. If reactions can be performed at significantly higher temperatures than normal, considerable savings in time and energy can be realized. With traditional laboratory glassware, however, when a temperature in the order of 200°C is desired, high boiling solvents are employed. This limits the choice of solvents and subsequent removal and recycling can create problems. Continuous and batch microwave reactors developed by our group [2,3], enable the use of low-boiling solvents under pressurized conditions to speed reactions and facilitate product isolation. With these systems, processes that are notoriously sluggish by traditional methods have been performed faster and in higher yield. Elevated temperatures also can be an attractive alternative to the use of aggressive reagents at lower temperatures. Some reactions proceed in the presence of less catalyst, with a milder catalyst or without addition of catalyst.
These and other advances in hardware for synthesis have revolutionised approaches to organic chemistry over the past few years. Chemists accustomed to traditional methods, however, are now asking such questions as “What time do I need to perform, with comparable results, the same reaction at 170°C instead of in five hours at 70°C?” or “I want to do the reaction in five minutes instead of five hours at 80°C, so what temperature do I need?”
Toward that end, it would be beneficial for established conditions from ambient to ca 120°C, to be transposable predictably into alternatives employing higher temperatures, without the need for extensive empirical investigation. Apparently, that concept has not been proposed previously, let alone explored. If achievable, it would facilitate the efficient performance by microwave or indeed, by other heating methods, of innumerable literature reactions reported over the past 150 years.
In that regard, this work had three sequential aims:
1. To develop predictive tools that would allow conditions for a conventionally performed reaction to be translated readily into protocols for the same reaction to be conducted at higher temperature with comparable results
2. To extend the applicability of the protocols developed in 1, to other reactions and
3. To develop predictive methods toward superior conditions for reactions that proceed poorly, modestly or moderately by known protocols.
Results and Discussion
We have gone some way toward achieving these objectives. The technology developed (termed chemical reaction optimization wand; CROW) for estimating optimal reaction conditions operates by a strategy of homing in on the desired conditions, instead of by painstakingly mapping the contours on the reaction surface. For a wide range of conditions estimated by CROW, the number of required confirmatory experiments is typically only one or two, regardless of the reaction. The first estimation produces a set of conditions that, when tested experimentally (the first iteration), usually give a yield or conversion close to that requested. If necessary, experimental results from this coarse-tuning can be used for fine-tuning. The fresh experimental data can be used to obtain a second estimation that also can be tested by experiment (the second iteration). Although rarely required, further iterations can be performed in a similar manner.
Starting with data from only a single experiment (reference data) for the subject reaction (e.g. Reaction A gives 20% yield in 2 hours at 80°C), CROW could estimate the time expected to give any specified yield, between 5 and 95%, for that reaction at the same temperature. Also, and somewhat surprisingly, with remarkable precision CROW could estimate alternative conditions of time (between 0.1 and 105 minutes) and temperature (between 70 and 270°C) that would be expected to return ANY NOMINATED yield or conversion between 5 and 95%.
Starting with the aforementioned set of reference data for Reaction A (20% yield in 2 hours at 80°C), CROW could estimate, for example, that Reaction A could proceed in 80% yield in 34 minutes at 150°C or in 80% yield after 5 minutes at 204°C. For both estimations, all of the variables of time, temperature and yield or conversion differ from those in the reference data set. Such estimations are not produced at random, but are based on parameters for conditions requested and sought by the investigator.
For each specific reaction, one set of experimentally determined reference data for temperature, time, yield or conversion is required, along with the desired reaction temperature or time and yield or conversion. This is a total of three experimentally established and two requested parameters.
CROW will estimate the one remaining unknown, be it the time, temperature, yield or conversion. The investigator may decide to test the conditions experimentally or to seek an alternative estimation by varying one or two of the three desired parameters if those returned by CROW are considered unsuitable.
Precision of the Estimations
We have studied 45 different reactions and made in excess of 200 estimations for specific yields or conversions. Estimations have been tested experimentally, including with replicates or by comparison with results in the open literature. Examples of reactions studied, conditions requested, as well as estimated and experimentally obtained results are presented in Table 1.
Table 1.
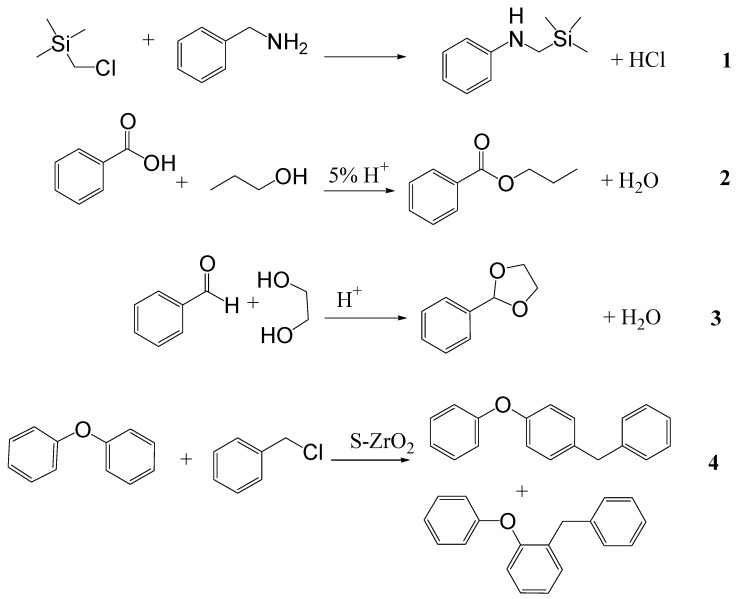
Examples of estimated conditions for specific conversions and the corresponding experimental results. Translation to higher temperature for comparable conversion (Reactions 1-2) [4] and translation to higher conversion at higher temperature (Reactions 3-4) [4,5]. Reactions 1-4 are shown in Figure 3.
| Reaction Number | Reference Conditions | Postulated Conditions | Experimental Conversion | Iteration |
|---|---|---|---|---|
| 1 | 100°C, 5 hours, | 170°C, 16.9 minutes | 82 | 1 |
| 82% conversion | 82% conversion | |||
| 2 | 97°C, 74 min, | 150°C, 7.7 min, | 86* | 1 |
| 90% conversion | 90% conversion | |||
| *150°C, 7.7 min, 86% conversion, become new reference conditions for the second iteration | ||||
| 2 | 150°C, 7.7 min, | 150°C, 8.8 min, | 89 | 2 |
| 86% conversion | 90 conversion | |||
| 3 | 120°C, 30 min, | 170°C, 24.3 min, | 90 | 1 |
| 32% conversion | 90% conversion | |||
| 4 | 90°C, 9.0 min, | 110°C, 36 min, | 83* | 1 |
| 20% conversion | 90% conversion | |||
| *110°C, 36 min, 83% conversion, become new reference conditions for the second iteration | ||||
| 4 | 110°C, 36 min, | 110°C, 46 min, | 89 | 2 |
| 83% conversion | 90% conversion | |||
For first estimations of all the processes studied (i.e. the coarse-tuning referred to above), the correlation coefficient between the predicted and experimentally obtained yields (i.e. the results from the first iteration) for conversions was 0.90 as shown in Graph 1.
Graph 1.
Correlation of Estimated and Experimental Conversions from the first iteration by CROW
When the experimental yield or conversion differed by more than 5% from that estimated, we usually entered results (as new reference data) obtained for the experimentally obtained parameters based on their first request, into CROW and made a second estimation (i.e. the fine-tuning referred to above). The correlation coefficient between the estimated and experimentally obtained result (the second iteration) was 0.98, as shown in Graph 2. Frequently, there was no need for a second iteration and rarely for subsequent estimations and iterations.
Graph 2.
Relationship between Estimated Conditions by CROW and Experimental Conversions subjected to a second iteration
Applicable Reactions
Optimization of conditions has been carried out with CROW for a broad range of reaction types. Most of the reactions have involved up to four reactants or products in total, although on occasions that number has been higher as has the number of components. Reaction mixtures in the experimental sample set have contained solvents (organic and inorganic) or been solvent-free. They have included homogeneous and heterogeneous systems. Processes have been catalysed by acid, base, metals (in solution or suspension), auto-catalysed and uncatalysed. Transformations studied so far have included esterification, transesterification, amidation, imidation, etherification, cyclisation, rearrangement, addition and elimination of water, hydrolysis of esters and amides, lactonisation, N-methylation, ring cleavage, acetalisation and O-alkylation. Examples that produced the data in Table 1 are presented in Figure 3.
Figure 3.
Conclusions
New technology has been developed to facilitate the estimation of alternative reaction conditions that would afford specific designated yields or conversions. We have successfully modelled >40 independent reactions by transposing reaction conditions to either elevated temperature for a comparable yield or to elevated temperature and increased yield. Analysis of these data gave R2 values of 0.901 (first iteration) and 0.981 (second iteration). The approach has been demonstrated on a variety of chemical processes undertaken within our research laboratories and from literature examples, employing a range of temperatures, times, yields and modes of heating.
Experimental
General.
Translation of known reaction protocols was made from a set of reference reaction conditions with experimentally defined temperature, time, yield or conversion. These conditions were presented to CROW with two desired variables from either temperature, time or conversion (or yield) and the third unknown variable was calculated.
Example:
Reference Reaction: 20% yield, 2 hours, 80°C
Desired Conditions: 80% yield, 5 minutes, requires what temperature??
CROW then calculates the temperature to be 204°C. In the absence of logistical constraints or safety issues, the conditions to attempt the reaction have been determined.
References and Notes
- 1.Search conducted 11/03 using Beilstein CrossFire.
- 2.Cablewski T, Faux A. F, Strauss C. R. J. Org. Chem. 1994;59:3408. [Google Scholar]
- 3.Raner K. D, C. R, Trainor R. W. J. Org. Chem. 1995;60:2456. doi: 10.1021/jo00113a028. [DOI] [Google Scholar]
- 4.Roberts B. A, Strauss C. R. Internal communication.
- 5.Yadav G. D, Sengupta S. Org. Process. Res. Dev. 2002;6:256. doi: 10.1021/op990099y. Figure 8 in this example. [DOI] [Google Scholar]