Abstract
Aims
This study aimed to evaluate in vitro antioxidant capacity of olive leaf extract (OLE), Olea europaea L., and its protective effect on peroxyl radical-induced oxidative damage in human erythrocytes.
Main methods
The OLE was evaluated by the following assays: i) total phenolic and flavonoid content; ii) oleuropein content; iii) Ferric reducing antioxidant power (FRAP); iv) antioxidant activity against ABTS•+, DPPH• and reactive oxygen and nitrogen species: superoxide anion (), hypochlorous acid (HOCl) and nitric oxide (NO•) and v) protective effect on peroxyl radical-induced oxidative damages in human erythrocytes as hemolysis, thiobarbituric acid reactive substances (TBARS) formation and oxyhemoglobin oxidation.
Key findings
Total phenolic and flavonoid contents were 131.7 ± 9.4 mg gallic acid equivalents/g dry weight (dw) and 19.4 ± 1.3 mg quercetin equivalents/g dw, respectively. Oleuropein content was 25.5 ± 5.2 mg/g dw. FRAP analysis was 281.8 ± 22.8 mg trolox equivalent/g dw and OLE inhibited ABTS•+ (50% effective concentration (EC50) = 16.1 ± 1.2 μg/mL) and DPPH• (EC50 = 13.8 ± 0.8 μg/mL). The extract demonstrated effective ability to scavenge (EC50 = 52.6 ± 2.1 μg/mL), NO• (EC50 = 48.4 ± 6.8 μg/mL) and HOCl (EC50 = 714.1 ± 31.4 μg/mL). The extract inhibited peroxyl radical-induced hemolysis (EC50 = 11.5 ± 1.5 μg/mL), TBARS formation (EC50 = 38.0 ± 11.7 μg/mL) and hemoglobin oxidation (EC50 = 186.3 ± 29.7 μg/mL) in erythrocytes.
Significance
OLE is an important source of natural antioxidants; it has effective antioxidant activity against different reactive species and protects human erythrocytes against oxidative damage.
Keywords: Nutrition, Food science, Food technology
1. Introduction
The Olea europaea L. tree, known as olive, belongs to the Oleaceae family and has great historical and commercial importance, especially in Mediterranean countries [1]. Recent studies have shown that olive leaves have high contents of bioactive compounds, including phenolic compounds, with diverse biological properties, such as antioxidant, antimicrobial, antiviral, anti-inflammatory, in addition to regulating blood pressure and cholesterol levels in animals [2, 3]. Thus, the research community's interest in olive leaves and their extracts has intensified in recent years because of the olive's great potential for use in medicine and the pharmaceutical industry [4, 5]. Furthermore, a previous study verified the effective antioxidant activity of olive leaf extract as a potential natural functional ingredient in food products [6].
Free radicals, such as ABTS•+ (2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)) and DPPH• (2,2-diphenyl-1-picrylhydrazyl) are used to evaluate the antioxidant activity of plant extracts [7]; however, these radicals are not present in biochemical oxidative processes. Considering the complexity of biological systems and the generation of numerous reactive intermediates of cellular metabolism, a combination of several techniques for estimating the activities of antioxidants may be favorable. Techniques to evaluate scavenging activity against reactive oxygen and nitrogen species that are produced in vivo, for example, hypochlorous acid (HOCl), superoxide anion () and nitric oxide (NO•), are used to predict antioxidant effects on biochemical processes [8].
Furthermore, peroxyl radicals, which are common products of chain reactions of oxidative processes in biological systems, can be produced in vitro by thermal decomposition of AAPH (2,2′-azobis (2-amidinopropane) dihydrochloride), a water soluble azo compound. This radical generator is used to verify the antioxidant properties of a sample against peroxyl radical-induced oxidative damage in cellular model systems, such as erythrocytes [9, 10]. These cells, also known as red blood cells, have a simple structure and are susceptible to oxidative damage, such as lipid and protein oxidation, and hemolysis [11].
In spite of the clear antioxidant potential of Olea europaea L. leaves, studies related to its scavenging capacity against reactive oxygen and nitrogen species and its protective effect against free radical-induced cellular oxidative damage are scarce. The aim of this study was to evaluate the in vitro antioxidant capacity of Olea europaea L. leaf extract as a scavenger of superoxide anion, hypochlorous acid and nitric oxide as well as its protective effect on peroxyl radical-induced oxidative damage in human erythrocytes. In addition, the contents of total phenolic and flavonoid compounds and oleuropein of olive leaf extract (OLE), their ferric reducing antioxidant power, and their scavenging capacity against ABTS•+ and DPPH• radicals were determined.
2. Materials and methods
2.1. Plants and chemicals
Commercial micronized powdered olive leaves were provided by Folhas de Oliva® (Brazil). All chemical compounds were obtained from Sigma-Aldrich (St. Louis, USA).
2.2. Olive leaf extract preparation
Ten grams of dried/micronized olive leaves were subjected to removal of n-hexane-soluble compounds using a Soxhlet extractor [12]. The sample was subjected to extraction with 200 mL of methanol/water (80:20, v/v), under agitation at 170 rpm (Shaker TE-420, Tecnal, Brazil), protected from light at 25 °C for 24 h, and was centrifuged at 1 970 g for 10 min. The supernatant was filtered through Whatman n.1 (11 μm) filter paper and methanol was removed under reduced pressure (≤50 °C) (Rotary evaporator SL-126, Solab, Brazil). The concentrated extract was diluted in water (final volume 200 mL), sonicated (Ultrasonic Disruptor Unique cells, Brazil) (2 cycles of 20 s at 100 W), and then centrifuged at 1 970 g for 5 min. The supernatant was frozen and freeze-dried (Lyophilizer K-202, Liobras, Brazil) to obtain dried OLE. The OLE was diluted in phosphate-buffered saline (PBS), pH 7.4. The OLE preparation was performed six times.
2.3. Determination of total phenolic, total flavonoid contents and oleuropein content
The Folin-Ciocalteau colorimetric method was used for estimation of total polyphenol content of the extract [13]. The OLE (0.2 mg/mL) was incubated for 2 min with Folin-Ciocalteau reagent, and then 0.708 mol/L sodium carbonate solution was added. After 1 h of incubation at 25 °C, the absorbance at 760 nm was determined. The results were expressed as gallic acid equivalent (GAE) per gram dry weight (dw).
The total flavonoid content was estimated according to Miliauskas et al. [14]. The extract (10 mg/mL) was incubated with 0.150 mol/L ethanolic aluminium chloride. The absorbance at 415 nm was determined after 40 min of incubation at 25 °C. The results were expressed as milligrams of quercetin equivalent (QE) per gram dw.
Oleuropein content of the extract was determined according to Altinyay and Altun [15] and Tayoub et al. [16] with modifications. The dried extract was dissolved in methanol HPLC grade at a final concentration of 10 mg/mL, and filtered through Whatman filter paper (0.45 μm). The solution was injected in a Shimadzu HPLC system consisted of a Shim-pack CLC-ODS column (5 μm) 4.6 × 250 mm, preceded by a precolumn Shim-pack (5 μm) 4 × 10 mm (Shimadzu Corporation, Japão), equipped with UV-visible detector at 280 nm that operated with LabSolutions software (Shimadzu Corporation, Japão). A gradient of water with 0.1% phosphoric acid as eluent A and methanol as eluent B was used in the following proportion: 60% A; 40% B. The flow rate was 1.0 mL/min. The retention time was 18 min and the total time was 30 min at room temperature. Absorbance at 280 nm was recorded.
2.4. Determination of antioxidant activity of extract
2.4.1. Ferric reducing antioxidant power (FRAP)
FRAP analysis was evaluated according to Benzie & Strain [17], with modifications. Reaction mixtures containing 50 μL of sample (0.2 g/mL) and 950 μL of FRAP reagent were incubated for 30 min at 37 °C and the absorbances were determined at 593 nm. Trolox was used as standard. The results were expressed as Trolox Equivalent Antioxidant Capacity (TEAC) in mg/g of dried extract.
2.4.2. ABTS•+ and DPPH• radical-scavenging activity
ABTS•+ scavenging activity was determined according to Re et al. [18] with modifications. ABTS•+ radical was obtained from reaction of 7.0 mmol/L ABTS with 2.45 mmol/L ammonium persulfate in the dark, under constant agitation at room temperature for 12–16 h. ABTS•+ solution was diluted in ethanol to obtain an absorbance of approximately 0.70 at 750 nm. Final reaction mixtures containing different concentrations of extract (3.3–26.7 μg/mL) and ABTS•+ solution were incubated for 6 min in the dark at room temperature and the absorbance determined at 750 nm. Inhibition percentage of ABTS•+ was calculated according to the following equation: ABTS•+ inhibition (%) = [1 − (A/AC0)] × 100, where AC0 is the absorbance of the control assay (no extract) at the initial time and A is the absorbance of the sample assay after 6 min of incubation.
The DPPH• radical-scavenging was determined using the method proposed by Brand-Williams et al. [19]. The reaction medium contained DPPH• (65 μmol/L) and different concentrations of extract (3.8–25.0 μg/mL). Absorbance measurements at 515 nm were started immediately (t0) and after 3 h (tf) of reaction time. The results were expressed in percentage of inhibition of DPPH• according to the following equation: DPPH• inhibition (%) = [1 − (A/ACo)] x 100, where ACo is the absorbance of the control assay (no extract) at t0 and A is the absorbance of the sample at tf. Methanolic solutions of Trolox were tested in both ABTS•+ and DPPH• assays.
2.4.3. Biological reactive species scavenging activity (, HOCl and NO•)
The scavenging activity of OLE was evaluated according Ewing & Janero [20], adapted by Valentão et al. [21] and Orak et al. [22], with some modifications. Reaction medium containing PBS, nicotinamide adenine dinucleotide reduced form, NADH (166 μmol/L), nitroblue tetrazolium, NBT (43 μmol/L), sample (20–160 μg/mL) and phenazine methosulfate, PMS (2.7 μmol/L) was incubated for 2 min at 25 °C and the absorbance was determined at 540 nm using a microplate reader (Multiskan FC Thermo Scientific, USA). The inhibition percentage of was calculated according to the following equation: inhibition (%) = [1 − (A/AC)] × 100, where AC is the absorbance of the control assay (no extract) and A is the absorbance of the sample.
The HOCl assay was performed according to Valentão et al. [21], with modifications. HOCl induces the oxidation of 5-thio-2-nitrobenzoic acid (TNB) to 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB). The reaction was evaluated by TNB absorbance at 412 nm. Reaction media containing 62.5 μmol/L TNB in the absence (control) and presence of OLE (100−1 400 μg/mL) at 25 °C were used to measure absorbance at 421 nm before (t0) and 5 min after (tf) the addition of HOCl. The inhibition percentage of HOCl was calculated according to the following equation: HOCl inhibition (%) = [1 − (Ai − Af)/(ACi − ACf)] × 100, where Ai and ACi are the absorbances of the sample and control, respectively, determined before HOCl addition, and Af and ACf are the absorbances of the sample and control, respectively, evaluated 5 min after HOCl addition.
NO• scavenging activity of OLE was determined according to Marcocci et al. [23] and Pooja et al. [24], based on the Griess reaction. The reaction medium contained 8.3 mmol/L sodium nitroprusside in phosphate buffer saline, pH 7.4 (PBS), prepared immediately before use, in the absence (control) and presence of OLE (10–200 μg/mL). The mixture was incubated for 3 h at 25 °C. formation was quantified by Griess reaction with sulphanilamide (58.0 mmol/L) and N-(1-naphthyl)-ethylenediamine (3.86 mmol/L) at 540 nm using a microplate reader. The concentration was obtained using a standard curve with sodium nitrite (1.5–100 μmol/L). The values were expressed as inhibition percentage of NO•, after 2 h of incubation, obtained by the equation NO• inhibition (%) = [1 − (A/AC)] × 100, where A is the absorbance of the sample and AC is the absorbance of the control.
Ascorbic acid (AA) was used as standard compound.
2.5. Determination of protective effect on peroxyl radical-induced oxidative damage on human erythrocytes
The protective effect of the extract on cellular oxidative damage was in vitro evaluated by hemolysis, TBARS formation and hemoglobin oxidation induced by AAPH in human erythrocytes. Besides, the protective effect of the extract was visually evaluated by optical microscopy and scanning electron microscopy (SEM). Peroxyl radicals were generated by thermal decomposition of AAPH, inducing free-radical chain oxidation in erythrocytes [11].
Blood was obtained from healthy volunteers who were non-smokers and not receiving any pharmacological treatment. Blood was centrifuged at 2 332 g for 10 min and the plasma and buffy coat were removed. Erythrocyte suspensions were washed three times with PBS [9]. The final cell suspension (5% hematocrit) was maintained at 4 °C and immediately used for the assays. The volunteers signed a Consent and Informed, previously approved by the Local Committee of Ethics in Human Research (number: 493.382).
2.5.1. Effect on hemolysis
For the hemolysis assay [11, 25], cells (1% hematocrit) were pre-incubated with PBS in the absence (control) and presence of OLE (1–25 μg/mL) for 30 min at 37 °C with agitation (100 rpm). Then, erythrocytes were incubated in the absence and presence of 5 mmol/L AAPH for 5 h. Every hour, aliquots were collected and centrifuged at 1 750 g for 5 min for quantification of hemolysis in the supernatant by absorbance of hemoglobin at 540 nm. Total hemolysis (100%) was obtained in 1% erythrocytes following incubation with water for 10 min at 37 °C. Ascorbic acid was used as a reference antioxidant.
The results were expressed as % hemolysis, and % inhibition of hemolysis after 5 h of incubation, according to the following equations: Hemolysis (%) = (A/A100%) × 100 and Hemolysis inhibition (%) = [1 − (A/AC)] × 100, respectively, where A is the absorbance in the OLE assay, A100% is the absorbance of total hemolysis and AC is the absorbance of the control.
2.5.2. Effect on erythrocytes by SEM
The effect on erythrocytes was verified by scanning electron microscopy (SEM) according to Pereira et al. [26] and Suwalsky et al. [27], with modifications. The same incubation conditions as for the hemolytic assay were employed, except for the concentrations of AAPH (10 mmol/L) and OLE (25 and 50 μg/mL). After 4 h of incubation, the reaction mixtures were centrifuged at 3 500 rpm for 10 min. Afterwards, cells were fixed for 17 h at 4 °C with glutaraldehyde 2.5% (in PBS). Cells were washed three times with PBS and dehydrated with sucessive washes in ascending sequence of acetone (25%, 50%, 75%, 95% (v/v): 10 min/each, and 99.5% (v/v): 10 min/each for 2 times). Aliquots (20 μL) were placed on glass cover, air-dried at room temperature, and examined in scanning electron microscopy (TM3000, Hitachi, Japan) at 15 kV and magnitude of 1 000–10 000×, using conductive carbon tape. Images were captured by the equipment.
2.5.3. Effect on TBARS formation
Formation of thiobarbituric acid reactive substances (TBARS) was evaluated according to Sato et al. [28], Simão et al. [25] and Chisté et al. [29], with modifications. Cells (5% hematocrit) were pre-incubated with PBS in the absence (control) and presence of OLE (25–250 μg/mL) for 30 min at 37 °C with agitation (100 rpm). Then, erythrocytes were incubated in the absence and presence of 50 mmol/L AAPH for 4 h. Every hour, aliquots were taken and added trichloroacetic acid (TCA) 12% (in the ratio 2.5:1) and, after vigorous homogenization, centrifuged at 1 4000 rpm for 10 min. Subsequently, the supernatant and the thiobarbituric acid (TBA) solution 0.7% (in the ratio 1:1.25) were incubated for 15 min at 100 °C. After 5 min on ice to stop the reaction, the absorbance was determined at 535 nm. Ascorbic acid was used as reference antioxidant. For dose-response studies, the action of antioxidant (OLE or ascorbic acid) on inhibition of TBARS formation after 3 h of cell incubation was evaluated.
The results were expressed as % inhibition of TBARS formation according to the following equation: TBARS inhibition (%) = [1 − (A/AC)] × 100, where AC is the absorbance of the control assay (no extract) and A is the absorbance of the sample.
2.5.4. Effect on oxyhemoglobin oxidation
The protective effect of OLE on oxyhemoglobin (OxyHb) oxidation was assessed as described by Chisté et al. [29] and Winterbourn [30], with modifications. Cells (5% hematocrit) were pre-incubated with PBS in the absence (control) and presence of OLE (25–250 μg/mL) for 30 min at 37 °C with agitation (100 rpm). Then, reaction mixtures were incubated in the absence and presence of 50 mmol/L AAPH for 4 h. Every hour, the formation of (MetHb) was determined by removing aliquots and adding water at a ratio of 1: 9 to induce the hemolysis. After gently homogenization, they were centrifuged at 5 000 rpm for 5 min and the absorption spectrum of the supernatant was verified between 500 and 700 nm. Ascorbic acid was used as reference antioxidant.
Furthermore, we evaluated the OxyHb oxidation on hemolysed and non-hemolysed erythrocytes after 4 h of incubation as previously described. Aliquots of the reaction mixtures were removed and placed on ice to stop the reaction. After centrifugation at 5 000 rpm for 5 min, the absorbance of supernatant (hemolysed erythrocytes) was determined at 630 and 577 nm. The pellet (non-hemolysed erythrocytes) was carefully washed with PBS twice and the cells were lysed with water. Then, after centrifugation at 5 000 rpm for 5 min, the absorbance was also determined at 630 and 577 nm.
The concentrations of OxyHb and MetHb were calculated as proposed by Winterbourn [30] by the following equations: OxyHb concentration (μmol/L) = 66A577 − 80A630, and MetHb concentration (μmol/L) = 279A630 − 3A577, where A577 is the absorbance at 577 nm and A630 is the absorbance at 630 nm. Results of OxyHb and MetHb concentration were corrected based on the hemoglobin content of each fraction considering that 5% hematocrit is equivalent to 5.5 × 108 cell/mL [31].
The results were expressed as % inhibition of OxyHb oxidation according to the following equation: OxyHb oxidation inhibition (%) = [1 − (A/AC)] × 100, where AC is the absorbance of the control assay (no extract) and A is the absorbance of the sample at 630 nm.
2.6. Statistical analysis
All assays were performed in triplicate and the results represent the means ± standard deviation from up to six independent extractions (n = 6). EC50 (50% effective concentration) values were determined using linear equations derived from inhibition (%) versus antioxidant concentrations. The data were evaluated by analysis of variance (ANOVA), t-test and Tukey's test for comparison of means, with a significance level of α = 0.05.
3. Results
3.1. Total phenolic, total flavonoid and oleuropein contents
In present study, OLE was characterized in terms of total phenolic, flavonoids and oleuropein. Total phenolic and flavonoid contents were 131.7 ± 9.4 mg GAE/g dw and 19.4 ± 1.3 mg QE/g dw, respectively. Oleuropein content of extract was 25.5 ± 5.2 mg/g dw. These results demonstrate the reducing power of OLE, suggesting high antioxidant capacity in subsequent assays. Hence, under the tested conditions, these values may indicate a high content of phenolics and flavonoids in hydrophilic OLE.
3.2. Antioxidant activity of extract
3.2.1. FRAP analysis and ABTS•+ and DPPH• radical-scavenging activity
The extract antioxidant activity measured as FRAP analysis was 281.8 ± 22.8 mg TE/g dw. Antioxidant activity of OLE evaluated by inhibition of ABTS•+ and DPPH• radicals was associated with extract concentration with determination coefficients (r2) of 0.9969 and 0.9983, respectively. The EC50 for ABTS•+ and DPPH• radicals was 16.1 ± 1.2 μg/mL and 13.8 ± 0.8 μg/mL, respectively. The extract showed lower antioxidant activity in comparison with the standard trolox that presented EC50 values of 2.3 ± 0.1 μg/mL and 3.0 ± 0.2 μg/mL against ABTS•+ and DPPH•, respectively.
3.2.2. , HOCl and NO• scavenging activity
A direct relation was observed between extract concentration and inhibition effect against , HOCl and NO•, and the mean values adapted a linear regression with an r2 of 0.9111, 0.9182 and 0.7564, respectively. The EC50 values of extract and AA for antioxidant activity against different reactive species are shown in Table 1. These values demonstrate the dose-dependent effect of OLE on biological reactive species.
Table 1.
EC50 values (μg/mL) of superoxide anion (), hypochlorous acid (HOCl) and nitric oxide (NO•) scavenging capacity by olive leaf extract and ascorbic acid.
| Olive leaf extract (n = 6) |
Ascorbic acid (n = 4) |
|
|---|---|---|
| Superoxide anion () | 52.6 ± 2.1a | 59.3 ± 9.2a |
| Hypochlorous acid (HOCl) | 714.1 ± 31.4a | 27.3 ± 1.3b |
| Nitric oxide (NO•) | 48.4 ± 6.8a | 43.9 ± 8.8a |
EC50, effective concentration, the concentration required to decrease the oxidative effect of reactive species by 50%.
Values are expressed as mean ± standard deviation.
Means with different letters in the same line were significantly different (P < 0.05).
Similar dose-response results were found for extract and ascorbic acid in the scavenging assay, with no statistical difference between EC50 values (Table 1). However, compared with AA, a higher concentration of OLE was necessary to obtain HOCl inhibition. According to EC50 values, the extract showed lower HOCl scavenging activity than ascorbic acid. Extract and ascorbic acid showed similar effect in the NO• scavenging assay with no statistical difference between EC50 values. These results indicate the good antioxidant potential of olive leaves in processes involving and NO• and lower HOCl-scavenging activity, which suggests that olive leaves are effective in preventing oxidative stress in biological systems.
3.3. Protective effect on peroxyl radical-induced oxidative damage on human erythrocytes
3.3.1. Effect on hemolysis
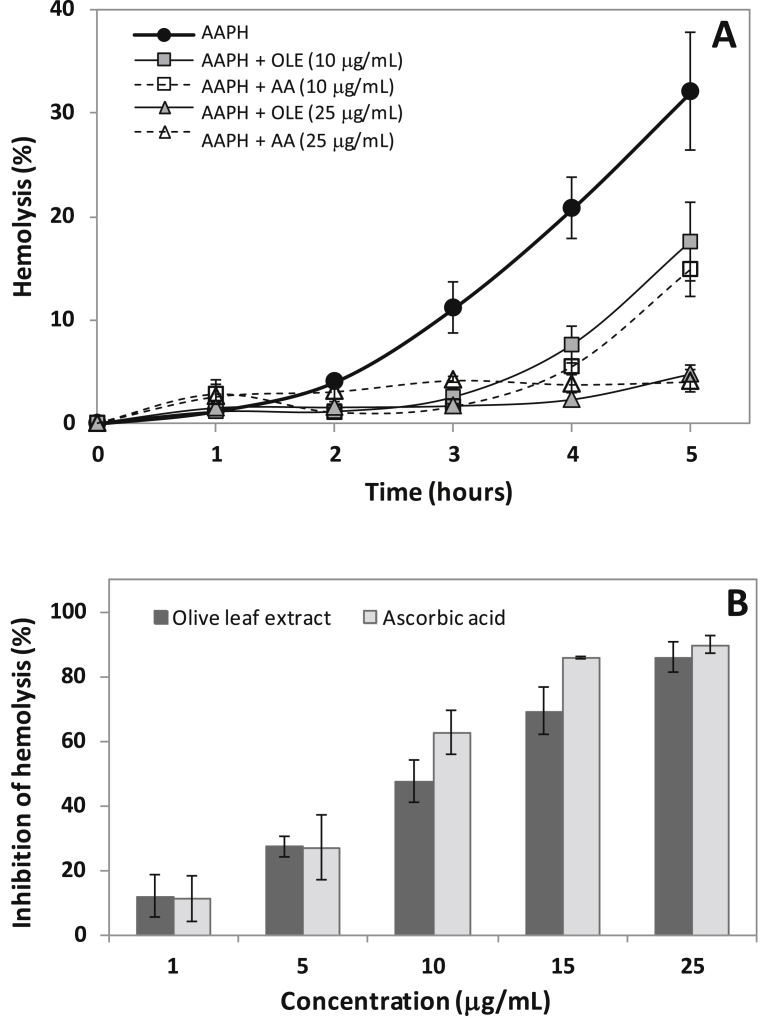
OLE and AA exert a protective effect against peroxyl radical-induced hemolysis in erythrocytes (Fig. 1). Erythrocytes exposed to peroxyl radicals generated by AAPH, showed progressive hemolysis after 2 h of incubation (Fig. 1A). This hemolytic process was inhibited in the presence of OLE and AA in a concentration dependent manner after 5 h of incubation (Fig. 1B) with r2 values of 0.9602 and 0.9895, respectively. This hemolysis study showed EC50 values after 5 h of incubation of 11.5 ± 1.5 μg/mL and 8.6 ± 0.6 μg/mL for OLE and AA, respectively. Hemolysis was not observed in erythrocytes incubated with extract in the absence of AAPH during 5 h of incubation, indicating that OLE does not cause hemolytic damage to erythrocytes (results not shown).
Fig. 1.
Effects of olive leaf extract (OLE) and ascorbic acid (AA) on AAPH-induced hemolysis in erythrocytes. (A) Time course of AAPH-induced hemolysis, (B) Percentage inhibition of hemolysis at 5 h of incubation. Erythrocyte suspension at 1% hematocrit was incubated with 5 mmol/L AAPH at 37 °C in the absence or presence of extract or ascorbic acid at the indicated concentrations. Values are expressed as mean ± standard deviation (n = 5).
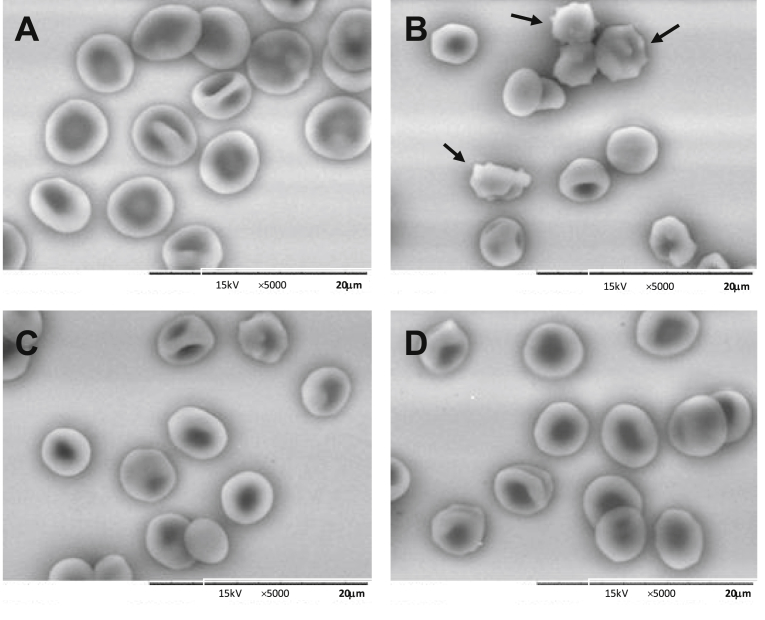
3.3.2. Effect on erythrocytes by SEM
The scanning electron microscopy analysis is shown in Fig. 2. In the absence of AAPH, RBC were in normal biconcave shape, and visually intact (Fig. 2A). When incubated in the presence of 10 mM AAPH the shape and size of cells have changed, with several protuberances in their surfaces (echinocytes), due to oxidation by peroxyl radicals. These morphological changes caused by AAPH were inhibited in the presence of OLE (50 μg/mL) (Fig. 2D). In presence of 25 μg/mL OLE only a few cells showed slight conformational changes (Fig. 2C).
Fig. 2.
Scanning electron microscopy (SEM) evaluation of the effects of olive leaf extract (OLE) on AAPH-induced hemolysis in erythrocytes. Erythrocyte suspension at 1% hematocrit was incubated for 4 h at 37 °C as follows: (A) erythrocytes alone (control); (B) erythrocytes plus 10 mmol/L AAPH; (C) erythrocytes plus 25 μg/mL OLE and 10 mmol/L AAPH; (D) Eryhtrocytes plus 50 μg/mL OLE and 10 mmol/L AAPH. 5000× magnification.
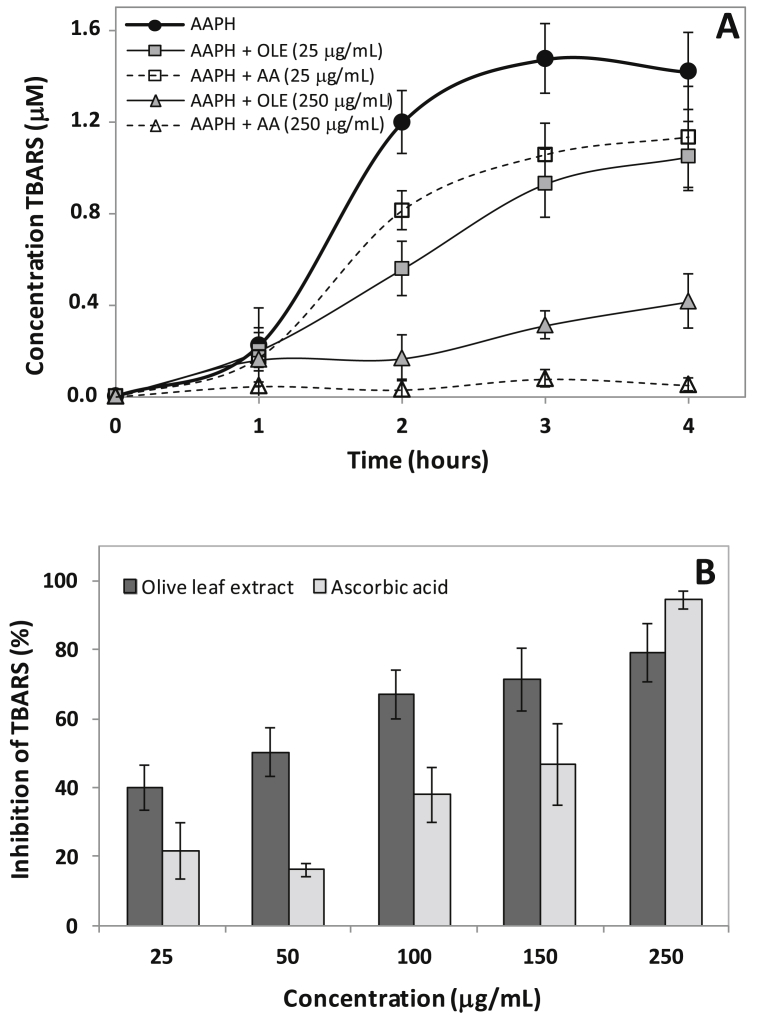
3.3.3. Effect on TBARS formation
The effect of OLE on TBARS formation on erythrocytes exposed to AAPH was shown in Fig. 3. The AA was used as standard antioxidant. In the presence of AAPH, the TBARS significantly increased in a time dependent manner, reaching a maximum value in 3 hours of incubation (1.47 ± 0.15 μmol/L). The TBARS was reduced in the presence of OLE (25–250 μg/mL) from 2 hours of incubation. Incubation of erythrocytes with OLE in the absence of AAPH showed TBARS values similar to the control, indicating that under the tested conditions, the extract does not cause lipid peroxidation in erythrocytes (results not shown).
Fig. 3.
Effects of olive leaf extract (OLE) and ascorbic acid (AA) on TBARS formation in erythrocytes. (A) Time course of AAPH-induced TBARS formation. (B) Percentage inhibition of TBARS formation at 3 h of incubation. Erythrocyte suspension at 5% hematocrit was incubated with 50 mmol/L AAPH at 37 °C in the absence or presence of extract or ascorbic acid at the indicated concentrations. Values are expressed as mean ± standard deviation (n = 5).
Dose-response curve shows that inhibition of TBARS formation in erythrocytes by OLE and AA after 3 h of incubation with AAPH (50 mmol/L) were linear relation with respective r2 0.8573 and 0.9799. The EC50 value of OLE (38.0 ± 11.7 μg/mL) was lower than AA (137.2 ± 24.1 μg/mL) (p < 0.01).
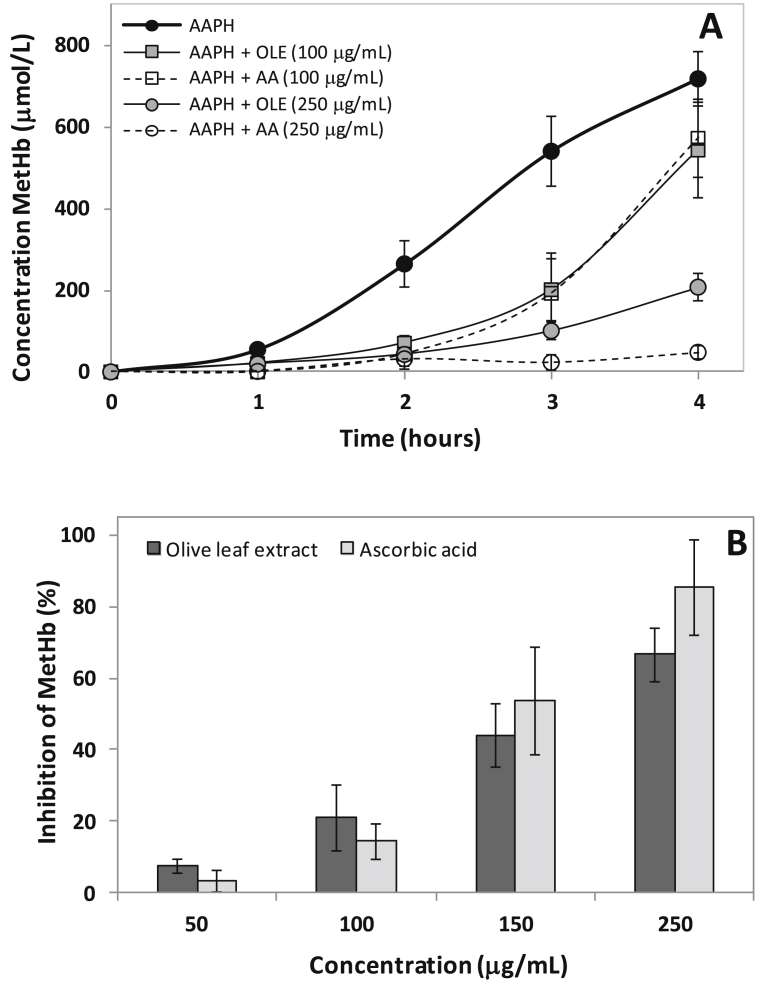
3.3.4. Effect on oxyhemoglobin oxidation
Fig. 4 shows OxyHb oxidation to MetHb induced by AAPH, over time, in absence and presence of OLE. The AA was used as standard antioxidant. Maximum MetHb concentration (718.1 ± 66.6 μM) after 4 h by AAPH erythrocytes exposure was seen. The formation of MetHb, as an indication of OxyHb oxidation, was reduced in presence of different concentrations of OLE or AA. Only OLE or AA did not induces OxyHb oxidation (result not shown).
Fig. 4.
Effects of olive leaf extract (OLE) and ascorbic acid (AA) on AAPH-induced oxyhemoglobin oxidation to methemoglobin in erythrocytes. (A) Time course of AAPH-induced hemoglobin oxidation, (B) Percentage inhibition of hemoglobin oxidation at 4 h of incubation. Erythrocyte suspension at 5% hematocrit was incubated with 50 mmol/L AAPH at 37 °C in the absence or presence of extract or ascorbic acid at the indicated concentrations. Values are expressed as mean ± standard deviation (n = 5).
The inhibition percentage of OLE and AA on MetHb formation was determinate after 4 h of AAPH erythrocytes exposure. Both OLE and AA inhibited the MetHb formation, as noted 66.6 ± 7.5% and 85.4 ± 13.3% of inhibition by 250 μg/mL of these antioxidants, respectively. A linear relationship was found between the inhibition percentage and antioxidant concentration with r2 0.9793 and 0.9608 for EFO and AA, respectively. Similar EC50 for OLE and AA were found, respective 186.3 ± 29.7 μg/mL and 157.0 ± 34.5 μg/mL, without significant difference (p < 0.05).
The erythrocyte OxyHb oxidation was evaluated in hemolysate from total hemoglobin content (supernatant + pellet) and apart supernatant (hemoglobin outside the cell) and pellet (hemoglobin inside the cell) (Table 2). Total hemoglobin content shows a small MetHb concentration in relation to OxyHb on erythrocytes alone (control). However in presence of AAPH the MetHb concentration is greater than OxyHb, probably due to the action of peroxyl radicals from AAPH decomposition. In supernatant, the control showed a less concentration of both OxyHb and MetHb, indicating no hemolysis and no oxidation of OxyHb. In presence of AAPH, hemoglobin was detected outside the cells, demonstrating the hemolytic action of this oxidant. Moreover, the results show that 80% of this hemoglobin was found as MetHb outside the cells. Control assay demonstrated higher hemoglobin concentration inside the cell (pellet) and small content when in presence of AAPH.
Table 2.
Meta-hemoglobin and oxy-hemoglobin formation in total hemoglobin content, supernatant (hemoglobin outside the cell) and pellet (hemoglobin inside the cell) after 4 hours of erythrocytes incubation (5% hematocrit) in the absence or presence of AAPH (50 mM).
| Experimental condition | Total hemoglobin content |
Supernatant |
Pellet |
|||
|---|---|---|---|---|---|---|
| MetHb (μM) |
OxyHb (μM) |
MetHb (μM) |
OxyHb (μM) |
MetHb (μM) |
OxyHb (μM) |
|
| Control | 19.6 ± 5.1∗∗ | 956.4 ± 40.6∗∗ | 3.3 ± 1.7∗∗ | 11.9 ± 3.2∗ | 28.8 ± 17.2 | 863.1 ± 36.7∗∗ |
| AAPH | 718.1 ± 66.6 | 171.1 ± 38.3 | 812.6 ± 94.7 | 189.1 ± 32.8 | 25.1 ± 4.6 | 7.4 ± 2.2 |
| AAPH +50 μg/mL OLE | 683.0 ± 70.1 | 250.3 ± 84.3 | 729.5 ± 47.5 | 281.4 ± 41.6∗ | 20.5 ± 7.5 | 7.5 ± 1.9 |
| AAPH +100 μg/mL OLE | 543.7 ± 117.1∗ | 447.6 ± 82.2∗∗ | 561.4 ± 78.7∗∗ | 371.7 ± 91.7∗∗ | 16.1 ± 5.5∗ | 13.2 ± 3.6 |
| AAPH +150 μg/mL OLE | 406.0 ± 93.2∗∗ | 612.4 ± 45.9∗∗ | 362.5 ± 17.6∗∗ | 387.8 ± 27.0∗∗ | 32.4 ± 18.8 | 172.9 ± 52.9∗ |
| AAPH +250 μg/mL OLE | 207.8 ± 33.5∗∗ | 712.0 ± 109.8∗∗ | 18.1 ± 6.1∗∗ | 4.1 ± 2.4∗ | 127.4 ± 31.4∗∗ | 635.9 ± 63.9∗∗ |
Values are expressed as mean ± standard deviation.
MetHb, meta-hemoglobin; OxyHb, oxy-hemoglobin; OLE, olive leaf extract.
Significantly different in relation to AAPH, in the same column: *p < 0.05; **p < 0.01 (t-test).
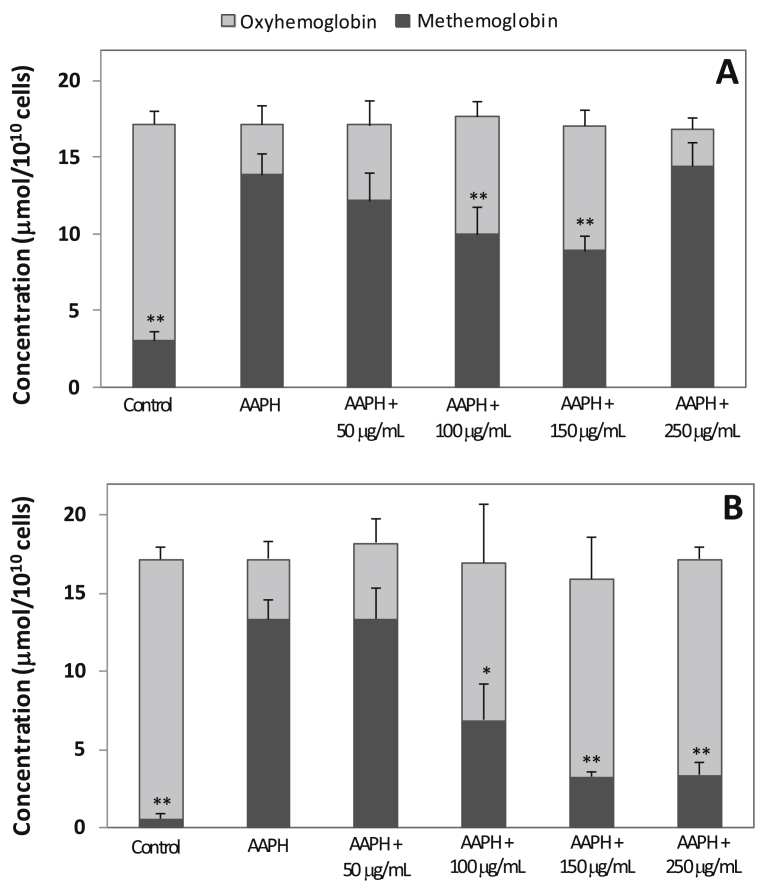
Inhibitory effect of OLE on OxyHb oxidation was found. In order to show OLE activity on OxyHb pre-hemolytic oxidation, the values of total hemoglobin content were corrected for cell numbers (5% hematocrit = 5.5 108 cells/mL) and shown in Fig. 5. Results show that AAPH induced OxyHb oxidation on cellular content (pellet), demonstrating the action of this compound on the oxidation of OxyHb to MetHb even within the cell. So, OLE at 100 μg/mL and 150 μg/mL inhibited the MetHb formation in both supernatant and pellet, but at 250 μg/mL the inhibitory effect was found only in the pellet.
Fig. 5.
Concentration of oxyhemoglobin and methemoglobin (μmol/1010 cells) in (A) supernatant and (B) pellet at 4 h of incubation of erythrocytes. Erythrocyte suspension at 5% hematocrit was incubated at 37 °C in the absence or presence of 50 mmol/L AAPH. Values are expressed as mean ± standard deviation (n = 5). Significantly different in relation to AAPH: *p < 0.05; **p < 0.01 (t-test).
Although the AAPH concentration is the same in all assays (50 mM), the ratio of AAPH:hemoglobin in the supernatant is dependent of hemolysis percentage. In absence of OLE, AAPH-induced hemolysis generates about 1 000 μM of hemoglobin in supernatant medium. However, in presence of 50, 100, 150 and 250 μg/mL of OLE the hemoglobin concentration is 984, 988, 747 and 21 μM, respectively, as a consequence of the anti-hemolytic action of the extract. Thus, the ratio of AAPH:hemoglobin was 0.05:1 in supernatant of erythrocytes exposure to AAPH only; however, the respective ratios 0.05:1, 0.05:1, 0.07:1 and 2.3:1 were found for assays with AAPH plus OLE (50, 100, 150 and 250 μg/mL). Except for assay containing 250 μg/mL of OLE, the ratios were approximately 0.05 of AAPH for each hemoglobin unit. So, in presence of 250 μg/mL of extract, the AAPH:hemoglobin ratio was 2.3:1, suggesting greater hemoglobin exposure to the AAPH effects which may explain the fact that there was no protection of the hemoglobin oxidation by OLE (Fig. 5A).
4. Discussion
The biological effects of olive leaves have been attributed to the composition of phenolic compounds including flavonoids [32], which can be influenced by cultivar, growing region and other factors [2, 22, 33]. Phenolic compounds of olive leaves from different extraction methods have been studied [33, 34, 35]. Studies with methanolic OLE showed total phenolic values of 12.7 and 24.1 mg GAE/g [35, 36]. In addition, the removal of hexane-soluble compounds has been shown to increase the efficiency of phenolic extraction [37]. The content of total flavonoids in this study was similar to other results (21.5 mg catechin equivalents/g dw) [35].
Results indicate that the extraction process with ethanol/water was efficient for obtaining an extract with reducing power, probably due to its phenolic and total flavonoids composition, including oleuropein (25.5 mg/g dw), which suggests an antioxidant potential. In fact, recent studies have shown high antioxidant power of oleuropein on NO generated by HepG2 cells [38]. Other studies showed that methanol/water extracted larger amount of polyphenols [34, 35]. They found 43.2 and 85.2 mg/g dw of oleuropein in methanolic OLE [39, 40], whereas other study observed 0.11 to 0.25 mg/g dw for two cultivars at different harvesting stages [41]. Studies with ethanol/water verified 74 and 6.5 mg of oleuropein/g dw [42, 43]. However, ethanolic extracts from different cultivars presented 44.6 to 87.2 mg of oleuropein/g dw [2].
FRAP analysis and ABTS•+ and DPPH• scavenging activities of OLE have been verified in previous studies [22, 35, 44, 45]. Study with methanolic OLE showed FRAP of 301 mg TE/g dw, similar to this study [6]. Other studies obtained TEAC of 379.3 mg TE/g dw and EC50 of 34.58 μg/mL, for ABTS•+ and DPPH• scavenging by methanolic OLE [44]. However, a lower EC50 (1.57 μg/mL) was observed in DPPH• assay [45]. Differences in these results may be related to different methodologies and extraction procedures used.
The antioxidant capacity of olive leaves is attributed mainly to the presence of phenolic compounds [32, 43, 46]. Several of these compounds were evaluated individually and the antioxidant effects were related to functional groups characteristic, amount and hydroxyl position in their structures, which gives them redox properties [32]. In addition, some studies suggest that phenolic compounds have a synergistic effect on antioxidant capacity when they are together, as in OLE, when compared to their individual effects [43, 47].
The antioxidant activity of a sample can be assessed by various chemical analysis methods [7]. In vitro determinations normally evaluate the scavenging ability of a compound against free radicals or other reactive species. Synthetic ABTS•+ and DPPH• radicals are normally used to evaluate antioxidant activity in vitro [35, 44]. However, in this study, interesting values were found for biological reactive species scavenging by OLE. Results of and NO• scavenging activity demonstrate that OLE have effects comparable to those of AA, indicating significant antioxidant potential.
Our results are similar to others, who showed variation of EC50 (44–386 μg/mL) for scavenging in aqueous OLE from several cultivars [22]. The antioxidant effect of plant aqueous extracts against has been studied and authors have reported that it may be due to the presence of hydroxyl groups in phenolic compounds [48].
There are no studies in literature with HOCl and NO• scavenging activity of OLE; however, some studies have verified that the main polyphenols from olive, such as oleuropein and hydroxytyrosol, have low HOCl scavenging capacity [49, 50]. A previous study evaluated 18 different Mediterranean plant species and observed EC50 from 51–604 μg/mL [51], which suggests that our OLE is an effective NO• scavenger in comparison with other plants. Studies reported that at 5 to 50 μmol/L, oleuropein inhibited approximately 100% of NO• formation [49], and 100 μmol/L of hydroxytyrosol inhibited 61.3% of NO• [52].
Peroxyl radicals attack erythrocyte membrane components, such as proteins and lipids, causing changes in their structure and function, which may result in hemolysis. Recent study showed that antioxidant compounds can protect both hemoglobin degradation and erythrocyte membrane during AAPH-induced hemolysis [53]. Oxidative processes in cell membrane, such as lipid peroxidation and protein oxidation, can cause blistering and protuberances, which normally result in membrane asymmetry and structural changes in erythrocytes [54]. Similarly, we observed the effects of peroxyl radicals initiated by AAPH on human erythrocytes lysis. Studies have shown an antioxidant effect of OLE on peroxyl radical-induced hemolysis in sheep erythrocytes [36]. These authors verified a relationship between the antihemolytic effect of extracts and their radical scavenging activity, measured by DPPH assay, suggesting that the mechanism of action in both cases is related to total phenol content. In fact, studies showed protective effect of the main phenolic compounds and their metabolites present in olive against oxidative hemolysis in human erythrocytes [55, 56].
Lipid peroxidation in cellular membrane together with oxidative damage to membrane proteins, have been related to structural and morphological alterations in erythrocytes [54, 57]. Therefore, our results obtained in biochemical analyzes by TBARS assays corroborates with the observations made in SEM analysis, suggesting that OLE can prevent lipid peroxidation. Although the TBARS contend in intact erythrocytes exposed to AAPH is related to a pool of substances reactive to thiobarbituric acid and not only to the product of lipid peroxidation [29].
Manna et al. [55] found that hydroxytyrosol prevents lipid peroxidation and hemolysis, induced by H2O2 in human erythrocytes. In vivo study observed reduction in TBARS values in liver, heart, kidneys and aorta of Wistar rats after oral administration of OLE rich in phenolic compounds such as oleuropein and hydroxytyrosol [39].
The mechanisms involved in oxidative processes induced by free radicals in erythrocytes are not fully understood. Studies have suggested that occurrence of hemolysis when erythrocytes are exposed to AAPH is due to the action of peroxyl radicals on cell membrane proteins and the induction of membrane lipid peroxidation [25, 28, 57]. However, some authors have verified that, prior to occurrence of AAPH-induced hemolysis, there is no significant lipid peroxidation and/or hemoglobin oxidation in erythrocytes, and suggest that AAPH has no ability to enter erythrocytes and access hemoglobin and polyunsaturated fatty acids located in the cytosolic phase of the membrane [58, 59]. Other studies have shown that AA is a potent inhibitor of peroxyl radicals action and significantly reduces hemolysis and other oxidative damages in erythrocytes [28, 60]. However, the OLE had a greater protective effect on lipid peroxidation than AA. Thus, results of this study demonstrate that OLE efficiently inhibits oxidative damages induced by peroxyl radicals in human erythrocytes.
In this study, OLE has shown a similar effect to AA on AAPH-induced hemoglobin oxidation in human erythrocytes, which shows an important antioxidant potential of the extract in prevention of cellular oxidative processes. These findings are consistent with previous study in which metabolites of hydroxytyrosol prevent the oxidation of hemoglobin to in human erythrocytes after AAPH exposure [61].
Pre-hemolytic oxidation of hemoglobin with MetHb formation was shown in Fig. 5. However, another study did not observe MetHb occurrence in intact cells after 4 hours of incubation with AAPH (5 mM), and suggested that hemoglobin oxidation does not appear to be part of the pre-hemolytic damages [25]. This difference in results may be related to the lower concentration of AAPH (5 mM) compared to this study (50 mM). Our results show that OLE (100 and 150 μg/mL) protected the hemoglobin oxidation in both extracellular medium (supernatant) and inside the erythrocyte. However, 250 μg/mL of extract did not protect hemoglobin oxidative effects in extracellular medium; instead the same concentration prevented the protein oxidation inside the cell.
In studies using phospholipid membrane models it has been observed that oleuropein can cross the lipid membrane and act as an internal antioxidant, whereas the hydroxytyrosol would act near the surface of the membranes [62]. In contrast, other studies have proposed a superficial localization of oleuropein in phospholipid bilayer membranes, where this compound would have an effective role as antioxidant [63, 64].
Regarding the process of MetHb formation inside the cell by AAPH exposure and the consequent protection of this effect by OLE, it should be pointed that peroxyl radicals from AAPH accessed the intracellular hemoglobin; this process caused Fe(II)-heminic oxidation of OxyHb producing Fe(III)-heminic protein or MetHb. Thus, antioxidants present in OLE neutralized the deleterious effects of AAPH on erythrocytes. However, studies suggest that AAPH-derivate peroxyl radicals do not induce significant oxidation in intracellular hemoglobin because of their low ability to penetrate the cell membrane of erythrocytes [58, 59]. Another possible explanation may be related to auto-oxidation process of OxyHb to MetHb, which occurs naturally in erythrocytes [30], and this process is increased in the presence of the oxidant.
In this study, to quantify the oxidative damages on biomolecules, 10-fold higher concentration of AAPH (50 mM) were used in relation to hemolysis assays. Therefore, lower concentrations of AAPH are able to induce hemolysis in erythrocytes, but were not sufficient to evaluate the induction of TBARS formation and hemoglobin oxidation.
To our knowledge, this is the first study evaluating the antioxidant effects of olive leaves on these reactive oxygen and nitrogen species (, HOCl and NO•), and against peroxyl radical-induced oxidative damage in human erythrocytes, which have great importance in biological systems. This antioxidant activity may be partly responsible for some medicinal effects of olive leaves related to the generation of free radicals, since excessive production of reactive oxygen and nitrogen species is involved in various diseases. Therefore, our results represent an important contribution to the understanding of the potential antioxidant activity of olive leaves.
5. Conclusion
Olive leaves are an important source of antioxidants, such as phenolic compounds and flavonoids, which display effective antioxidant activity when various methodologies are used. OLE inhibits the action of reactive species that participate in cellular biochemical processes and protects human erythrocytes against oxidative damage. These results show that olive leaves are effective antioxidant in biological systems, suggesting that their intake may be related to prevention of oxidative stress in vivo, with consequent health benefits. Moreover, OLE have the potential to be used as natural antioxidants in preservation of food products, pharmaceuticals and cosmetics, in which chain reactions mediated by free radicals result in oxidative alterations.
The compounds responsible for the antioxidant effects of OLE are not yet fully understood. Thus, additional studies are required for identification and isolation of these compounds and their efficiency. In addition, further in vivo studies should be performed to confirm the results obtained so far.
Declarations
Author contribution statement
Patricia Goldschmidt Lins, Silvana Marina Piccoli Pugine, Antonio Márcio Scatolini, Mariza Pires de Melo: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We are grateful to the Folhas de Oliva (Brazil) for supplying the olive leaves. The authors are grateful for the review manuscript done by “Writing Services Uniwork Inc”, Department of Proof-Editing.com under order 82122533 payments.
References
- 1.Bianco A., Ramunno A. The chemistry of Olea europaea. Stud. Nat. Prod. Chem. 2006;33:859–903. [Google Scholar]
- 2.Guinda Á., Castellano J.M., Santos-Lozano J.M., Delgado-Hervás T., Gutiérrez-Adánez P., Rada M. Determination of major bioactive compounds from olive leaf. LWT – Food Sci. Technol. 2015;64:431–438. [Google Scholar]
- 3.Lockyer S., Yaqoob P., Spencer J., Rowland I. Olive leaf phenolics and cardiovascular risk reduction: physiological effects and mechanisms of action. Nutr. Aging. 2012;1:125–140. [Google Scholar]
- 4.Dekanski D., Mihailovic-Stanojevic N., Milanovic G., Jovovic D., Miloradovic Z. Effects of high dose olive leaf extract on haemodynamic and oxidative stress parameters in normotensive and spontaneously hypertensive rats. J. Serb. Chem. Soc. 2014;79:1085–1097. [Google Scholar]
- 5.Paiva-Martins F., Barbosa S., Silva M., Monteiro D., Pinheiro V., Mourão J.L., Fernandes J., Rocha S., Belo L., Santos-Silva A. The effect of olive leaf supplementation on the constituents of blood and oxidative stability of red blood cells. J. Funct. Foods. 2014;9:271–279. [Google Scholar]
- 6.Hayes J.E., Stepanyan V., Allen P., O'Grady M.N., Kerry J.P. Evaluation of the effects of selected plant-derived nutraceuticals on the quality and shelf-life stability of raw and cooked pork sausages. LWT – Food Sci. Technol. 2011;44:164–172. [Google Scholar]
- 7.Krishnaiah D., Sarbatly R., Nithyanandam R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011;89:217–233. [Google Scholar]
- 8.López-Alarcón C., Denicola A. Evaluating the antioxidant capacity of natural products: a review on chemical and cellular-based assays. Anal. Chim. Acta. 2013;763:1–10. doi: 10.1016/j.aca.2012.11.051. [DOI] [PubMed] [Google Scholar]
- 9.Gião M.S., Leitão I., Pereira A., Borges A.B., Guedes C.J., Fernandes J.C., Belo L., Santos-Silva A., Hogg T.A., Pintado M.E., Malcata F.X. Plant aqueous extracts: antioxidant capacity via haemolysis and bacteriophage P22 protection. Food Contr. 2010;21:633–638. [Google Scholar]
- 10.Zhang J., Hou X., Ahmad H., Zhang H., Zhang L., Wang T. Assessment of free radicals scavenging activity of seven natural pigments and protective effects in AAPH-challenged chicken erythrocytes. Food Chem. 2014;145:57–65. doi: 10.1016/j.foodchem.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 11.Yang H.-L., Chen S.-C., Chang N.-W., Chang J.-M., Lee M.-L., Tsai P.-C., Fu H.-H., Kao W.-W., Chiang H.-C., Wang H.-H., Hseu Y.-C. Protection from oxidative damage using Bidens pilosa extracts in normal human erythrocytes. Food Chem. Toxicol. 2006;44:1513–1521. doi: 10.1016/j.fct.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Virot M., Tomao V., Colnagui G., Visinoni F., Chemat F. New microwave-integrated Soxhlet extraction. An advantageous tool for the extraction of lipids from food products. J. Chromatogr. A. 2007;1174:138–144. doi: 10.1016/j.chroma.2007.09.067. [DOI] [PubMed] [Google Scholar]
- 13.Singleton V.L., Rossi Junior J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965;16:144–158. [Google Scholar]
- 14.Miliauskas G., Venskutonis P.R., van Beek T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004;85:231–237. [Google Scholar]
- 15.Altinyay C., Altun M.L. HPLC analysis of oleuropein in Olea europaea L. J. Fac. Pharm. Ank. 2006;35:1–11. [Google Scholar]
- 16.Ghaleb Tayoub M.A., Sulaiman Huda, Hassan Abdul Hadi. Determination of oleuropein in leaves and fruits of some Syrian olive varieties. Int. J. Med. Aromat. Plants. 2012;2:428–433. [Google Scholar]
- 17.Benzie I.F.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 18.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 19.Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT – Food Sci. Technol. 1995;28:25–30. [Google Scholar]
- 20.Ewing J.F., Janero D.R. Microplate superoxide dismutase assay employing a nonenzymatic superoxide generator. Anal. Biochem. 1995;232:243–248. doi: 10.1006/abio.1995.0014. [DOI] [PubMed] [Google Scholar]
- 21.Valentão P., Fernandes E., Carvalho F., Andrade P.B., Seabra R.M., Bastos M.L. Antioxidative properties of cardoon (Cynara cardunculus L.) infusion against superoxide radical, hydroxyl radical, and hypochlorous acid. J. Agric. Food Chem. 2002;50:4989–4993. doi: 10.1021/jf020225o. [DOI] [PubMed] [Google Scholar]
- 22.Orak H.H., Isbilir S.S., Yagar H. Determination of antioxidant properties of lyophilized olive leaf water extracts obtained from 21 different cultivars. Food Sci. Biotechnol. 2012;21:1065–1074. [Google Scholar]
- 23.Marcocci L., Maguire J.J., Droy-Lefaix M.T., Packer L. The nitric oxide-scavenging properties of ginkgo biloba extract EGb 761. Biochem. Biophys. Res. Commun. 1994;201:748–755. doi: 10.1006/bbrc.1994.1764. [DOI] [PubMed] [Google Scholar]
- 24.Pooja P.S., Samanta K.C., Garg V. Evaluation of nitric oxide and hydrogen peroxide scavenging activity dalbergia sissoo roots. Pharmacophore. 2010;1:77–88. [Google Scholar]
- 25.Simão A.N.C., Suzukawa A.A., Casado M.F., Oliveira R.D., Guarnier F.A., Cecchini R. Genistein abrogates pre-hemolytic and oxidative stress damage induced by 2,2′-Azobis (Amidinopropane) Life Sci. 2006;78:1202–1210. doi: 10.1016/j.lfs.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 26.Pereira W.A.B., Hatayde M.R., Faria J.L.M. Uso da técnica de microscopia eletrônica de varredura para determinação de alterações eritrocitárias em ovinos intoxicados experimentalmente com cobre. Vet. Zootec. 2011;18:275–283. [Google Scholar]
- 27.Suwalsky M., Oyarce K., Avello M., Villena F., Sotomayor C.P. Human erythrocytes and molecular models of cell membranes are affected in vitro by Balbisia peduncularis (Amancay) extracts. Chem. Biol. Interact. 2009;179:413–418. doi: 10.1016/j.cbi.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 28.Sato Y., Kamo S., Takahashi T., Suzuki Y. Mechanism of free radical-induced hemolysis of human erythrocytes: hemolysis by water-soluble radical initiator. Biochemistry. 1995;34:8940–8949. doi: 10.1021/bi00028a002. [DOI] [PubMed] [Google Scholar]
- 29.Chisté R.C., Freitas M., Mercadante A.Z., Fernandes E. Carotenoids inhibit lipid peroxidation and hemoglobin oxidation, but not the depletion of glutathione induced by ROS in human erythrocytes. Life Sci. 2014;99:52–60. doi: 10.1016/j.lfs.2014.01.059. [DOI] [PubMed] [Google Scholar]
- 30.Winterbourn C.C. Oxidative reactions of hemoglobin. In: Abelson J.N., Simon M.I., Colowick S.P., Kaplan N.O., Packer L., Glazer A.N., editors. Methods in Enzymology. 1990. pp. 265–272. [DOI] [PubMed] [Google Scholar]
- 31.Vallada E.P. Hematócrito. In: Atheneu, editor. Manual de Técnicas Hematológicas. São Paulo; 1997. pp. 31–34. [Google Scholar]
- 32.Goulas V., Papoti V.T., Exarchou V., Tsimidou M.Z., Gerothanassis I.P. Contribution of flavonoids to the overall radical scavenging activity of olive (Olea europaea L.) leaf polar extracts. J. Agric. Food Chem. 2010;58:3303–3308. doi: 10.1021/jf903823x. [DOI] [PubMed] [Google Scholar]
- 33.Bilgin M., Şahin S. Effects of geographical origin and extraction methods on total phenolic yield of olive tree (Olea europaea) leaves. J. Taiwan Inst. Chem. Eng. 2013;44:8–12. [Google Scholar]
- 34.Brahmi F., Mechri B., Dabbou S., Dhibi M., Hammami M. The efficacy of phenolics compounds with different polarities as antioxidants from olive leaves depending on seasonal variations. Ind. Crop. Prod. 2012;38:146–152. [Google Scholar]
- 35.Abaza L., Ben Youssef N., Manai H., Mahjoub Haddada F., Methenni K., Zarrouk M. Chétoui olive leaf extracts: influence of the solvent type on phenolics and antioxidant activities. Grasas Aceites. 2011;62:96–104. [Google Scholar]
- 36.Ferreira I.C.F.R., Barros L., Soares M.E., Bastos M.L., Pereira J.A. Antioxidant activity and phenolic contents of Olea europaea L. leaves sprayed with different copper formulations. Food Chem. 2007;103:188–195. [Google Scholar]
- 37.Rodríguez-Rojo S., Visentin A., Maestri D., Cocero M.J. Assisted extraction of rosemary antioxidants with green solvents. J. Food Eng. 2012;109:98–103. [Google Scholar]
- 38.Sherif I.O., Al-Gayyar M.M.H. Oleuropein potentiates anti-tumor activity of cisplatin against HepG2 through affecting proNGF/NGF balance. Life Sci. 2018;198:87–93. doi: 10.1016/j.lfs.2018.02.027. [DOI] [PubMed] [Google Scholar]
- 39.Jemai H., Bouaziz M., Fki I., El Feki A., Sayadi S. Hypolipidimic and antioxidant activities of oleuropein and its hydrolysis derivative-rich extracts from Chemlali olive leaves. Chem. Biol. Interact. 2008;176:88–98. doi: 10.1016/j.cbi.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 40.Tóth G., Alberti Á., Sólyomváry A., Barabás C., Boldizsár I., Noszál B. Phenolic profiling of various olive bark-types and leaves: HPLC–ESI/MS study. Ind. Crop. Prod. 2015;67:432–438. [Google Scholar]
- 41.Brahmi F., Mechri B., Dhibi M., Hammami M. Variations in phenolic compounds and antiradical scavenging activity of Olea europaea leaves and fruits extracts collected in two different seasons. Ind. Crop. Prod. 2013;49:256–264. [Google Scholar]
- 42.Ahmad-Qasem M.H., Cánovas J., Barrajón-Catalán E., Micol V., Cárcel J.A., García-Pérez J.V. Kinetic and compositional study of phenolic extraction from olive leaves (var. Serrana) by using power ultrasound. Innovat. Food Sci. Emerg. Technol. 2013;17:120–129. [Google Scholar]
- 43.Xie P., Huang L., Zhang C., Zhang Y. Phenolic compositions, and antioxidant performance of olive leaf and fruit (Olea europaea L.) extracts and their structure–activity relationships. J. Funct. Foods. 2015;16:460–471. [Google Scholar]
- 44.Hayes J.E., Allen P., Brunton N., O’Grady M.N., Kerry J.P. Phenolic composition and in vitro antioxidant capacity of four commercial phytochemical products: olive leaf extract (Olea europaea L.), lutein, sesamol and ellagic acid. Food Chem. 2011;126:948–955. [Google Scholar]
- 45.Bouaziz M., Sayadi S. Isolation and evaluation of antioxidants from leaves of a Tunisian cultivar olive tree. Eur. J. Lipid Sci. Technol. 2005;107:497–504. [Google Scholar]
- 46.Rahmanian N., Jafari S.M., Wani T.A. Bioactive profile, dehydration, extraction and application of the bioactive components of olive leaves. Trends Food Sci. Technol. 2015;42:150–172. [Google Scholar]
- 47.Lee O.-H., Lee B.-Y. Antioxidant and antimicrobial activities of individual and combined phenolics in Olea europaea leaf extract. Bioresour. Technol. 2010;101:3751–3754. doi: 10.1016/j.biortech.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 48.Govindan P., Muthukrishnan S. Evaluation of total phenolic content and free radical scavenging activity of Boerhavia erecta. J. Acute Med. 2013;3:103–109. [Google Scholar]
- 49.Czerwinska M., Kiss A.K., Naruszewicz M. A comparison of antioxidant activities of oleuropein and its dialdehydic derivative from olive oil, oleacein. Food Chem. 2012;131:940–947. [Google Scholar]
- 50.Rietjens S.J., Bast A., Haenen G.R.M.M. New insights into controversies on the antioxidant potential of the olive oil antioxidant hydroxytyrosol. J. Agric. Food Chem. 2007;55:7609–7614. doi: 10.1021/jf0706934. [DOI] [PubMed] [Google Scholar]
- 51.Conforti F., Marrelli M., Carmela C., Menichini F., Valentina P., Uzunov D., Statti G.A., Duez P., Menichini F. Bioactive phytonutrients (omega fatty acids, tocopherols, polyphenols), in vitro inhibition of nitric oxide production and free radical scavenging activity of non-cultivated Mediterranean vegetables. Food Chem. 2011;129:1413–1419. [Google Scholar]
- 52.Ammendola S., Giusti A.M., Masci A., Mosca L., Saso L., Bovicelli P. Antioxidant properties of hydroxytyrosyl acetate compared with hydroxytyrosol and their protective capacity against oxidative stress in human neuroblastoma cells. J. Sci. Ind. Res. 2011;70:929–937. [Google Scholar]
- 53.Bellik Y., Iguer-Ouada M. Concurrent measurement of cellular turbidity and hemoglobin to evaluate the antioxidant activity of plants. Food Chem. 2016;190:468–473. doi: 10.1016/j.foodchem.2015.05.126. [DOI] [PubMed] [Google Scholar]
- 54.Ansari F.A., Ali S.N., Mahmood R. Sodium nitrite-induced oxidative stress causes membrane damage, protein oxidation, lipid peroxidation and alters major metabolic pathways in human erythrocytes. Toxicol. Vitro. 2015;29:1878–1886. doi: 10.1016/j.tiv.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 55.Manna C., Galletti P., Cucciolla V., Montedoro G., Zappia V. Olive oil hydroxytyrosol protects human erythrocytes against oxidative damages. J. Nutr. Biochem. 1999;10:159–165. doi: 10.1016/s0955-2863(98)00085-0. [DOI] [PubMed] [Google Scholar]
- 56.Paiva-Martins F., Fernandes J., Rocha S., Nascimento H., Vitorino R., Amado F., Borges F., Belo L., Santos-Silva A. Effects of olive oil polyphenols on erythrocyte oxidative damage. Mol. Nutr. Food Res. 2009;53:609–616. doi: 10.1002/mnfr.200800276. [DOI] [PubMed] [Google Scholar]
- 57.Zou C.-G., Agar N.S., Jones G.L. Oxidative insult to human red blood cells induced by free radical initiator AAPH and its inhibition by a commercial antioxidant mixture. Life Sci. 2001;69:75–86. doi: 10.1016/s0024-3205(01)01112-2. [DOI] [PubMed] [Google Scholar]
- 58.Celedón G., Rodriguez I., España J., Escobar J., Lissi E. Contribution of haemoglobin and membrane constituents modification to human erythrocyte damage promoted by peroxyl radicals of different charge and hydrophobicity. Free Radic. Res. 2001;34:17–31. doi: 10.1080/10715760100300031. [DOI] [PubMed] [Google Scholar]
- 59.Celedón G., González G., Lissi E.A., Hidalgo G. Free radical-induced protein degradation of erythrocyte membrane is influenced by the localization of radical generation. IUBMB Life. 2001;51:377–380. doi: 10.1080/152165401753366140. [DOI] [PubMed] [Google Scholar]
- 60.Raval J.S., Fontes J., Banerjee U., Yazer M.H., Mank E., Palmer a F. Ascorbic acid improves membrane fragility and decreases haemolysis during red blood cell storage. Transfus. Med. (Oxford, England) 2013;23:87–93. doi: 10.1111/tme.12013. [DOI] [PubMed] [Google Scholar]
- 61.Paiva-Martins F., Silva A., Almeida V., Carvalheira M., Serra C., Rodrigues-Borges J.E., Fernandes J., Belo L., Santos-Silva A. Protective activity of hydroxytyrosol metabolites on erythrocyte oxidative-induced hemolysis. J. Agric. Food Chem. 2013;61:6636–6642. doi: 10.1021/jf4016202. [DOI] [PubMed] [Google Scholar]
- 62.Saija A. “In vitro” evaluation of the antioxidant activity and biomembrane interaction of the plant phenols oleuropein and hydroxytyrosol. Int. J. Pharm. 1998;166:123–133. [Google Scholar]
- 63.Paiva-Martins F., Gordon M.H., Gameiro P. Activity and location of olive oil phenolic antioxidants in liposomes. Chem. Phys. Lipids. 2003;124:23–36. doi: 10.1016/s0009-3084(03)00032-x. [DOI] [PubMed] [Google Scholar]
- 64.Caturla N., Pérez-Fons L., Estepa A., Micol V. Differential effects of oleuropein, a biophenol from Olea europaea, on anionic and zwiterionic phospholipid model membranes. Chem. Phys. Lipids. 2005;137:2–17. doi: 10.1016/j.chemphyslip.2005.04.003. [DOI] [PubMed] [Google Scholar]