Abstract
A simple, efficient, and general method has been developed for the synthesis of various α-aminophosphonate derivatives 4a-4l by treatment of substituted benzaldehydes and aniline with bis(2-methoxyethyl)- or bis(2-ethoxyethyl) phosphite under microwave irradiation without solvents and catalysts. The products were characterized by elemental analysis, IR, 1H-NMR, 13C- and 31P-NMR spectra. The X-ray crystallographic data of the representative compound 4l was determined. The new α-aminophosphonate derivatives were found to possess moderate to good antiviral activity.
Keywords: Aminophosphonates, microwave irradiation, synthesis, antiviral activity
Introduction
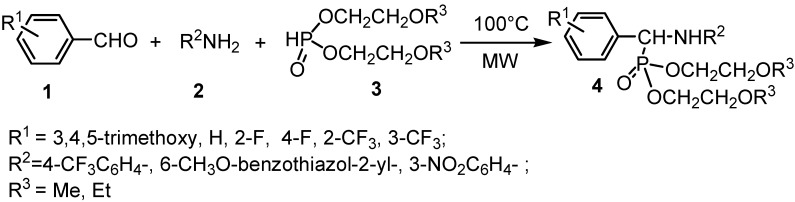
α-Aminophosphonate derivatives have attracted much attention in medicinal and pesticide chemistry over the past two decades due to their biological activities. Some of them have been used as potent enzyme inhibitors [1], antimicrobial [2], antitumor [3] and antiviral agents [4]. In our search for new potential plant antiviral agents, we have reported the design and synthesis of a series of α-aminophosphonate compounds, among which some were found to possess moderate to good bioactivity [5,6]. Recently, attention has been focused on their potential as antiviral agents after the discovery of the activity of compounds containing the methoxyethyl moiety [7]. We now report the design and synthesis of a series of novel α-aminophosphonate compounds containing alkoxyethyl moieties and the investigation of their bioactivity. The synthetic route is shown in Scheme 1. The structures of compounds 4a-4l were firmly established by IR, 1H-, 13C- and 31P-NMR and elemental analysis. The X-ray crystallographic data of compound 4l is also provided. Preliminary bioassay tests showed that some of these compounds displayed in vivo antiviral activity against Tobacco Mosaic Virus (TMV) at 500 μg/mL.
Scheme 1.
Synthetic route to the title compounds.
Results and Discussion
In order to optimize the reaction conditions, the synthesis of 4a was carried out under a variety of conditions. First, it was determined that microwave irradiation should be used, as the reactions without microwave activation were much slower and, for example, the product was obtained in a yield of only 64.9 %, after 4h of conventional heating as compared with the yield of 83.5% obtained after 10 min. under microwave irradiation (Table 1, entries 2, 11). In addition, we also examined the effects of reaction time on the reactions. When the reaction times were increased from 5 min. to 10 min., 15 min. and 20 min., 4a was obtained in 78.9, 83.5, 82.7 and 81.8 % yield, respectively (Table 1, entries 1-4). When the reaction time was prolonged further to 25 min. (entry 5), no additional improvement was noted (82.8 % yield) as compared to that obtained after 10 min. (83.5 % yield, entry 2).The effect of reaction temperature was also studied. It could be seen that the yields were relatively lower when the reactions were carried out at 60 or 80 °C (Table 1, entries 6, 7) than at 100 °C (entry 2). A lower yield was also observed when the reaction system was heated to 120 °C, and no yield was observed when the reaction system was heated to 140 °C (Table 1, entries 9, 10). Consequently, it is better for the reaction to be carried out at 100 °C than at lower or higher temperatures.
Table 1.
Different reaction conditions used for the microwave irradiation synthesis of 4a.
| Entry | Reaction time / min | Power (W) | Reaction temperature / °C | Yield c / % |
|---|---|---|---|---|
| 1 a | 5 | 50 | 100 | 78.9 |
| 2 a | 10 | 50 | 100 | 83.5 |
| 3 a | 15 | 50 | 100 | 82.7 |
| 4 a | 20 | 50 | 100 | 81.8 |
| 5 a | 25 | 50 | 100 | 82.8 |
| 6 a | 10 | 50 | 60 | 5.0 |
| 7 a | 10 | 50 | 80 | 51.2 |
| 8 b | 10 | - | 100 | 0 |
| 9 a | 10 | 50 | 120 | 65.7 |
| 10 a | 10 | 50 | 140 | 0 |
| 11 b | 240 | - | 100 | 64.9 |
a Reaction conditions: stirred under microwave irradiation in a DiscoveryTM LabMate instrument at 50W; b stirred with no microwave irradiation; c Yields of isolated products.
Using the optimized conditions, the best results were obtained when bis(2-methoxyethyl) phosphite or bis(2-ethoxyethyl) phosphite were treated with 1 equiv. of substituted benzaldehyde and 1 equiv. of substituted aniline under microwave conditions without solvent or catalyst at 100 °C for 10 min. Under these conditions, the Mannich reactions proceeded smoothly, and the results are summarized in Table 2.
Table 2.
Yields of the title compounds 4 a.
| Compd. | R1- | R2- | R3- | Yields /% b |
|---|---|---|---|---|
| 4a | 2-F | 4-CF3C6H5 | CH3 | 83.5 |
| 4b | H | 4-CF3C6H5 | CH3 | 81.3 |
| 4c | 2-F | 6-methoxybenzothiazole-2-yl | CH3 | 76.5 |
| 4d | 2-F | 3-NO2C6H5 | CH3 | 79.8 |
| 4e | 3,4,5-trimethoxy | 4-CF3C6H5 | CH3 | 70.6 |
| 4f | 4-F | 4-CF3C6H5 | CH3 | 73.6 |
| 4g | 2-CF3 | 4-CF3C6H5 | CH3 | 56.8 |
| 4h | 3-CF3 | 4-CF3C6H5 | CH3 | 79.2 |
| 4i | 3,4,5-trimethoxy | 4-CF3C6H5 | C2H5 | 78.6 |
| 4j | 2-F | 4-CF3C6H5 | C2H5 | 81.6 |
| 4k | H | 4-CF3C6H5 | C2H5 | 82.8 |
| 4l | 2-F | 3-NO2C6H5 | C2H5 | 80.5 |
a Reaction conditions: stirred under microwave irradiation in a DiscoveryTM LabMate instrument at 50W; b Yields of isolated products.
The structures of title compounds 4a-4l were established on the basis of their spectroscopic data. They showed IR absorption bands at 3200-3500 (NH) and 1520-1616 cm-1(C=C) (aromatic ring skeletal vibration), while the absorption at 1220~1250 cm-1 was assigned to the P=O stretching absorption bands and that at 990~1100 cm-1 to the C-O stretching absorption bands in the P-O-C group. In the 1H-NMR all phenyl protons showed multiplets at 6.19-7.78 ppm. The chemical shifts of the ester PCH were about 4.85-6.17 ppm, respectively.
Antifungal activity bioassay
The antifungal bioassay results are given in Table 3. It can be seen that the newly synthesized alkoxyethyl α-aminophosphonate derivatives 4a-4l exhibit weak antifungal activities at 500 μg/mL towards Fusarium oxysporum, Valsa mali and Gibberella zeae, which were obviously lower than that of a hymexazol standard.
Table 3.
Inhibitory effects on phytopathogenic fungi.
| Compd (500 μg/mL) | Fusarium oxysporum | Gibberella zeae | Valsa mali |
|---|---|---|---|
| 4a | 51.2 | 42.8 | 50.3 |
| 4b | 36.7 | 30.2 | 33.5 |
| 4c | 8.4 | 11.8 | 8.9 |
| 4d | 12.4 | 17.9 | 15.5 |
| 4e | 6.1 | 10.7 | 5.7 |
| 4f | 2.1 | 6.9 | 3.2 |
| 4g | 0 | 0 | 0 |
| 4h | 12.1 | 23.1 | 11.0 |
| 4i | 10.0 | 0 | 2.0 |
| 4j | 23.1 | 9.0 | 30.9 |
| 4k | 33.0 | 2.9 | 40.1 |
| 4l | 20.0 | 1.0 | 43.4 |
| Hymexazol | 100 | 90.0 | 82.3 |
Antiviral activity bioassay
The results of the in vivo bioassay against Tobacco Mosaic Virus (TMV) are given in Table 4. Ningnanmycin was used as the reference antiviral agent. The results indicated that the antiviral activity depended on the substituents present. When R1 is H, R2 is a 4-trifluoromethyl group and R3 is CH3, (4b) the compound showed a 56.5 % curative rate against TMV at 500μg/mL, slightly higher than that of reference (53.8 %). The inhibitory rates of compounds 4i, 4d, 4e, 4c and 4g at the same 500μg/mL concentration were 53.1 %, 51.6 %, 51.3 %, 49.2 % and 46.0%, respectively. These compounds all have an antiviral activity slightly lower than that of the reference compound. The other compounds 4a, 4k, 4f, 4h, 4j and 4l exhibited a weak anti-TMV bioactivity, with inhibitory rates of 38.8%, 34.3 %, 34.2%, 25.0 %, 16.2 % and 13.8 %, respectively, at 500μg/mL.
Table 4.
The curative effects of the new compounds 4a-4l against TMV in vivo*.
| Compound | Ningnanmycin | 4a | 4b | 4c | 4d | 4e | 4f |
|---|---|---|---|---|---|---|---|
| Inhibition rate (%) | 53.8 | 38.8 | 56.5 | 49.2 | 51.6 | 51.3 | 34.2 |
| Compound | 4g | 4h | 4i | 4j | 4k | 4l | |
| Inhibition rate (%) | 46.0 | 25.0 | 53.1 | 16.2 | 34.3 | 13.8 |
* Concentration: 500 mg/L.
Crystal Structure Analysis
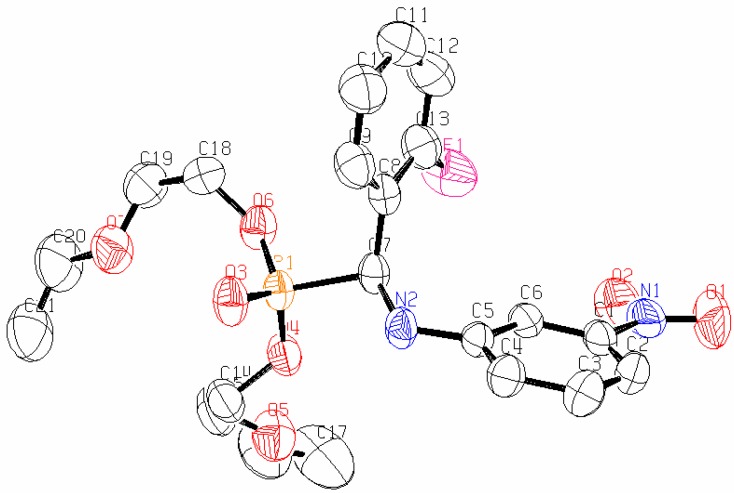
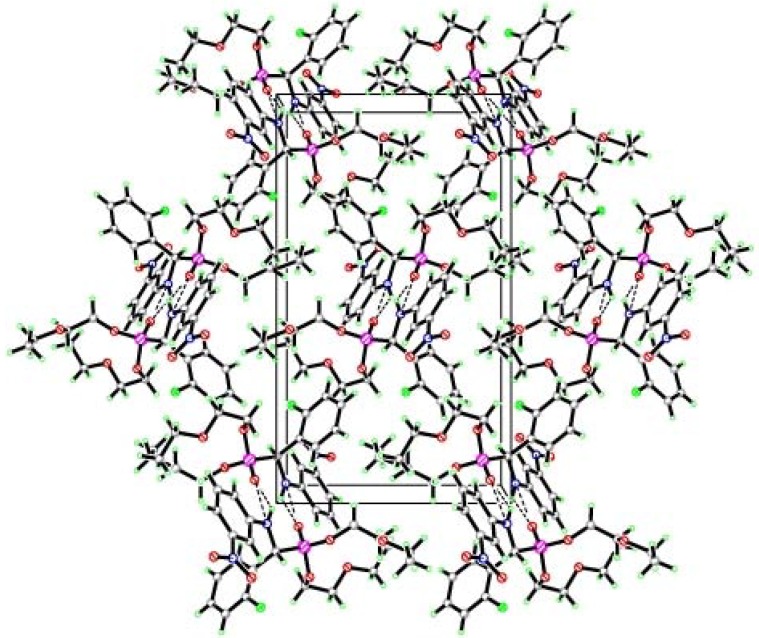
As it can be seen from the X-ray single crystal analysis of 4l (Figure 1), the dihedral angle between the C(8)-C(7)-N(2)-C(5) plane is -72.3(2)°. The N-H···O type of intermolecular interactions plays a major role in stabilizing the molecules in the unit cell. As shown in the packing diagram of this compound (Figure 2), there is a N(2)-H(2)···O(3), intermolecular hydrogen bond interaction in which N(2)-H(2) = 0.860 Å, H(2)..O(2) = 2.1866, N(2))···O(3) = 2.892(3) Å, N(2)-H(2)···O(3) = 139.81°, -x, -y+1/2, -z+1/2). In the solid state, the above hydrogen bonds connecting the molecules form hydrogen bond networks which stabilize the crystal structure.
Figure 1.
The molecular structure of compound 4l.
Figure 2.
Packing diagram of the unit cell of compound 4l.
Conclusions
A new method is presented for the formation under microwave irradiation conditions of α-aminophosphonates containing alkoxyethyl moieties. The method offers several advantages: an easy, rapid, one-pot reaction, environmental-friendliness and good yields. It was also found that the title compounds 4b, 4i, 4d and 4e displayed good antiviral activity.
Experimental
General
The melting points of the products were determined on a XT-4 binocular microscope (Beijing Tech Instrument Co., P.R. China) and are uncorrected. IR spectra (KBr disks) were recorded on a Bruker Vector 22 spectrometer. 1H- and 13C-NMR spectra (solvent CD3COCD3) were recorded at room temperature on a JEOL-ECX 500 NMR spectrometer operating at 500 and 125 MHz, respectively, using TMS as internal standard. 31P-NMR spectra were measured with 85% H3PO4 as external reference.. The reference sample was prepared by sealing a capillary containing 85% H3PO4 in a 5 mm NMR tube inside which a suitable amount of CDCl3 was added for field locking. Elemental analysis was performed on an Elementar Vario-III CHN analyzer. Microwave reactions were performed on a DiscoveryTM LabMate Focused Microwave Synthesizer (50 W power). Analytical TLC was performed on silica gel GF254. All reagents were analytical reagent grade or chemically pure. Solvents were dried, deoxygenated and redistilled before use. Dialkyoxyethyl phosphites were synthesized according to the literature method [8].
Preparation of Alkyloxyethyl-containing α-Aminophosphonates 4a-4l
Substituted benzaldehyde (5 mmol) and aniline (5 mmol) and di(2-methoxyethyl) phosphite or di(2-ethoxyethyl) phosphite were placed in a microwave tube, which was the sealed and placed in the Discovery TM synthesizer and irradiated at 100 °C and 50 W for 10 min. Completion of the reaction was checked by TLC. The reaction mixture was cooled and the crude product was recrystallized from 95% ethanol to give title compounds 4a-4l.
N-(4-trifluoromethylphenyl)-α-amino-α-(2-fluorophenyl)-O,O-di(2-methoxyethyl)phosphonate (4a). White crystals; yield 83.5%; m.p. 67~68 °C; IR: 3273.2, 1537.3, 1238.3, 1064.7 cm-1; 1H-NMR δ: 3.07 (s,1H, N-H), 3.28 (s, 6H, 2CH3O), 3.44~4.27(m, 8H, 4CH2), 5.37 (d, J= 25.2 Hz, 1H, CH), 6.89~7.68(m, 8H, Ar-H); 13C-NMR δ: 47.74, 49.01, 58.69, 58.72, 66.67, 66.73, 72.06, 75.15, 113.68, 115.90, 116.06, 125.41, 127.16, 127.20, 130.24, 150.74, 150.86, 160.73, 162.73; 31P-NMR δ: 22.19, 22.21; Anal. Calcd for C20H24F4NO5P: C, 51.62; H, 5.20; N, 3.01. Found: C, 51.92; H, 5.17; N, 3.15.
N-(4-trifluoromethylphenyl)-α-amino-α-phenyl-O,O-di(2-methoxyethyl)phosphonate (4b). White crystals; yield 81.3%; m.p.71~73 °C; IR: 3309.9, 1539.2, 1230.6, 983.7 cm-1; 1H-NMR δ: 3.09 (s, 1H, N-H), 3.27 (s, 6H, 2CH3O), 3.43~4.21 (m, 8H, 4CH2), 5.14 (d, J= 25.2Hz, 1H, CH), 6.91~7.62 (m, 9H, Ar-H); 13C-NMR δ: 54.87, 56.09, 58.69, 66.50, 66.55, 72.13, 72.17, 113.90, 118.89, 126.98, 127.01, 128.58, 129.16, 129.23, 137.09, 151.17, 151.27; 31P NMR δ: 23.01; Anal. Calcd for C20H25F3NO5P: C, 53.69; H, 5.63; N, 3.13. Found: C, 53.40; H, 5.39; N, 3.30.
N-(6-methoxybenzothiazole-2-yl)-α-amino-α-(2-fluorophenyl)-O,O-di(2-methoxyethyl)phosphonate (4c). White crystals; yield 76.5%; m.p. 74~75 °C; IR: 3286.7, 1521.8, 1234.4, 1041.6 cm-1; 1H-NMR δ: 3.08 (s,1H, N-H), 3.26 (s, 6H, 2CH3O), 3.47~3.56 (m, 4H, 2CH2), 3.77 (s, 3H, CH3O), 4.04~4.29 (m, 4H, 2CH2), 6.12 (d, J= 22.4 Hz, 1H, CH), 6.85~7.75 (m, 7H, Ar-H); 13C-NMR δ: 48.46, 49.70, 56.00, 58.75, 66.77, 66.83, 72.04, 72.09, 105.98, 114.12, 115.93, 116.12, 120.28, 125.24, 130.34 , 130.71, 133.14, 147.03, 156.35, 160.34, 162.25, 164.40, 164.48; 31P-NMR δ: 20.67, 20.69; Anal. Calcd for C21H26FN2O6PS: C, 52.06; H, 5.41; N, 5.78. Found: C, 52.09; H, 5.29; N, 5.99.
N-(3-nitrophenyl)-α-amino-α-(2-fluorophenyl)-O,O-di(2-methoxyethyl)phosphonate (4d). Yellow crystals; yield 79.8%; m.p. 108~109 °C; IR: 3211.5, 1544.1, 1222.9, 1055.0 cm-1; 1H-NMR δ: 3.07(s, 1H, N-H), 3.27 (s, 6H, 2CH3O), 3.45~4.31 (m, 8H, 4CH2), 5.42 (d, J = 25.2 Hz, 1H, CH), 7.17~7.73 (m, 8H, Ar-H); 13C-NMR δ: 47.89, 49.17, 58.74, 58.77, 66.85, 66.88, 72.12, 72.18, 108.18, 112.95, 115.97, 116.15, 120.08, 125.50, 130.32, 130.83, 130.90, 148.91, 149.02, 150.16, 160.83, 162.78, 162.84; 31P-NMR δ: 21.90, 21.92; Anal. Calcd for C19H24FN2O7P: C, 51.40; H, 5.81; N, 2.61. Found: C, 51.50; H, 5.54; N, 2.85.
N-(4-trifluorophenyl)-α-amino-α-(3, 4, 5-trimethoxyphenyl)-O,O-di(2-methoxyethyl)phosphonate (4e). White crystals; yield 70.6 %; m.p. 104~106 °C; IR: 3311.8, 1537.3, 1221.7, 1043.4 cm-1; 1H-NMR δ: 3.08 (s,1H, N-H), 3.28 (s, 6H, 2CH3O), 3.46~3.56 (m, 4H, 2CH2), 3.70 (s, 9H, 3CH3O), 3.96~4.22 (m, 4H, 2CH2), 5.09 (d, J = 24.6 Hz, 1H, CH), 6.95~7.39 (m, 6H, Ar-H); 13C-NMR δ: 56.40, 58.72, 60.49, 66.53, 66.59, 72.22, 72.27, 106.61, 106.65, 113.98, 118.97, 119.22, 125.06, 127.01, 127.21, 132.46, 138.65, 151.31, 151.42, 154.26; 31P-NMR δ: 22.89; Anal. Calcd for C23H31F3NO8P: C, 51.50; H, 5.54; N, 2.61. Found: C, 51.50; H, 5.54; N, 2.85.
N-(4-trifluoromethylphenyl)-α-amino-α-(4-fluorophenyl)-O,O-di(2-methoxyethyl)phosphonate (4f). Colorless crystals; yield 73.6%; m.p. 45~47 °C. IR: 3298.3, 1606.7, 1238.3, 1064.7 cm-1; 1H NMR δ: 3.00 (s, 1H, N-H), 3.33 (s, 6H, 2CH3O), 3.48~3.56 (m, 4H, 2CH2), 4.02~4.19 (m, 4H, 2CH2), 4.87 (d, J = 32.0Hz, 1H, CH), 6.57~7.46 (m, 8H, Ar-H); 13C-NMR δ: 54.41, 55.62, 58.78, 58.83, 66.01, 66.04, 71.39, 71.43, 112.97, 115.65, 115.81, 126.51, 126.54, 129.43, 129.47, 129.54, 148.94, 149.05, 161.55, 163.54; 31P-NMR δ: 22.60, 22.63; Anal. Calcd for C20H24F4NO5P: C, 51.62; H, 5.20; N, 3.01. Found: C, 51.65; H, 5.26; N, 3.13.
N-(4-trifluoromethylphenyl)-α-amino-α-(2-trifluoromethylphenyl)-O,O-di (2-methoxyethyl)phos-phonate (4g). White crystals; yield 56.8%; m.p. 47~48 °C; IR: 3319.5, 1616.3, 1323.2, 1060.9 cm-1; 1H-NMR δ: 3.06 (s, 1H, N-H), 3.27 (s, 6H, 2CH3O), 3.53~3.55 (m, 8H, 4CH2), 5.37 (d, J = 24.05 Hz, 1H, CH), 6.68~7.78 (m, 8H, Ar-H); 13C-NMR δ: 50.72, 51.93, 58.83, 58.94, 66.23, 66.26, 113.00, 126.51, 126.56, 126.64, 126.67, 128.34, 128.98, 129.00, 132.51, 148.49, 148.60; 31P-NMR δ: 22.06; Anal. Calcd for C21H24F6NO5P: C, 48.94; H, 4.69; N, 2.72. Found: C, 48.95; H, 4.80; N, 2.71.
N-(4-trifluoromethylphenyl)-α-amino-α-(3-trifluoromethylphenyl)-O,O-di(2-methoxyethyl) phos-phonate (4h). White crystals; yield 79.2%; m.p. 46~48 °C. IR: 3296.4, 1614.4, 1327.0, 1064.7 cm-1; 1H-NMR δ: 3.09 (s, 1H, N-H), 3.34 (s, 6H, 2CH3O), 3.47~4.20 (m, 8H, 4CH2), 4.96 (d, J = 25.2Hz, 1H, CH), 6.58~7.75 (m, 8H, Ar-H); 13C-NMR δ: 54.99, 56.21, 58.83, 58.89, 66.16, 66.21, 66.27, 71.42, 71.46, 112.99, 124.71, 125.06, 126.67, 126.70, 129.26, 129.28, 131.17, 131.21, 148.89, 14901; 31P- NMR δ: 21.66; Anal. Calcd for C21H24F6NO5P: C, 48.94; H, 4.69; N, 2.72. Found: C, 48.96; H, 4.65; N, 2.82.
N-(4-trifluorophenyl)-α-amino-α-(3, 4, 5-trimethoxyphenyl)-O,O-di(2-ethoxyethyl)phosphonate (4i). White crystals; yield 78.6%; m.p.103~104 °C; IR: 3309.8, 1614.4, 1332.8, 1060.85 cm-1; 1H-NMR δ: 1.18~1.20 (m, 6H, 2CH3), 3.04 (s, 1H, NH), 3.46~3.52 (m, 8H, 4CH2), 3.82~3.52 (s, 9H, 3CH3O), 3.97~4.21 (4H, 2CH2), 4.78 (d, J = 31.4Hz, 1H, CH), 6.62~7.37 (m, 6H, Ar-H); 13C-NMR δ: 56.23, 60.91, 66.63, 66.65, 69.48, 69.53, 104.76, 104.80, 113.06, 119.94, 120.20, 123.76, 125.90, 126.59, 126.62, 130.85, 137.79, 149.23, 149.35, 153.52; 31P- NMR δ: 22.78; Anal. Calcd for C25H35NO8P: C, 53.55; H, 5.27; N, 2.84. Found: C, 53.29; H, 6.11; N, 2.55.
N-(4-trifluoromethylphenyl)-α-amino-α-(2-fluorophenyl)-O,O-di(2-ethoxyethyl)phosphonate (4j). White crystals; yield 81.6%; m.p. 74~75 °C; IR: 3296.4, 1541.1, 1228.7, 1043.5 cm-1; 1H-NMR δ: 1.16~1.19 (m, 6H, 2CH3O), 3.07 (s, 1H, N-H), 3.47~4.20 (m, 12H, 6CH2O), 5.29 (d, 1H, J=25.2 Hz, CH ), 6.63~7.35(m, 8H, Ar-H); 13C-NMR δ: 47.46, 48.71 66.28, 66.57, 66.60, 77.04, 77.30, 112.75, 115.33, 115.50, 124.64, 126.57, 126.60, 128.81, 148.65, 148.76, 159.74,159.80, 161.71; 31P-NMR δ: 22.06; Anal. Calcd for C22H28F4NO5P: C, 53.10; H, 6.24; N, 2.48. Found: C, 53.38; H, 6.12; N, 2.43.
N-(4-trifluoromethylphenyl)-α-amino-α-(phenyl)-O,O-di(2-ethoxyethyl)phosphonate (4k). White crystals; yield 82.8%; m.p. 59~60 °C; IR: 3298.3 , 1537.3, 1226.7, 1035.8 cm-1; 1H-NMR δ: 1.18~1.21 (m, 6H, 2CH3O), 3.07 (s, 1H, N-H), 3.46~4.18 (m, 12H, 6CH2O), 4.88 (d, 1H, J=25.2 Hz, CH), 6.60~7.34 (m, 8H, Ar-H); 13C-NMR δ: 55.19, 56.39, 66.18, 66.59, 66.64, 76.84, 77.10, 77.36, 113.03, 126.55, 126.58, 127.87, 127.92, 128.22, 128.24, 128.78, 135.31, 149.13, 149.25; 31P-NMR δ: 22.86; Anal. Calcd for C22H29F3NO5P: C, 55.58; H, 6.15; N, 2.95. Found: C, 55.71; H,5.96; N, 3.09.
N-(3-nitrophenyl)-α-amino-α-(2-fluorophenyl)-O,O-di(2-eethoxyethyl)phosphonate (4l). Yellow crystals; yield 80.5 %; m.p. 75~76 °C; IR: 3284.8, 1543.1, 1251.8, 1022.3 cm-1; 1H-NMR δ: 1.16~1.20 (m, 6H, 2CH3O), 3.07 (s, 1H, N-H), 3.49~4.28 (m, 12H, 6CH2O), 5.32 (d, 1H, J=25.2 Hz, CH), 7.10~7.51 (m, 8H, Ar-H); 13C-NMR δ: 47.83, 49.06, 66.41, 66.53, 66.68, 76.87, 77.38, 108.10, 113.02, 115.49, 115.67, 118.88, 124.72, 128.90, 129.91, 129.96, 147.18, 147.29, 149.30, 159.87, 159.93, 161.90; 31P-NMR δ: 21.73; Anal. Calcd for C21H28FN2O7P: C, 53.62; H, 6.00; N, 5.95. Found: C, 53.89; H, 5.86; N, 6.00.
Bioassays: Antifungal Bioassays
The antifungal activity of all synthesized compounds 4a-l was tested against three pathogenic fungi, namely Fusarium oxysporum, Gibberella zeae, and Valsa mali, by the poison plate technique [9]. Test compounds were dissolved in acetone (10 mL) before mixing with Potato Dextrose Agar (PDA, 90 mL). The final concentration of compounds 4a-l in the medium was fixed at 500 μg/mL. Three kinds of fungi were incubated in PDA at 25±1 °C for 5 days to get new mycelium for antifungal assay, then a mycelia disk of approximately 0.45 cm diameter cut from the culture medium was picked up with a sterilized inoculation needle and inoculated in the center of PDA plate. The inoculated plates were incubated at 25±1°C for 5 days. Acetone in sterilized distilled water served as control, while hymexazole was used as positive control For each treatment, three replicates were carried out. The radial growth of the fungal colonies was measured on the sixth day and the data were statistically analyzed. The in vitro inhibiting effects of the test compounds on the fungi were calculated by the formula CV = , where A represents the diameter of fungi growth on untreated PDA, B represents the diameter of fungi on treated PDA, and CV represents the rate of inhibition.
Antiviral Bioassays
Growing leaves on Nicotiana tabacum. L of the same ages were selected. The tobacco mosaic virus (6×10-3 mg/mL) and inoculated on the whole leaves by dipping, then the leaves were washed with water and dried. The compound solution was smeared on the left side and the solvent was smeared on the right side for control. The local lesion numbers were then recorded 3-4 days after inoculation [10]. For each compound, three repetitions were conducted to ensure the reliability of the results.
Crystal structure determination.
For the structure determination of a single crystal of 4l X-ray intensity data were recorded on a Rigaku Raxis-IV diffraction meter using graphite monochromated MoKα radiation (λ = 0.71073 Å). In the range of 2.14 º ≤ θ ≤ 25.01º, 3641 independent reflections were obtained. Intensities were corrected for Lorentz and polarization effects and empirical absorption, and all data were corrected using SADABS [11] program. The structure was solved by direct methods SHELXS-97 program [12]. All the non-hydrogen atoms were refined on F2 anisotropically by full-matrix least squares method. The hydrogen atoms were located from the difference Fourier map, but their position were not refined. The contributions of these hydrogen atoms were included in structure-factor calculations. The final least-square cycle gave wR=0.1254, R=0.0511 for 5779 reflection with I>2σ(I); the weighting scheme, w = 1 / [σ2(Fo2) + ( 0.0603P ) 2 + 0.7083P], where P = [( Fo2) + 2Fc2 ] / 3. The max and min difference peaks and holes are 0.337 and -0.348 e.A-3, respectively. s=1.038. Atomic scattering factors and anomalous dispersion corrections were taken from International Table for X-ray Crystallography [13]. Crystallographic data (excluding structure factors) for the structure have been deposited with the Cambridge Crystallographic Data Center as supplementary publication No. CCDC-616465. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via the website www.ccdc.cam.ac.uk/data_request/cif.
Acknowledgements
This work was financially supported by the National Nature Science Foundation of China (Grant No. 20362004) and the Foundation for New Century Excellent Talent in University of China (Grant No. NCET-04-0912).
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Du S. C., Faiger H., Belakhov V., Timor Baasov T. Towards the Development of Novel Antibiotics: Synthesis and Evaluation of a Mechanism-Based Inhibitor of Kdo8P Synthase. Bioorg. Med. Chem. 1999;7:2671–2682. doi: 10.1016/S0968-0896(99)00233-3. [DOI] [PubMed] [Google Scholar]
- 2.Zomova A. M., Molodykh Zh. V., Kudryavtseva L. A., Teplyakova L. V., Fedorov S. B., Ivanov B. E. Antimicrobial activity of O,O-diethyl N-alkylaminomethylphosphonates and O-ethyl N-alkylaminomethylphosphonic acids. Pharm. Chem. J. 1986;20:774–777. [Google Scholar]
- 3.Jin L. H., Song B. A., Zhang G. P., Xu R. Q., Zhang S. M., Gao X. W., Hu D. Y., Song Yang S. Synthesis, X-ray crystallographic analysis, and antitumor activity of N-(benzothiazole-2-yl)-1-(fluorophenyl)-O,O-dialkyl-α-aminophosphonates. Bioorg. Med. Chem. Lett. 2006;16:1537–1543. doi: 10.1016/j.bmcl.2005.12.041. [DOI] [PubMed] [Google Scholar]
- 4.Kukhar V. P., Hudson H. R. Aminophosphonic and Aminophosphinic Acids-Chemistry and Biological Activity. John Wiley & Sons; Chichester: 2000. [Google Scholar]
- 5.Zhang G. P., Song B. A., Xue W., Jin L. H., Hu D. Y., Wan Q. Q., Lu P., Yang S., Li Q. Z., Liu G. Synthesis and Biological Activities of Novel dialkyl 1-(4-trifluoromethylphenylamino)-1-(4-trifluoromethyl or 3- fluorophenyl)methylphosphonate. J. Fluor. Chem. 2006;127:48–53. [Google Scholar]
- 6.Song B. A., Wu Y. L., Yang S., Hu D. Y., He X. Q., Jin L. H. Synthesis and bioactivity of 2-aminophosphonate containing fluorine. Molecules. 2003;8:186–192. doi: 10.3390/80100186. [DOI] [Google Scholar]
- 7.Zideka Z., Kmoničkovaa E., Holy A. Cytotoxicity of pivoxil esters of antiviral activity nucleoside phosphonates: adefovir dipivoxil versus adefovir. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2005;149:315–319. doi: 10.5507/bp.2005.049. [DOI] [PubMed] [Google Scholar]
- 8.Huang R. Q., Wang H. L., Zhou J. The Preparation of Organic Intermediates. Chemical Industrial Press; Beijing: 2001. [Google Scholar]
- 9.Song S. Q., Zhou L. G., Li D., Tang D., Li J. Q., Jiang W. B. Antifungal activity of five plants from Xinjiang. Nat. Prod. Res. Dev. 2004;16:157–159. [Google Scholar]
- 10.Li S. Z., Wang D. M., Jiao S. M. Pesticide Experiment Methods-Fungicide Sector. Agriculture Press of China; Beijing: 1991. pp. 93–94. [Google Scholar]
- 11.Sheldrick G. M. Program for Empirical Absorption Correction of Area Detector Data. University of Gottingen; Gottingen, Germany: 1996. [Google Scholar]
- 12.Sheldrick G. M. SHELXTL V5.1, Software Reference Manual. Bruker AXS, Inc.; Madison, Wisconsin, USA: 1997. [Google Scholar]
- 13.Wilson A. J. International Table for X-ray Crystallography. vol. C. Kluwer Academic Publishers; Dordrecht: 1992. Tables 6.1.1.4 (pp. 500-502) and 4.2.6.8 (pp. 219-222), respectively. [Google Scholar]