Abstract
The nitro group of methyl 3-nitropyridine-4-carboxylate (1) has successfully been replaced by fluoride anion via nucleophilic aromatic substitution to give the 3-fluoro-pyridine-4-carboxylate 2.
Keywords: Methyl 3-fluoropyridine-4-carboxylate, methyl 3-nitropyridine-4-carboxylate, fluoride anion, nucleophilic aromatic substitution
1. Introduction
Compounds containing C-F bonds are rarely found in nature, in contrast to the abundant fluoride anions [1,2]. The fluoride ion is extensively hydrated and therefore relatively unreactive as a nucleophile, inhibiting C-F bond formation in nature. However, fluorinated organic compounds play an important role and are widely used in the synthesis of pharmaceuticals and agrochemicals due to their favourable chemical and biological properties.
In general, the introduction of a fluorine atom in a molecule does not result in a considerable change in the size or the shape of the compound. Due to the minimal conformational effects, the fluorinated molecule generally also fits in the active site of the receptor and the fluorine atom is thus a suitable isosteric substitution of hydrogen. The incorporation of fluorine into organic molecules may change the solubility properties and enhance the lipophilicity and thus increase the rate of cell penetration and transport of a drug to an active site. The higher electronegativity of fluorine may also alter the physical and chemical properties of a molecule, and the higher polarizability due to the new C-F bond may give new possibilities for binding to the receptor. The C-F bond is stronger than the original C-H bond and fluorinated compounds are more resistant to metabolic degradation. On this background, the development of new and efficient methods for the preparation of fluoroorganic compounds has got increased attention in recent years.
As a general fact, aromatic substrates are very unreactive towards nucleophilic substitution. However, substitution is accelerated by the presence of electron-withdrawing groups o- or p- to the leaving group. A hetero-N atom in the ring is also activating. Surprisingly, the nitro group, which is not generally lost in aliphatic systems, is a particularly good leaving group in nucleophilic aromatic substitutions [3,4,5].
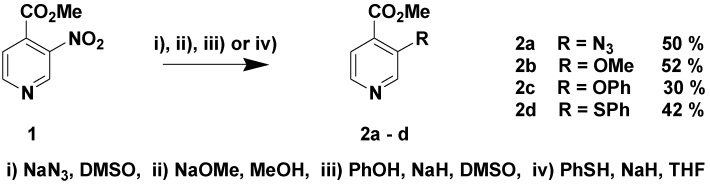
These effects and the synthetic potential for the replacement of a nitropyridine group have been demonstrated by our results for the nucleophilic aromatic substitution of the nitro group in 3-nitro-4-carboxylate (1) since the electron-poor pyridyl aromatic system in combination with the anion stabilizing carbonyl group in the ortho position makes the 3-nitro substituent a good leaving group. We have previously obtained promising results from the nucleophilic aromatic substitution of the nitro group in methyl 3-nitropyridine-4-carboxylate (3-nitroisonicotinic acid methyl ester, 1) with heteroatomic nucleophiles [6], since oxygen, nitrogen and sulphur nucleophiles yielded the new products 2a-d as shown in Scheme 1.
Scheme 1.
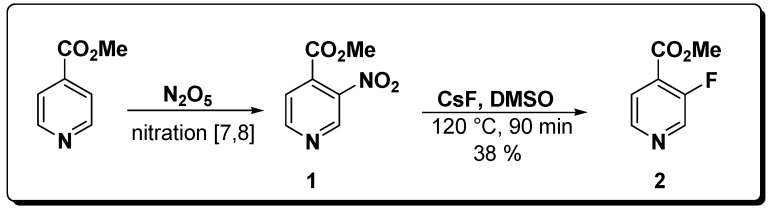
A number of substituted 3-nitropyridines have become readily available through an improved nitration method [7,8] and an investigation of the chemistry of nitropyridines is now in progress in our laboratories. Based on our experience with the good leaving group ability of the nitro group in carboxylate 1, we have now investigated it as a potential substrate for the preparation of the 3-fluoro-pyridine product 2 (see Scheme 2).
Scheme 2.
2. Results and Discussion
We have succeeded in achieving the nucleophilic aromatic substitution of the nitro group in 3-nitro-pyridyl carboxylate 1 with fluoride anion, thus obtaining methyl 3-fluoropyridine-4-carboxylate (2, see Scheme 2) in 38 % yield. 1H-NMR showed complete conversion of the 3-nitro substrate 1 into the 3-fluoro substitution product 2 after 1.5 hours reflux with CsF in dry DMSO. 1H-, 13C-, and 19F-NMR spectra of the product 2 showed the characteristic features of fluorinated aryl compounds. Four signals were observed in 1H-NMR for product 2 and the spectrum had the expected proton coupling patterns and the 3JHF and 4JHF coupling constants were in accordance with the respective JHF observed in the 19F‑ NMR spectrum. 13C-NMR confirmed the fluoropyridine structure, especially based on the most characteristic observation represented by the large C-F coupling constants (1JCF = 268 Hz, 2JCF = 25.3 Hz). The shift values and the coupling patterns in the respective 19F-NMR spectra also supported the proposed structure 2. To our knowledge, the 3-fluoropyridyl compound 2 has not previously been described.
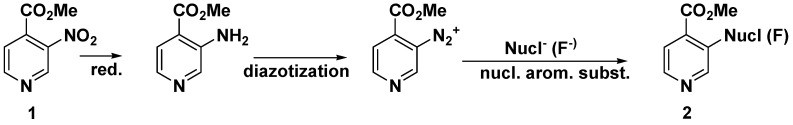
For some purposes this direct nitro substitution may be a more convenient and rapid pathway than the traditional three-step procedure via diazotization as shown in Scheme 3.
Scheme 3.
3. Conclusions
Methyl 3-fluoropyridine-4-carboxylate (2) is readily available from isonicotinic acid methyl ester in two steps. The product 2 was prepared (in 38 % yield) by nucleophilic aromatic substitution of the nitro group in methyl 3-nitro-4-pyridylcarboxylate (1) by fluoride anion after nitration of isonicotinic acid methyl ester. The results confirm [6] the good leaving group ability of the nitro group in methyl 3-nitropyridine-4-carboxylate (1) and demonstrate that the nitropyridine pathway may represent a convenient strategy for the nucleophilic aromatic substitution of pyridines. This one-step nitro group substitution reaction may be a superior alternative to the traditional three-step procedure going via the amine and the standard substitution of a diazonium salt.
4. Experimental
General
Chemicals: CsF (Acros); Solvents: pro analysi quality, distilled and stored under N2 over 4Å molecular sieves. 1H-, 13C- and 19F-NMR: Bruker Avance DPX 300 and 400 MHz spectrometers, chemical shifts are reported in ppm downfield from TMS. Hexafluorobenzene was used as reference for 19F-NMR. J values are given in Hz. MS: Finnigan MAT 95 XL (EI / 70 eV). IR: Nicolet 20SXC FT-IR spectrophotometer. Flash chromatography: Silica (sds, 60 Å, 40-63 μm). Methyl 3-nitro-4-pyridinecarboxylate (1) was prepared from methyl 4-pyridinecarboxylate by nitration according to the literature [7,8].
Methyl 3-fluoropyridine-4-carboxylate (2).
Methyl 3-nitropyridine-4-carboxylate (1, 120 mg, 0,681 mmol) and CsF (517 mg, 3,406 mmol, 5 equivalents) was added to dry DMSO (6 mL) under N2. The reaction mixture was heated at 120 °C for 90 minutes. TLC (4:1 EtOAc/pentane) showed complete conversion to the fluoro substituted product. Distilled water (20 mL) was added to the reaction mixture, which was then extracted with EtOAc (3x20 mL) and concentrated in vacuo. The crude product was purified by flash chromatography (4:1 EtOAc/pentane) yielding 40 mg ( 38 %) of an oily product, pure by 1H- and 13C-NMR. 1H-NMR (400 MHz, CDCl3): δ 3.98 (d, JHF 2.0, 3H, OCH3), 7.77 (ddd, J 0.4, 4.8, 4JHF 6.0, 1H, H-5), 8.55 (dd, J 0.8, 4.8, 1H, H-6), 8.62 (d, 3JHF 2.4, 1H, H-6); 13C-NMR (100 MHz, CDCl3): δ 53.0 (s), 124.4 (d, 3JCF 1.5 C5), 125.2 (d, 2JCF 8.0, C4), 140.4 (d, 3JCF 25.3, C2), 157.1 (d, 1JCF 268.0, C3), 163.3 (d, JCF 3.0, C=O); 19F-NMR (376 MHz, C6D6): δ -125.5 (dd, JHF 2.3, 6.0, F3); IR (film) υmax: 3020w, 2926m, 1744s, 1538m, 1438w, 1352m, 1277m, 758s cm-1; MS: m/z 155 (M+, 24%), 149 (11), 124 (43), 111 (13), 109 (13), 96 (23); Anal. Calcd. for C7H6FNO2: C, 54.20; H, 3.90; N, 9.03. Found: C, 53.96; H, 3.68; N, 8.86.
Footnotes
Sample Availability: Available from the authors.
References
- 1.Wakefield B. Fluorinated Pharmaceuticals. Innov. Pharmaceut. Tech. 2000:74–78. [Google Scholar]
- 2.Chambers R. D. Fluorine in Organic Chemistry. Blackwell; Oxford: 2004. [Google Scholar]
- 3.Effenberger F., Koch M., Streicher W. Nucleophilic substitution of nitrite in nitrobenzenes, nitrobiphenyls and nitronaphthalenes. Chem. Ber. 1991;124:163–173. doi: 10.1002/cber.19911240125. [DOI] [Google Scholar]
- 4.Beck J. R. Nucleophilic displacement of aromatic nitro groups. Tetrahedron. 1978;34:2057–2068. doi: 10.1016/0040-4020(78)89004-8. [DOI] [Google Scholar]
- 5.Kuduk S. D., DiPardo R. M., Bock M. G. Tetrabutylammonium Salt Induced Denitration of Nitropyridines: Synthesis of Fluoro-, Hydroxy-, and Methoxypyridines. Org. Lett. 2005;7:577–579. doi: 10.1021/ol047688v. [DOI] [PubMed] [Google Scholar]
- 6.Holt J., Tjosås F., Bakke J., Fiksdahl A. Nucleophilic aromatic substitution of methyl 3-nitropyridine-4-carboxylate. J. Heterocyclic Chem. 2004;41:987–989. [Google Scholar]
- 7.Bakke J. M., Hegbom I., Øvreeide K., Aaby K. Nitration of aromatic and heteroaromatic compounds by dinitrogen pentaoxide. Acta Chem. Scand. 1994;48:1001–1006. [Google Scholar]
- 8.Bakke J. M., Ranes E. A new efficient synthesis of 3-nitropyridine and substituted derivatives. Synthesis. 1997:281–283. doi: 10.1055/s-1997-4463. [DOI] [Google Scholar]