Abstract
Background: Angiolymphoid hyperplasia with eosinophilia (ALHE) is a distinctive benign vascular disease that can be challenging to treat due to inconsistent results for various treatment modalities such as surgical excision, corticosteroids, radiotherapy, laser therapy, and other therapies, so novel approaches are needed to improve treatment outcomes.
Materials and Methods: ALHE on the right auricle of a 54-year-old Chinese woman underwent brachytherapy using 32P simple drug membranes for five times. The 32P brachytherapy involving simple drug membranes of brachytherapy began by diluting a 32P solution with 0.9% NaCl solution to produce a radioactivity of 69.2–74.7 MBq/mL(1.87–2.02 mCi/mL). The drug membranes were removed between 48 and 72h after application. There were intervals ranging from 65 to 72d between the membrane application periods, and the last treatment was in June 2010.
Results: After the 32P brachytherapy, follow-up results over the course of 8 years were promising. The regional symptoms disappeared, the right preauricular swelling decreased, the subcutaneous nodules decreased in size, the exudate disappeared, and the skin appearance improved.
Conclusions: This case indicated that 32P brachytherapy may represent a novel ALHE treatment method that produces a favorable long-term outcome.
Keywords: : angiolymphoid hyperplasia with eosinophilia (ALHE), brachytherapy, phosphorus-32
Introduction
Angiolymphoid hyperplasia with eosinophilia (ALHE), first described in 1969 by Wells and Whimster,1 is a rare benign vascular disease. Despite its benign nature, ALHE presents a therapeutic dilemma,2 as persistent or recurrent lesions with clear symptoms may require treatment. Various modalities such as surgical excision, corticosteroids, radiotherapy, laser therapy, and other therapies have been used to treat ALHE. Surgical excision appears to be the most effective treatment for ALHE, but clinicians should anticipate an ∼40% recurrence rate; so novel approaches are needed to improve treatment outcomes for this condition.3 In this study, the authors report a case of ALHE occurring on the right auricle that had improved presentation for over 8 years after 32P brachytherapy.
Case Presentation
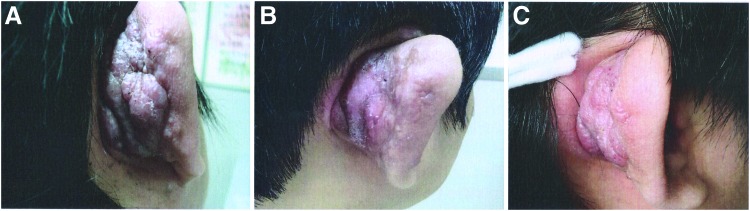
A 54-year-old Chinese woman with a 5-year history of multiple nodules under the subcutaneous tissues on the right auricle (Fig. 1A) was referred to their hospital in April 2009. The patient also suffered from itching and occasional tingling in the right auricle. Topical corticosteroids and oral antihistamines were prescribed, but the condition repeatedly recurred. On examination, erythematous or violaceous papules and nodules were present in the right dermis and subcutaneous tissues, and auricle swelling was observed. No regional lymphadenopathy or other pathological findings were evident.
FIG. 1.
Multiple nodules under the subcutaneous tissues on the right auricle (A). Three- (B) and eight-year (C) follow-up of ALHE on the right auricle treated with 32P brachytherapy. ALHE, angiolymphoid hyperplasia with eosinophilia. Color images available online at www.liebertpub.com/cbr
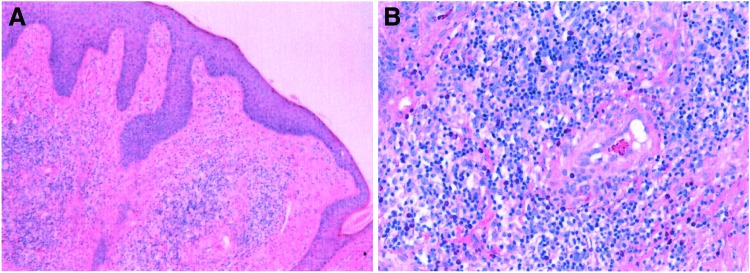
Laboratory data, including eosinophil count and total serum immunoglobulin (Ig)E, were within normal limits. A biopsy was performed on the lesion, and the pathological diagnosis was ALHE (Fig. 2).
FIG. 2.
Small blood vessels proliferated in the dermis and were accompanied by infiltrating eosinophils and lymphocytes, epidermal hyperplasia with hyperkeratosis, and epithelial foot extensions. Hematoxylin and eosin staining at (A) low (40 × ) and (B) high power (200 × ) magnification. Color images available online at www.liebertpub.com/cbr
After the patient presented to their department, brachytherapy with 32P simple-drug membranes was performed on the lesions. The patient underwent 32P brachytherapy treatment five times. The 32P brachytherapy involving simple-drug membranes of brachytherapy began by diluting a 32P solution (Beijing Atoms High-Tech Ltd. Co.) with 0.9% NaCl solution to produce a radioactivity of 69.2–74.7 MBq/mL (1.87–2.02 mCi/mL). The lesion area was covered with transparent plastic film and cellulose qualitative filter paper (Grade 1) as a medicine film. The size of the 32P simple-drug membranes was determined using carbon paper, and the membranes were prepared by evenly applying the diluted 32P to filter paper, which was then allowed to dry. Electric soldering was used to close the transparent plastic film. The treatment area was disinfected using iodine tincture, and the prepared 32P simple-drug membranes were pressed tightly to the lesion. The drug membranes were removed between 48 and 72 h after application, and the membranes were properly disposed of as radioactive waste. There were intervals ranging from 65 to 72 d between the membrane application periods, and the last treatment was in June 2010. After the 32P brachytherapy, follow-up results over the course of 8 years were promising. The regional symptoms disappeared, the right preauricular swelling decreased, the subcutaneous nodules decreased in size, the exudate disappeared, and the skin appearance improved (Fig. 1B, C).
Discussion
ALHE, also called epithelioid hemangioma, is a rare benign proliferative disease. The most common locations for ALHE are the ear and periauricular area (36.3%).3 The lesions typically present as well-circumscribed papules or nodules that are red, brown, or purple in color,4 but the diagnosis should be histologically confirmed and not confused with Kimura's disease.4–6 More than half of ALHE patients (53.4%) present with a single lesion, and the remainder have multiple lesions. Only 15.4% of ALHE cases are asymptomatic, whereas the majority of ALHE patients suffer from at least one symptom, including pruritus (36.8%), bleeding (25.3%), or pain (20.2%).3 Furthermore, some lesions can become infected,7 pulsatile, or ulcerated. Thus, timely and effective treatment of ALHE is necessary for symptomatic relief, to address cosmetic concerns and to prevent tissue deterioration.
Many approaches have been used to treat ALHE, including surgical excision,2,3,5,8–10 steroids, and other drugs,11–16 as well as biological and physical therapy.17–22 Surgery is the mainstay of ALHE treatment, although surgery is less successful for cases with poorly delineated multiple nodules. Moreover, surgery can be disfiguring and technically difficult, especially in the periauricular region. Other ALHE treatments also have both advantages and disadvantages. Regardless of the treatment method, recurrence and incomplete resolution of the disease are frequent. Indeed, a study by Adler et al.3 documented that the rate of treatment success was low with excision (40.8%), pulsed dye laser (50.0%), or carbon dioxide laser (54.6%); intermediate for argon laser (66.7%), intralesional corticosteroids (79.1%), or cryotherapy (80.5%); and high for systemic (87.8%) and topical corticosteroids (98.2%), oral pentoxifylline, and isotretinoin (100%). Given the availability of several different treatment modalities, the use of multiple treatments for ALHE is proposed in the dermatologic literature. Selection of appropriate methods should be predicated on parameters such as the number of lesions, the lesion size and site, and previous treatments.
Radiotherapy is currently widely applied for the treatment of tumors, but there are few studies concerning the treatment of ALHE with radiotherapy. Conill et al.23 successfully used orthovoltage radiation therapy to treat an ALHE patient who had multiple nodules involving the skin, subcutaneous tissue, and bone of the distal phalanx of the fingers, but this approach carries a risk of impaired function and radiation damage to surrounding normal tissues.
32P is a pure β-particle-emitting radionuclide that has a half-life of 14.3 d. The biologic effects of ionizing radiation,24–26 benign proliferative diseases,27 and malignant tumors can be treated using 32P brachytherapy, a form of radiation therapy.25,26 In this technique, the radiation source is placed close to the area to be treated,24–27 similar to other surface-mold isotope brachytherapies.28 The range of electrons emitted by 32P is limited in that the maximum range in the tissue is 7.5 mm. The therapeutic range of 32P brachytherapy in tissue is about 2 mm, and the accumulated dose falls to subcritical levels within 10 mm of the radioactive source, thus making this approach safe for treatment of lesions in humans26–27 and ensuring that surrounding healthy tissue receives only minimal radiation doses. 32P brachytherapy may be superior to conventional external beam radiation for treating patients who have small, primary, and/or superficial lesions in terms of providing excellent functional and cosmetic results.28 In the case that the authors described in this study, the lesions were located in the preauricular region and its base. The lesions were poorly delineated multiple modules that impeded local surgical removal. 32P brachytherapy was performed with improved presentation over 8 years of follow-up. Thus, 32P brachytherapy may be a promising new treatment option for ALHE that produces favorable long-term outcomes.
Conclusions
ALHE is an uncommon, benign, and idiopathic vascular lesion that often has a chronic course with frequent relapses. Many approaches that have both advantages and disadvantages for treating ALHE have been attempted, and there is ongoing debate regarding optimal management for this condition. 32P brachytherapy, a form of radiation therapy that places the radiation source close to the area to be treated, could represent a novel method to treat ALHE that produces favorable long-term outcomes. 32P brachytherapy may thus be a good choice for recurrent ALHE and for ALHE patients who refused other treatments.
Disclosure Statement
No competing financial interests exist.
References
- 1.Wells GC, Whimster IW. Subcutaneous angiolyrnphoid hyperplasia with eosinophilia. Br J Dermatol 1969;81:1. [DOI] [PubMed] [Google Scholar]
- 2.Bahloul E, Amouri M, Charfi S, et al. Angiolymphoid hyperplasia with eosinophilia: Report of nine cases. Int J Dermatol 2017;56:1373. [DOI] [PubMed] [Google Scholar]
- 3.Adler BL, Krausz AE, Minuti A, et al. Epidemiology and treatment of angiolymphoid hyperplasia with eosinophilia (ALHE): A systematic review. J Am Acad Dermatol 2016;74:506.e11. [DOI] [PubMed] [Google Scholar]
- 4.Buder K, Ruppert S, Trautmann A, et al. Angiolymphoid hyperplasia with eosinophilia and Kimura's disease—A clinical and histopathological comparison. J Dtsch Dermatol Ges 2014;12:224. [DOI] [PubMed] [Google Scholar]
- 5.Jun R, Liu XK, Zeng K. Successful treatment of angiolymphoid hyperplasia with eosinophilia and Kimura's disease in the same patient with surgery. Dermatol Ther 2014;27:36. [DOI] [PubMed] [Google Scholar]
- 6.Seregard S. Angiolymphoid hyperplasia with eosinophilia should not be confused with Kimura's disease. Acta Ophthalmol Scand 2001;79:91. [DOI] [PubMed] [Google Scholar]
- 7.Chou Ch-Y, Lee W-R, Tseng JTP. Case of angiolymphoid hyperplasia with eosinophilia associated with scabies infestation. J Dermatol 2012;39:102. [DOI] [PubMed] [Google Scholar]
- 8.Guo R, Gavino ACP. Angiolymphoid hyperplasia with eosinophilia. Arch Pathol Lab Med 2015;139:683. [DOI] [PubMed] [Google Scholar]
- 9.Baghestani S, Firooz A, Ghazisaidi MR. A refractory case of angiolymphoid hyperplasia with eosinophilia successfully treated by surgery. J Dermatolog Treat 2011;22:49. [DOI] [PubMed] [Google Scholar]
- 10.Zaraa I, Mlika M, Chouk S, et al. Angiolymphoid hyperplasia with eosinophilia: Analysis of 7 cases. Dermatol Online J 2011;17:1. [PubMed] [Google Scholar]
- 11.Lembo S, Balato A, Cirillo T, et al. A long-term follow-up of angiolymphoid hyperplasia with eosinophilia treated by corticosteroids: When a traditional therapy is still up-to-date. Case Rep Dermatol 2011;3:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nouchi A, Hickman G, Battistella M, et al. Treatment of angiolymphoid hyperplasia with eosinophilia (ALHE) using topical tacrolimus: Two cases. Ann Dermatol Venereol 2015;142:360. [DOI] [PubMed] [Google Scholar]
- 13.Isohisa T, Masuda K, Nakai N, et al. Angiolymphoid hyperplasia with eosinophilia successfully treated with imiquimod. Int J Dermatol 2014;53:e43. [DOI] [PubMed] [Google Scholar]
- 14.Sayed FE, Dhaybi R, Ammoury A, et al. Angiolymphoid hyperplasia with eosinophilia: Efficacy of isotretinoin? Head Face Med 2006;2:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horst C, Kapur N. Propranolol: A novel treatment for angiolymphoid hyperplasia with eosinophilia. Clin Exp Dermatol 2014;39:810. [DOI] [PubMed] [Google Scholar]
- 16.Alaidarous A, Bouissou X, Mazereeuw-Hautier J, et al. Angiolymphoid hyperplasia with eosinophilia treated with low-dose methotrexate. J Am Acad Dermatol 2015;1:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ali FR, Madan V. Facial angiolymphoid hyperplasia with eosinophilia: Sustained remission following treatment with carbon dioxide laser. Clin Exp Dermatol 2016;41:96. [DOI] [PubMed] [Google Scholar]
- 18.Sotiriou E, Apalla Z, Patsatsi A, et al. Angiolymphoid hyperplasia with eosinophilia: Good response to photodynamic therapy. Clin Exp Dermatol 2009;34:e629. [DOI] [PubMed] [Google Scholar]
- 19.González JA, Boixeda P, Díez MTT, et al. Angiolymphoid hyperplasia with eosinphilia treated with vascular laser. Lasers Med Sci 2011;26:285. [DOI] [PubMed] [Google Scholar]
- 20.Sagi L, Halachmi S, Levi A, et al. Combined pulsed dye and CO2 lasers in the treatment of angiolymphoid hyperplasia with eosinophilia. Lasers Med Sci 2016;31:1093. [DOI] [PubMed] [Google Scholar]
- 21.Bito T, Kabashima R, Sugita K, et al. Angiolymphoid hyperplasia with eosinophilia on the leg successfully treated with T-helper cell 2 cytokine inhibitor suplatast tosilate. J Dermatol 2011;38:300. [DOI] [PubMed] [Google Scholar]
- 22.Tambe SA, Nayak CS. Successful management of angiolymphoid hyperplasia with eosinophilia by radiofrequency. J Cutan Aesthet Surg 2017;10:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conill C, Toscas I, Mascaro Jr JM, et al. Angiolymphoid hyperplasia with eosinophilia of the nail bed and bone: Successful treatment with radiation therapy. J Eur Acad Dermatol Venereol 2004;18:584. [DOI] [PubMed] [Google Scholar]
- 24.Kassis AI. Therapeutic radionuclides: Biophysical and radiobiologic principles. Semin Nucl Med 2008;38:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salgueiro MJ, Duran H, Palmieri M, et al. Bioevaluation of 32P patch designed for the treatment of skin diseases. Nucl Med Biol 2008;35:233. [DOI] [PubMed] [Google Scholar]
- 26.Salgueiro MJ, Collia N, Durán H, et al. Biological effects of brachytherapy using a (32)P-patch on the skin of Sencar mice. Appl Radiat Isot 2009;67:1769. [DOI] [PubMed] [Google Scholar]
- 27.Oestreicher E, Bartsch H, Mayr D, et al. Preclinical study investigating the potential of low-dose-rate brachytherapy with 32P stents for the prevention of restenosis of paranasal neo-ostia. Brachytherapy 2017;16:207. [DOI] [PubMed] [Google Scholar]
- 28.Alam M, Nanda S, Mittal BB, et al. The use of brachytherapy in the treatment of nonmelanoma skin cancer: A review. J Am Acad Dermatol 2011;65:377. [DOI] [PubMed] [Google Scholar]