Abstract
Dexmedetomidine (DEX) is a sedative and analgesic agent that acts via the alpha-2 adrenoreceptor and is associated with reduced anesthetic requirements, as well as attenuated blood pressure and heart rate in response to stressful events. A previous study reported that cat gingival blood flow was controlled via sympathetic alpha-adrenergic fibers involved in vasoconstriction. In the present study, experiment 1 focused on the relationship between the effects of DEX on alpha adrenoreceptors and vasoconstriction in the tissues of the oral cavity and compared the palatal mucosal blood flow (PMBF) in rabbits between general anesthesia with sevoflurane and sedation with DEX. We found that the PMBF was decreased by DEX presumably because of the vasoconstriction of oral mucosal vessels following alpha-2 adrenoreceptor stimulation by DEX. To assess if this vasoconstriction would allow decreased use of locally administered epinephrine during DEX infusion, experiment 2 in the present study monitored the serum lidocaine concentration in rabbits to compare the absorption of lidocaine without epinephrine during general anesthesia with sevoflurane and sedation with DEX. The depression of PMBF by DEX did not affect the absorption of lidocaine. We hypothesize that this is because lidocaine dilates the blood vessels, counteracting the effects of DEX. In conclusion, despite decreased palatal blood flow with DEX infusion, local anesthetics with vasoconstrictors should be used in implant and oral surgery even with administered DEX.
Key Words: Dexmedetomidine, Alpha-2 adrenoreceptor, Palatal mucosal blood flow, Lidocaine, Sedation, General anesthesia
Adrenoreceptors are mainly divided into 2 types: alpha-1 adrenoreceptor and alpha-2 adrenoreceptor. The alpha-1 adrenoreceptor mediates sympathetic vasoconstriction of the blood vessels,1 and the alpha-2 adrenoreceptor induces a hypnotic-anesthetic effect in rats via the activation of central alpha-2 adrenoreceptors.2 Alpha-2 adrenoreceptors are divided into 3 subtypes (alpha-2A, alpha-2B, and alpha-2C). Alpha-2A adrenoreceptors exert inhibitory effects on the central sympathetic outflow while alpha-2B adrenoreceptors induce vasoconstriction of the peripheral blood vessels. In contrast, alpha-2C adrenoreceptors exert minimal cardiovascular effects but may be involved in therapies for disorders associated with enhanced startle responses and sensorimotor gating deficits, such as schizophrenia, attention-deficit hyperactivity disorder, posttraumatic stress disorder, and drug withdrawal states.3
Dexmedetomidine (DEX) is a sedative and analgesic agent that acts via the alpha-2 adrenoreceptor and is associated with reduced anesthetic requirements as well as attenuated blood pressure and heart rate in response to stressful events.4–8 DEX has a relatively high ratio of alpha-2 to alpha-1 activity (1620:1) in comparison with clonidine (220:1). Stimulation of alpha-2 adrenoreceptors within the spinal cord modulates pain pathways, thereby providing some degree of analgesia.9–11 In addition, DEX induces a sedative response that exhibits properties similar to natural sleep without significant respiratory depression, unlike other hypnotic anesthetics.12 DEX can therefore be used in sedation for oral implant surgery.13
However, oral surgery can cause significant bleeding because of an abundance of blood vessels in the region, and this can disrupt the surgical procedure. Several interesting reports14–16 have been published regarding vasoconstriction in the oral cavity. Izumi et al14 reported that cat gingival blood flow was controlled by the sympathetic alpha-adrenergic fibers involved in vasoconstriction, and Michael15 found that electrical stimulation of the cervical sympathetic nerve trunk uniformly induced vasoconstriction in tissues of the oral cavity in all species studied. Alpha adrenoreceptors are clearly involved in vascular constriction in the gingiva of cats and rats.
For this reason, we hypothesized that oral mucosal blood flow would be reduced because of the peripheral activation of alpha-2 adrenoreceptors by DEX. Two studies in rabbits were completed. In experiment 1, we focused on the relationship between the effects of intravenous DEX on oral mucosal blood flow in the oral cavity. If DEX was indeed found to reduce the oral mucosal blood flow in experiment 1, we hypothesized that the systemic absorption of lidocaine without epinephrine would be delayed because of peripheral vasoconstriction by DEX. Accordingly, in experiment 2, we planned to monitor serum lidocaine concentration under DEX sedation versus sevoflurane general anesthesia to determine if DEX affected the absorption of lidocaine without epinephrine.
METHODS
Experiment 1: The Effect of DEX on Palatal Mucosal Blood Flow
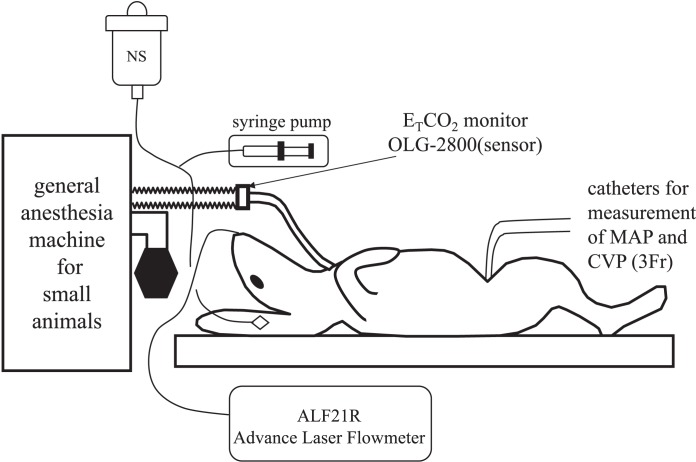
Japanese white male rabbits (n = 22) anesthetized with 5% sevoflurane and 3 L/min oxygen underwent tracheotomy, after which general anesthesia was maintained with 3% sevoflurane and 3 L/min oxygen. An intravenous line was placed in an ear vein, and a normal saline infusion was delivered at 30 mL/h. Catheters (3 Fr; Atom Medical, Tokyo, Japan) were then inserted via the femoral vein and femoral artery, and the tip of the catheter in the femoral vein was placed in the right atrium while that of the femoral artery was placed in the thoracic aorta. A laser Doppler flowmeter probe (ALF21RTM; Advance, Tokyo, Japan) was fixed onto the palatal mucosal surface using a piece of sponge to continuously monitor the palatal mucosal blood flow (PMBF). The rabbits were observed in a supine position for at least 5 minutes until the cardiovascular parameters had stabilized (change in the vital signs less than 10%) (Figure 1).
Figure 1.
Preparation for experiment 1. General anesthesia in both groups was induced and maintained with sevoflurane in oxygen for a tracheotomy and placement of femoral artery and vein catheters. Then, a laser Doppler flowmeter probe (ALF21RTM; Advance, Tokyo, Japan) was fixed onto the palatal mucosal surface using a piece of sponge to monitor the palatal mucosal blood flow (PMBF) continuously. After the preparation (tracheotomy and placement of catheters), at least 5 minutes elapsed until the cardiovascular parameters had stabilized when control (time 0) measurements were made.
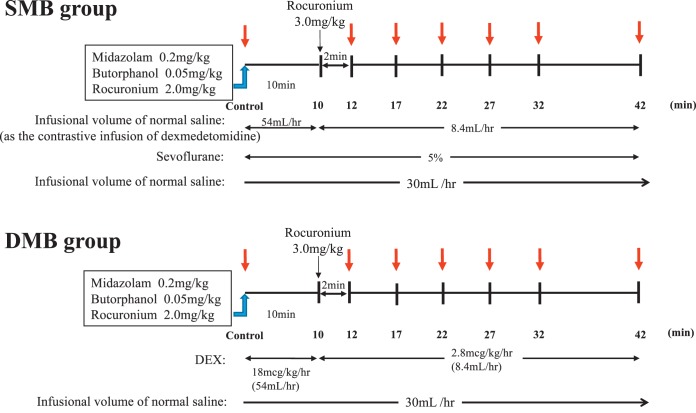
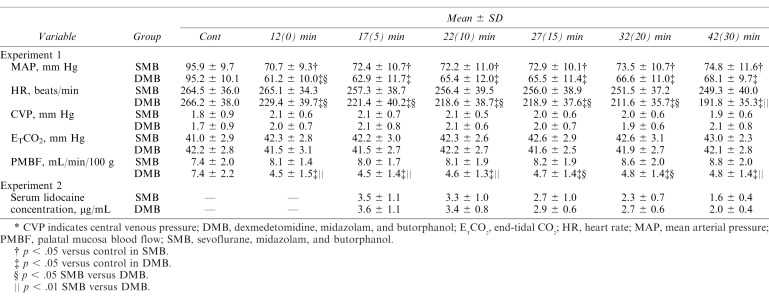
The rabbits were then randomly divided into 2 groups (n = 11 each). One group was managed with sevoflurane, midazolam, and butorphanol (SMB group), and the other group was managed with DEX, midazolam, and butorphanol (DMB group). In the SMB group, after each baseline parameter was measured (used as the control value), rabbits were given 0.2 mg/kg midazolam, 0.05 mg/kg butorphanol, and 2 mg/kg rocuronium intravenously and were subsequently maintained with 5% sevoflurane. An additional dose of rocuronium (3 mg/kg) was given at 10 minutes after measurement of the baseline control values, as the rocuronium dose for rabbits is much higher than humans. Normal saline infusion was given at the same infusion speed as DEX in the DMB group. In the DMB group, inhalation of sevoflurane was discontinued after baseline control values were obtained. At the same time, 0.2 mg/kg midazolam, 0.05 mg/kg butorphanol, and 2 mg/kg rocuronium was administered followed by a loading dose of DEX of 18 μg/kg/h to maintain sedation for rabbits in the DMB group. The same additional dose of rocuronium (3 mg/kg) was given at 10 minutes after measurement of the baseline control values. At this time, the continuous infusion dose of DEX was changed from 18 to 2.8 μg/kg/h. The mean arterial pressure (MAP), heart rate (HR), central venous pressure (CVP), end-tidal CO2 (ETCO2), and palatal mucosal blood flow were measured at 0 (baseline), 12, 17, 22, 27, 32, and 42 minutes after the start of the experimental maintenance phase in both the sevoflurane and DEX groups (Figure 2).
Figure 2.
Time course of experiment 1. Mean arterial pressure (MAP), heart rate (HR), central venous pressure (CVP), end-tidal CO2 (ETCO2), and palatal mucosal blood flow (PMBF) were measured in both groups at 12, 17, 22, 27, 32, and 42 minutes after administration of 0.2 mg/kg midazolam, 0.05 mg/kg butorphanol, and 2 mg/kg rocuronium (time 0). The SMB group was maintained with sevoflurane in oxygen. The DMB group was maintained with dexmedetomidine. SMB indicates sevoflurane, midazolam, and butorphanol; DMB, dexmedetomidine, midazolam, and butorphanol.
Experiment 1 was performed in accordance with the Animal Experiment Regulations of Ohu University (permit No. 2013-42, 2015-9).
Experiment 2: The Effect of DEX on the Absorption of Lidocaine
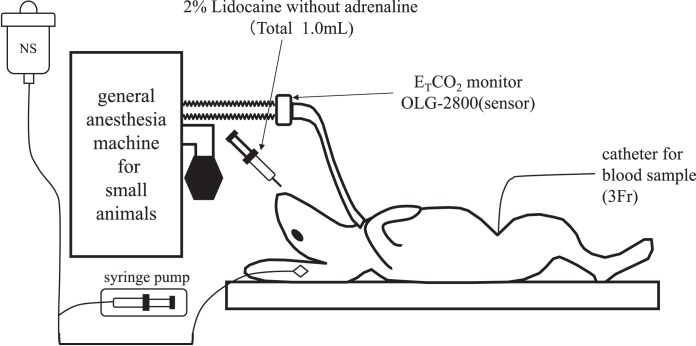
Japanese white male rabbits (n = 16) anesthetized with 5% sevoflurane and 3 L/min oxygen underwent tracheotomy, after which general anesthesia was maintained with 3% sevoflurane and 3 L/min oxygen. An intravenous line was placed in an ear vein, and a 3-Fr catheter was inserted via the femoral artery under general anesthesia with 3% sevoflurane and 3 L/min oxygen. The tip of the catheter in the femoral artery was placed in the thoracic aorta to take blood samples (Figure 3).
Figure 3.
Preparation for experiment 2. General anesthesia in both groups was induced and maintained with sevoflurane in oxygen for a tracheotomy. A 3-Fr catheter was then inserted via the femoral artery with the tip placed in the thoracic aorta to take blood samples.
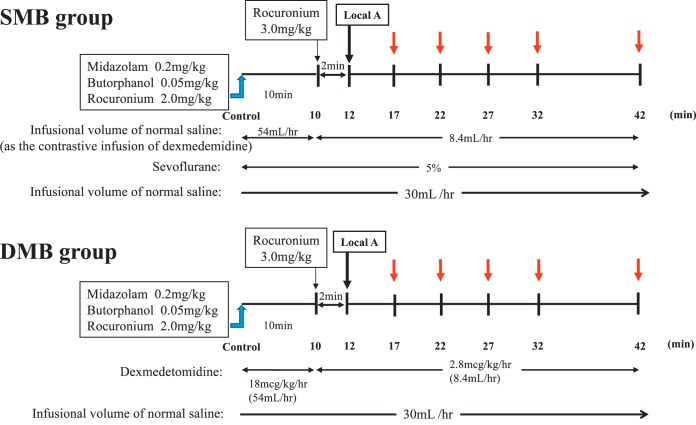
The rabbits were then randomly divided into 2 groups, as in experiment 1. One group was managed with sevoflurane, midazolam, and butorphanol (SMB group), and the other group was managed with DEX, midazolam, and butorphanol (DMB group). The same anesthetic protocol was followed in the SMB and DMB groups as in experiment 1 with regard to drug dosing and timing. In both groups, 0.5 mL each of 2% lidocaine without epinephrine was injected submucosally into the right and left palatal mucosa at 12 minutes after the measurement of the control value (Figure 4). The total dosage of the 1 mL lidocaine administered was therefore 20 mg. Blood samples (3 mL) were taken at 17, 22, 27, 32, and 42 minutes after the measurement of the control value (Figure 4).
Figure 4.
Time course of the investigation in experiment 2. Experiment 2 followed the same anesthetic protocol as experiment 1, except that in both the SMB and DMB groups, 0.5 mL of 2% lidocaine without adrenaline was injected submucosally into both the right and left palatal mucosa (total 20 mg) at 12 minutes after administration of 0.2 mg/kg midazolam, 0.05 mg/kg butorphanol, and 2 mg/kg rocuronium (time 0). Three milliliters of blood samples were then taken at 17, 22, 27, 32, and 42 minutes. SMB indicates sevoflurane, midazolam, and butorphanol; DMB, dexmedetomidine, midazolam, and butorphanol.
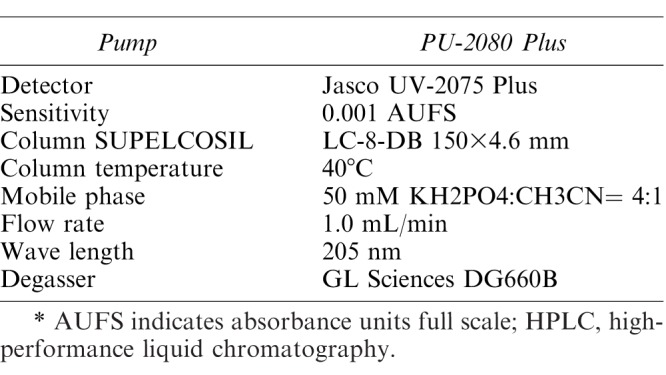
Serum was separated from the blood samples by centrifugation (3000 rpm; KUBOTA 5910, Tokyo, Japan) for 10 minutes. After centrifugal separation, the lidocaine concentration in the serum sample was measured via high-speed liquid chromatography (Jasco PU-2080 Plus Intelligent HPLC Pump, Jasco AS-2050 Plus Intelligent Autosampler, Jasco UV-2075 Plus Intelligent UV/Vis Detector; Jasco Corporation, Ontario, Canada). The conditions for the high-performance liquid chromatography analysis of lidocaine are shown in Table 1.
Table 1.
Conditions for HPLC Analysis of Lidocaine*
Experiment 2 was performed in accordance with the Animal Experiment Regulations of Ohu University (permit No. 2013-42, 2016-14).
Statistical Analyses
The parameters in Experiment 1 were compared between the groups using the Friedman test followed by Wilcoxon t test with Bonferroni correction. The Mann-Whitney U test was used between the groups. The serum lidocaine concentrations in experiment 2 were compared between the groups using the Mann-Whitney U test. A p value of <.05 was considered statistically significant in both experiments.
RESULTS
Experiment 1
Animal Weight.
The mean weight (± standard deviation) of the rabbits for both groups was 3.2 ± 0.2 kg. There was no significant difference in the weight between the 2 groups.
MAP.
The MAP (Table 2) was significantly lower than the control values in both groups (p < .05). On comparing the 2 groups, the value in the DMB group was significantly lower than in the SMB group at 12 minutes after induction (SMB group: 70.7 ± 9.3 mm Hg vs DMB group: 61.2 ± 10.0 mm Hg, p < .05).
Table 2.
MAP, HR, CVP, ETCO2, and PMBF in Experiment 1 and Serum Lidocaine Concentrations in Experiment 2*
HR.
The HR (Table 2) in the DMB group was significantly lower than the control values (p < .05). On comparing the 2 groups, the value in the DMB group was significantly lower than that in the SMB group from 10 to 42 minutes after induction (DMB values at 12, 17, 22, 27, and 32 minutes decreased [p < .05], and the DMB values at 42 minutes decreased [p < .01]).
CVP.
No significant differences in the CVP were noted in either group or between the groups (Table 2).
ETCO2.
No significant differences in the ETCO2 were noted in either group or between the groups (Table 2).
PMBF.
The PMBF in the DMB group was significantly lower than the control values (p < .05; Table 2). On comparing the 2 groups, the PMBF in the DMB group was significantly lower than in the SMB group from 12 to 42 minutes after induction (DMB values decreased at 12, 17, 22, and 42 minutes [p < .05] and at 27 and 32 minutes [p < .01]).
Experiment 2
Animal Weight.
The mean weight (± standard deviation) of the rabbits was 2.7 ± 0.4 kg in the SMB group and 2.8 ± 0.3 kg in the DMB group. There was no significant difference in the weight between the 2 groups.
Serum Lidocaine Concentration.
There were no significant differences in the serum lidocaine concentration between the SMB group and the DMB group from 17 (5) to 42 (30) minutes after induction (Table 2).
DISCUSSION
DEX is a sedative used for procedural sedation and management of intubated patients in intensive care units. It provides a unique sedative effect similar to natural sleep.12 Agitation and cognitive thought rarely occur in patients sedated with DEX.4,12,13 DEX has been used as a sedative during dental or oral surgeries. However, oral surgery is accompanied by a risk of bleeding, because of an abundance of blood vessels in this region. Significant bleeding during surgery can also disrupt oral surgical procedures. Experiment 1 in the present study compared the oral mucosal blood flow between general anesthesia with sevoflurane and sedation with DEX. Experiment 2 focused on the absorption of lidocaine (without epinephrine) due to oral mucosal vasoconstriction by DEX by investigating the serum lidocaine concentration.
Sedation with DEX alone has a weak amnestic action according to the findings of one report,17 which observed that an increase in bispectral index was noted immediately after stimulation to subjects. Kawaai et al13 then reported that amnesia was able to be improved by adding midazolam and butorphanol intravenously to sedation with DEX in implant surgery. In the present study, DEX, midazolam, and butorphanol were used with reference to that report, and sevoflurane was used as a drug to compare to DEX.
Regarding sevoflurane and DEX infusion in the present study, 5% sevoflurane was given to maintain the end-tidal sevoflurane at a maximum of 3.9%.18,19 The 5% sevoflurane used in the present study was 2 to 3 times the minimum alveolar concentration of humans as is common for rabbits. For this reason, we set the infusion dose of DEX at 3 times that in humans.
MAP
The MAP in both groups was significantly lower at each point assessed than at baseline; however, there were no significant differences in the values between the groups, except at 12 minutes after induction. MAP is determined by the cardiac output and peripheral vascular resistance, and the left ventricular afterload reflects the peripheral vascular resistance in MAP. Sevoflurane reduces the left ventricular afterload,20 but Ebert et al21 found that sevoflurane maintains a normal cardiac output because sevoflurane minimally inhibits myocardial contractility. This is suspected to be the reason underlying the decreased MAP in the SMB group.
Similarly, the MAP in the DMB group was also decreased. When DEX is administered as a single dose, a biphasic cardiovascular response has been shown. Regarding this point, Bloor et al22 reported that an initial 1-μg/kg dose of DEX results in a transient increase in blood pressure and a reflexive decrease in heart rate. The increase in blood pressure after initial DEX administration is attributed to the direct effects of alpha-2B adrenoreceptor stimulation on vascular smooth muscle. After the transient increase in blood pressure, a decrease in blood pressure occurs due to the central effects of alpha-2A adrenoreceptors, inducing inhibition of sympathetic outflow that overrides the direct effects of DEX on the vasculature. This is a common effect due to the time differential between direct binding to peripheral vascular receptors and diffusion into the central nervous system.17 However, transient hypertension was not observed during infusion of the loading dose of DEX (for 10 minutes) in the present study. Kawar et al,23 and others,24 have reported that MAP decreased significantly following an dose of 0.3 mg/kg midazolam with reductions in the systemic arterial pressure and pulmonary arterial pressure. It is speculated that midazolam given at induction with the loading dose of DEX may have inhibited transient DEX-induced hypertension. As a result, the MAP in the DMB group decreased significantly from 12 to 42 minutes after the start of DEX infusion.
HR
The HR in the DMB group was significantly lower than the control value (p < .05) from 12 to 42 minutes, although the SMB group showed no significant change. In addition, the value in the DMB group was significantly lower than that in the SMB group at all time points examined. This lowered HR was thought to be due to stimulation by central alpha-2A adrenoreceptor agonists such as DEX. MacDonald et al3 found that the bradycardic effect induced by alpha-2 adrenoreceptor agonists is partly mediated by alpha-2A adrenoreceptors and partly by the baroreceptor reflex. These finding suggest that DEX may be beneficial for sedation of patients with heart disease, such as angina,25,26 because its bradycardic effect reduces the myocardial oxygen consumption. This may offset the tachycardia, which has been reported in patients undergoing dental treatment with local anesthetics containing epinephrine.27
CVP and ETCO2
There were no significant differences in the CVP and ETCO2 between the 2 groups, indicating that both groups were examined under the same conditions in circulatory and respiratory management. CVP is used as an indicator of circulating blood volume and influences the MAP. Given that we noted no significant differences in the CVP between the 2 groups, the fluid management was deemed to be adequate. Regarding the ETCO2, a previous report28 stated that changes in the ETCO2 during DEX infusion affected the oral tissue blood flow in rabbits. We therefore set up a general anesthesia machine to maintain ETCO2 to be approximately 40 mm Hg and monitored ETCO2 in the present study. Our data showed that the ETCO2 was generally between 41 mm Hg and 43 mm Hg. We found no marked differences in this parameter between the groups, suggesting that the respiratory management in the present study had no effects on the oral tissue blood flow.
PMBF
The PMBF in the DMB group was significantly lower than at baseline. In addition, on comparing the 2 groups, the PMBF in the DMB group was also significantly lower than in the SMB group. Oral mucosal blood vessels reportedly contract due to alpha adrenoreceptor stimulation14; we therefore surmised that the PMBF was mainly reduced due to vasoconstriction in response to the alpha-2B adrenoreceptor stimulation of DEX. Regarding the cardiovascular system, Kawaai et al16 reported that the reduction in the cardiac output by HR depression due to DEX could partially contribute to the reduction in the oral mucosal regional blood flow. In the present study, we observed HR depression due to the infusion of DEX. Our PMBF results therefore suggest that the management of sedation and general anesthesia with DEX may contribute to a reduction in total blood loss during operation.
Serum Concentration of Lidocaine
The absorption of lidocaine in the DMB group was predicted to be delayed based on the results of experiment 1. However, in experiment 2, the serum concentration of lidocaine showed no significant difference between the 2 groups, indicating that the PMBF depression by DEX did not affect the absorption of lidocaine. We suggest that this is likely because of the dilating effect of lidocaine on blood vessels.
Burton and Samuel29 reported that lidocaine exerted significant dose-dependent dilation of the pial terminal arterioles in an experiment measuring rat cerebral spinal fluid lidocaine concentration. In their study, 0.1 to 20 mg/mL lidocaine in 0.1 mL was injected into the vessels in the pial terminal arterioles. Consequently, the arterioles dilated with a percentage change of 15 to 45%. The concentration of lidocaine used in our study (20 mg/mL) was the same as the maximum concentration in Burton's experiment. While we cannot conduct a simple comparison of the data in our and Burton's experiments, oral mucosal blood vessels may have undergone a similar vasodilation.
It is well known that the plasma concentration of lidocaine, based on absorption studies, is lower when epinephrine is added.30,31 In the present study, the serum lidocaine concentration showed no significant difference between the SMB and DMB groups, implying that the vasoconstriction effect of DEX was weaker than the vasodilating effect of lidocaine in the oral mucosal blood vessels. As such, local anesthetic with a vasoconstrictor should be used even under sedation and general anesthesia with DEX.
CONCLUSION
In a rabbit model, anesthetic management with DEX may contribute to a reduction in total blood loss during oral surgical operations, as DEX significantly decreased palatal mucosal blood flow. In addition, our results appear to indicate that the vasodilating effect of lidocaine is greater than the vasoconstricting effect of DEX. Therefore, lidocaine with a vasoconstrictor should be used during oral surgical operations under anesthetic management with DEX.
Conflicts of Interest
The authors of this article have no conflicts of interest to declare.
REFERENCES
- 1.Drew GM, Whiting SB. Evidence for two distinct types of postsynaptic α-adrenoceptor in vascular smooth muscle in vivo. Br J Pharmacol. 1979;67:207–215. doi: 10.1111/j.1476-5381.1979.tb08668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doze VA, Chen BX, Maze M. Dexmedetomidine produces a hypnotic-anesthetic action in rats via activation of central alpha-2 adrenoceptors. Anesthesiology. 1989;71:75–79. doi: 10.1097/00000542-198907000-00014. [DOI] [PubMed] [Google Scholar]
- 3.MacDonald E, Kobilka BK, Schenin M. Gene targeting—homing in on α2 adrenoceptor—subtype function. Trends Pharmacol Sci. 1997;18:211–219. doi: 10.1016/s0165-6147(97)01063-8. [DOI] [PubMed] [Google Scholar]
- 4.Venn RM, Grounds RM. Comparison between dexmedetomidine and propofol for sedation in the intensive care unit: patient and clinician perceptions. Br J Anaesth. 2001;87:684–690. doi: 10.1093/bja/87.5.684. [DOI] [PubMed] [Google Scholar]
- 5.McSPI-EUROPE Research Group. Perioperative sympatholysis. Beneficial effects of the α2-adrenoceptor agonist mivazerol on hemodynamic stability and myocardial ischemia. Anesthesiology. 1997;86:346–363. [PubMed] [Google Scholar]
- 6.Quintin L, Bonnet F, Macquin I, Szekely B, Becquemin P, Ghignone M. Aortic surgery: effect of clonidine on intraoperative catecholaminergic and circulatory stability. Acta Anaesthesiol Scand. 1990;34:132–137. doi: 10.1111/j.1399-6576.1990.tb03057.x. [DOI] [PubMed] [Google Scholar]
- 7.Aho M, Lehitinen AM, Erkola O, Kallio A, Kortina K. The effect of intravenously administered dexmedetomidine on perioperative hemodynamics and isoflurane requirements in patients undergoing abdominal hysterectomy. Anesthesiology. 1991;74:997–1002. doi: 10.1097/00000542-199106000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Proctor LT, Shmeling WT, Roerig D, Kampine JP, Warltier DC. Oral dexmedetomidine attenuates hemodynamic responses during emergence from general anesthesia in chronically instrumented dogs. Anesthesiology. 1991;74:108–114. doi: 10.1097/00000542-199101000-00018. [DOI] [PubMed] [Google Scholar]
- 9.Spaulding TC, Fielding S, Venafro JJ, Lal H. Antinociceptive activity of clonidine and its potentiation of morphine analgesia. Eur J Pharmacol. 1979;58:19–25. doi: 10.1016/0014-2999(79)90335-2. [DOI] [PubMed] [Google Scholar]
- 10.Bonnet F, Boico O, Rostaing S, Loriferne JF, Saada M. Clonidine-induced analgesia in postoperative patients: epidural versus intramuscular administration. Anesthesiology. 1990;72:423–427. doi: 10.1097/00000542-199003000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Segal IS, Jarvis DJ, Duncan SR, White PF, Maze M. Clinical efficacy of oral-transdermal clonidine combinations during the perioperative period. Anesthesiology. 1991;74:220–225. doi: 10.1097/00000542-199102000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Nelson LE, Lu J, Guo T, Franks NP, Maze M. The α2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98:428–436. doi: 10.1097/00000542-200302000-00024. [DOI] [PubMed] [Google Scholar]
- 13.Kawaai H, Tomita S, Nakaike Y, Ganzberg S, Yamazaki S. Intravenous sedation for implant surgery: midazolam, butorphanol, and dexmedetomidine versus midazolam, butorphanol and propofol. J Oral Implantol. 2014;40:94–102. doi: 10.1563/AAID-JOI-D-11-00200. [DOI] [PubMed] [Google Scholar]
- 14.Izumi H, Kuriwada S, Karita K, Sasano T, Sanjo D. The nervous of gingival blood flow in cats. Microvasc Res. 1990;39:94–104. doi: 10.1016/0026-2862(90)90061-u. [DOI] [PubMed] [Google Scholar]
- 15.Michael CK. Differential neural activation of vascular α-adrenoceptors in oral tissues of cats. Eur J Pharmacol. 2002;440:53–59. doi: 10.1016/s0014-2999(02)01364-x. [DOI] [PubMed] [Google Scholar]
- 16.Kawaai H, Yoshida K, Tanaka E, et al. Dexmedetomidine decreases the oral mucosal blood flow. Br J Oral Maxilofac Surg. 2013;51:928–931. doi: 10.1016/j.bjoms.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small-dose of dexmedetomidine infusions. Anesth Analg. 2000;90:699–705. doi: 10.1097/00000539-200003000-00035. [DOI] [PubMed] [Google Scholar]
- 18.Terada Y, Ishiyama T, Asano N, et al. Optimal dose of sevoflurane and propofol in rabbits. BMC Res Notes. 2014:820. doi: 10.1186/1756-0500-7-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin Y, Yan M, Zhu T. Minimum alveolar concentration of sevoflurane in rabbits with liver fibrosis. Anesth Analg. 2012;114:561–565. doi: 10.1213/ANE.0b013e31823feca7. [DOI] [PubMed] [Google Scholar]
- 20.Lowe D, Hettrick DA, Pagel PS, Warltier DC. Influence of volatile anesthetics on left ventricular afterload in vivo. Anesthesiology. 1996;85:112–120. doi: 10.1097/00000542-199607000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Ebert TJ, Harkin CP, Muzi M. Cardiovascular responses to sevoflurane: a review. Anesth Analg. 1995;81:S11–S22. doi: 10.1097/00000539-199512001-00003. [DOI] [PubMed] [Google Scholar]
- 22.Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology. 1992;77:1134–1142. doi: 10.1097/00000542-199212000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Kawar P, Carson IW, Clarke RS, Dundee JW, Lyons SM. Haemodynamic changes during induction of anaesthesia with midazolam and diazepam (Valium) in patients undergoing coronary artery bypass surgery. Anaesthesia. 1985;40:767–771. doi: 10.1111/j.1365-2044.1985.tb11002.x. [DOI] [PubMed] [Google Scholar]
- 24.Samuelson PN, Reves JG, Kouchoukos NT, Smith LR, Dole KM. Hemodynamic responses to anesthetic induction with midazolam or diazepam in patients with ischemic heart disease. Anesth Analg. 1981;60:802–809. [PubMed] [Google Scholar]
- 25.Cheng XY, Gu XY, Gao Q, Zong QF, Li XH, Zhang Y. Effects of dexmedetomidine postconditioning on myocardial ischemia and the role of the PI3K/Akt-dependent signaling pathway in reperfusion injury. Mol Med Rep. 2016;14:797–803. doi: 10.3892/mmr.2016.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kunisawa T, Nagata O, Iwasaki H. Postoperative management and extubation under sedation using dexmedetomidine in a patient with old myocardial infarction after carotid endarterectomy. Masui. 2005;54:1051–1055. [PubMed] [Google Scholar]
- 27.Abraham-Inpijn L, Borgmeijer-Hoelen A, Gortzak RA. Changes in blood pressure, heart rate, and electrocardiogram during dental treatment with use of local anesthesia. J Am Dent Assoc. 1988;16:531–536. doi: 10.14219/jada.archive.1988.0318. [DOI] [PubMed] [Google Scholar]
- 28.Okada R, Matsuura N, Kashara M, Ichinohe T. Effect of changes in end-tidal carbon dioxide tension on oral tissue blood flow during dexmedetomidine infusion in rabbits. Eur J Oral Sci. 2015;123:24–29. doi: 10.1111/eos.12162. [DOI] [PubMed] [Google Scholar]
- 29.Burton MA, Samuel L. Perivascular action of the local anaesthetic, lidocaine, on pial terminal arterioles: direct observations on the microcirculation. Br J Pharmacol. 1981;73:577–579. doi: 10.1111/j.1476-5381.1981.tb16788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braid DP, Scott DB. The systemic absorption of local analgesic drugs. Br J Anaesth. 1965;37:394–404. doi: 10.1093/bja/37.6.394. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka E, Yoshida K, Kawaai H, Yamazaki S. Lidocaine concentration in oral tissue by the addition of epinephrine. Anesth Prog. 2016;63:17–24. doi: 10.2344/15-00003R2.1. [DOI] [PMC free article] [PubMed] [Google Scholar]