Abstract
Background: Obesity is prevalent among HIV-infected individuals on antiretroviral therapy (ART). Cross-sectional studies have suggested that HIV-infected women are more likely to be overweight than men, but observational studies evaluating sex differences in body mass index (BMI) increases following ART initiation are conflicting.
Materials and Methods: We pooled data from three randomized clinical trials of ART initiation in persons with HIV in the United States. BMIs were compared between 760 women and 3041 men to test whether BMI changes in the first 96 weeks following initiation of ART differed by sex at birth. Linear regression estimated the relationship between sex and change in BMI from pre-ART initiation to week 96.
Results: After 96 weeks, women gained an average of 1.91 kg/m2 (95% confidence interval [CI] 1.64–2.19), men gained an average of 1.39 kg/m2 (95% CI 1.30–1.48); p for sex difference <0.001; the sex difference persisted within each pre-ART initiation BMI subgroup. After adjusting for pre-ART initiation age, CD4+ count, HIV-1 viral load, race/ethnicity, study, and ART regimen, mean BMI change for women was 0.59 kg/m2 (95% CI 0.37–0.81) more than for men (p < 0.001). Statistical interactions were observed between sex and both pre-ART CD4+ count and HIV-1 viral load and suggest that for subgroups with higher viral load and lower CD4+ at baseline, the estimated BMI changes in women are even larger than the average estimated difference.
Conclusions: HIV-1-infected women experienced a significantly greater increase in BMI following ART initiation than men. These differences are a problem of clinical significance to women living with HIV.
Keywords: : HIV, BMI, sex differences, obesity
Background
Despite improvements in survival with antiretroviral therapy (ART),1–4 life expectancy for those living with HIV is still lower compared with age-matched HIV-uninfected individuals.1 Non-HIV-related events such as cardiovascular disease (CVD) and diabetes are more prevalent in ART-treated HIV-infected persons compared to age-matched HIV-uninfected individuals.4–13 Obesity is an independent risk factor for CVD and obese individuals living in the United States are nearly twice as likely to experience CVD, even after adjustment for other traditional risk factors.14 Cross-sectional studies reveal a high prevalence of obesity among HIV-infected individuals15–17 and research has noted increases in weight gain after ART initiation, with up to 20% of patients moving into an overweight or obese body mass index (BMI) category within 2 years of ART initiation.18 Importantly, short-term gains in BMI following ART initiation have been directly linked to increases in the long-term risk of both CVD and diabetes.19
Several cross-sectional studies have suggested that HIV-infected women are more likely to be overweight or obese than HIV-infected men.14,20,21 However, observational cohort studies evaluating differences in BMI increases following ART initiation in men and women have yielded conflicting results.18,19,22–25 These observational studies evaluated patients on a variety of ART regimens that are no longer preferred, and may not reflect the current experience.
In this study, we pooled data from three randomized controlled trials of treatment-naive participants initiating ART with modern regimens in the United States, to assess changes in BMI over 96 weeks and explore the relationship between sex and changes in BMI.
Materials and Methods
Parent studies
We accessed participant-level data from three Phase 3, AIDS Clinical Trials Group (ACTG) ART initiation trials in treatment-naive persons, in which BMI data were collected. We included data from ACTG A5142 (NCT#00050895, enrolled 2003–2004),26 A5202 (NCT#00118898, enrolled 2005–2007),27 and A5257 (NCT# 00811954, enrolled 2009–2011).28
ACTG 5142 randomized 757 participants to one of three class-sparing regimens: lopinavir/ritonavir and two nucleoside reverse transcriptase inhibitors (NRTIs) (lamivudine plus zidovudine, stavudine, or tenofovir disoproxil fumarate [TDF]), efavirenz and two NRTIs, or lopinavir/ritonavir and efavirenz26; A5202 included 1858 participants randomized to atazanavir/ritonavir or efavirenz combined with either abacavir/lamivudine or TDF/emtricitabine (FTC); and A5257 included 1809 participants randomized to atazanavir/ritonavir, darunavir (DRV)/ritonavir, or raltegravir (RAL) combined with TDF/emtricitabine.
We included participants from these studies if they had pre-ART initiation and week 96 BMI data, were enrolled in a research site in the United States (applicable to A5142 only), started ART, had matching sex at birth and current gender identity (an indication that participant was not transgender), and did not become pregnant during follow-up. We investigated the change in BMI at 96 weeks because this is the latest time point at which all studies evaluated BMI.
Statistics
Linear regression was used to evaluate the relationship between sex and change in BMI from pre-ART initiation to 96 weeks. The modeling process included the following demographic and clinical characteristics assessed just before ART initiation: sex, age, reproductive status of women, race/ethnicity, geographical region of United States, CD4+ cell count (per mm3), plasma HIV-1 viral load (log10 copies/mL), BMI, and assigned ART regimen.
Characteristics with some association (p-value <0.2) in single covariate models were entered into a multivariable modeling process using stepwise selection (and p-value of 0.05 to remain in the model). Each two-way statistical interaction between sex and the characteristics selected into the main effects model was tested. We also evaluated the outcome of change to a worse BMI category from baseline to week 96. Primary analysis was modified intention to treat among those who started ART. A sensitivity analysis was performed among participants who were virologically suppressed (HIV-1 viral load <200 copies/mL at both weeks 48 and 96). A subgroup analysis was performed within the A5257 clinical trial subgroup to adjust for additional potentially prognostic pre-ART initiation characteristics available only within this one study, including socioeconomic status (SES) defined by highest education level attained, binge alcohol episodes, illicit drug use, smoking status, and history of metabolic syndrome.
p Values <0.05 were considered statistically significant. Statistical analyses were conducted using SAS software (version 9.4).
Results
Analysis sample derivation and baseline characteristics
Of the 4422 participants enrolled in the parent studies, 3801 had pre-ART and week 96 BMIs and met other inclusion criteria. The primary reasons for exclusion were missing data or loss to follow-up (568), but a total of 53 individuals were excluded by study design. Twenty were enrolled in a research site outside of the United States, 22 did not start study ART, 11 did not have matching sex at birth and current gender identity, and 28 became pregnant during follow-up. Patients with active opportunistic infections were excluded from the parent studies; however, 20% of participants in the parent studies had AIDS diagnoses at study entry. Table 1 displays the pre-ART characteristics of the included subjects. Women, compared to men, were slightly older (mean of 40.5 years vs. 37.7 years). Women also had a higher mean BMI before starting ART (28.4 vs. 25.2 kg/m2). The mean pre-ART CD4+ count did not differ between the sexes (260 vs. 261 cells/mm3). Before starting ART, women had slightly lower HIV-1 viral loads, mean of 4.54 log10 copies/mL in women compared to 4.74 log10 copies/mL in men. Women were more likely to be black and men were more likely to be white. Finally, the proportion of women who enrolled to each trial varied; women represented 18% of participants in A5142, 17% in A5202, and 24% in A5257. Each of these observed differences between sexes is a typically observed difference within ART-naive study samples and does not represent novel or unanticipated differences.
Table 1.
Pre-Antiretroviral Therapy Initiation Characteristics of Participants
| Women (n = 760) | Men (n = 3041) | Total (n = 3801) | p-Value | |
|---|---|---|---|---|
| Age (years), mean | 40.5 | 37.7 | 38.3 | <0.001* |
| BMI (kg/m2), mean | 28.4 | 25.2 | 25.9 | <0.001* |
| BMI category | <0.001* | |||
| Underweight (<18.5 kg/m2) | 37 (5%) | 89 (3%) | 126 (3%) | |
| Normal (18.5–24.9 kg/m2) | 245 (32%) | 1557 (51%) | 1802 (47%) | |
| Overweight (25–29.9 kg/m2) | 223 (29%) | 1007 (33%) | 1230 (32%) | |
| Obese (≥30 kg/m2) | 255 (34%) | 388 (13%) | 643 (17%) | |
| Race/ethnicity | <0.001** | |||
| White non-Hispanic | 127 (17%) | 1320 (43%) | 1447 (38%) | |
| Black non-Hispanic | 439 (58%) | 951 (31%) | 1390 (37%) | |
| Hispanic | 180 (24%) | 669 (22%) | 849 (22%) | |
| Other | 14 (2%) | 101 (3%) | 115 (3%) | |
| CD4+ cell count(/mm3), mean | 260.2 | 261.2 | 261 | 0.9* |
| Plasma HIV-1 viral load (log10 copies/mL), mean | 4.54 | 4.74 | 4.70 | <0.001* |
| Randomized clinical trial source (enrollment and 96-week follow-up) [ART regimens] | <0.001** | |||
| ACTG A5142 (January 2003–February 2006) [EFV + NRTIs vs. LPV/r + NRTIs vs. EFV + LPV/r] | 104 (14%) | 465 (15%) | 569 (15%) | |
| ACTG A5202 (September 2005–September 2009) [EFV vs. ATV/r + ABC/3TC vs. TDF/FTC] | 267 (35%) | 1325 (44%) | 1592 (42%) | |
| ACTG A5257 (May 2009–June 2013) [TDF/FTC + ATV/r vs. TDF/FTC + DRV/r vs. TDF/FTC + RAL] | 389 (51%) | 1251 (41%) | 1640 (43%) |
t-Test with unequal variance.
Chi-square test.
BMI, body mass index; ATV, atazanavir; rtv, ritonavir; TDF, tenofovir disoproxil fumarate; FTC, emtricitabine; RAL, raltegravir; EFV, efavirenz; DRV, darunavir; ABC, abacavir; 3TC, lamivudine; LPV, lopinavir; NRTI, nucleoside reverse transcriptase inhibitor; ART, antiretroviral therapy.
BMI observed data
After 96 weeks, women had both larger absolute and relative changes in BMI than men (mean of 1.91 kg/m2 vs. 1.39 kg/m2 or mean increase of 7.65% vs. 5.92%). The mean observed difference in absolute BMI increases between women and men is 0.52 kg/m2 (95% confidence interval [CI 0.29–0.75]); p for sex difference <0.001. Most of the weight change observed over 96 weeks occurred within the first year of follow-up: the magnitude of the change in BMI from baseline to week 48 (mean of 1.53 kg/m2 or 6.2% for women vs. 1.15 kg/m2 or 4.9% for men) was more than half of the magnitude of change from baseline to week 96.
Table 2 displays the observed mean change in BMI by pre-ART initiation BMI category (underweight, normal, overweight, and obese). The significant sex difference was seen within each pre-ART initiation BMI subgroup.
Table 2.
Mean Body Mass Index (Body Mass Index in kg/m2 with 95% Confidence Intervals) Changes
| n (%) | Overall (n = 3801) | Women (n = 760) | Men (n = 3041) | Sex difference (women–men) | p-Value | |
|---|---|---|---|---|---|---|
| Overall observed (i.e., unadjusted) | ||||||
| Thru week 48 | 3801 (100%) | 1.23 (1.15–1.31) | 1.53 (1.31–1.75) | 1.15 (1.07–1.23) | 0.38 (0.19–0.57) | 0.002 |
| Thru week 96 | 3801 (100%) | 1.50 (1.40–1.59) | 1.91 (1.64–2.19) | 1.39 (1.30–1.48) | 0.52 (0.29–0.75) | <0.001 |
| Observed by pre-ART initiation BMI category, through week 96 | ||||||
| Underweight (<18.5) | 126 (3%) | 2.53 (2.08–2.97) | 3.51 (2.48–4.54) | 2.12 (1.66–2.88) | 1.4 (0.44–2.35) | 0.016 |
| Normal (18.5 to <25) | 1802 (47%) | 1.77 (1.65–1.89) | 2.37 (1.93–2.81) | 1.68 (1.55–1.80) | 0.69 (0.33–1.05) | 0.003 |
| Overweight (25 to <30) | 1230 (32%) | 1.17 (1.01–1.32) | 1.71 (1.22–2.22) | 1.04 (0.89–1.20) | 0.67 (0.27–1.08) | <0.001 |
| Obese (≥30) | 643 (17%) | 1.16 (0.88–1.44) | 1.41 (0.89–1.95) | 0.99 (0.68–1.29) | 0.43 (−0.14 to 1.00) | <0.001 |
BMI modeling
In single characteristic modeling, we found that age, baseline CD4+ count, baseline HIV-1 viral load, race/ethnicity, study, and study treatment group were each significantly associated with the absolute increase in BMI after 96 weeks of ART. After adjusting for these variables, mean BMI change for women was on average 0.59 kg/m2 (95% CI 0.37–0.81) more than for men (p < 0.001) (Table 3).
Table 3.
Linear Regression on Outcome of Body Mass Index Changes from Pre-Antiretroviral Therapy to Week Ninety Six
| Model | Estimated sex difference in BMI change to week 96 |
|---|---|
| Single covariate: sex | 0.52 (0.29, 0.75) |
| Two-covariate models | |
| Sex and age | 0.49 (0.26, 0.72) |
| Sex and CD4 cell count | 0.51 (0.30, 0.73) |
| Sex and HIV-1 viral load | 0.80 (0.58, 1.01) |
| Sex and race/ethnicity | 0.40 (0.17, 0.64) |
| Sex and AR regimen within trial | 0.56 (0.33, 0.79) |
| Main effects model—all covariates listed above | 0.59 (0.37, 0.81) |
| Main effects plus interactions for sexa CD4 and sexa HIV-1 viral loada | 0.62 (0.40, 0.84), for CD4 = 260 and VL = 4.7 |
| 1.12 (0.83, 1.40), for CD4 = 120 and VL = 5.2 | |
| 0.53 (0.13, 0.93), for CD4 = 400 and VL = 5.2 | |
| 0.12 (−0.15, 0.39), for CD4 = 400 and VL = 4.2 | |
| Subgroup analysis (A5257 trial) | |
| Sex alone | 0.59 (0.27, 0.91) |
| Main effects model (see above) | 0.59 (0.28, 0.90) |
| Main effects and education (as SES) | 0.57 (0.25, 0.88) |
| Main effects and binge alcohol consumption | 0.52 (0.19, 0.85) |
| Main effects and illicit drug use | 0.52 (0.20, 0.84) |
| Main effects and metabolic syndrome | 0.57 (0.25, 0.88) |
| Main effects plus binge alcohol consumption, sex,a and smoking status interaction | 0.85 (0.38, 1.33) among non-smokers |
| 0.52 (−0.21, 1.24) among former smokers | |
| −0.04 (−0.55, 0.47) among current smokers | |
See Figure 1 for display of more sex difference estimates over the observed ranges of CD4 and HIV-1 viral load.
AR, antiretroviral; SES, socioeconomic status; VL, HIV-1 viral load.
Outcome of change to a worse BMI category
We defined change to a worse BMI category as the change in BMI category from underweight to overweight or obese, or from normal to overweight or obese, or change from overweight to obese in the first 96 weeks following the initiation of ART. While similar proportions of women and men moved to a worse BMI category (23.16% and 24.17%, respectively), we found that among participants moving to a worse BMI category, women gained more weight than men (mean absolute increase in BMI of 5.57 [95% CI 5.07–6.07] for women vs. 4.03 [95% CI 3.85–4.21] for men).
Statistical interactions between sex and baseline CD4+ count and HIV-1 viral load
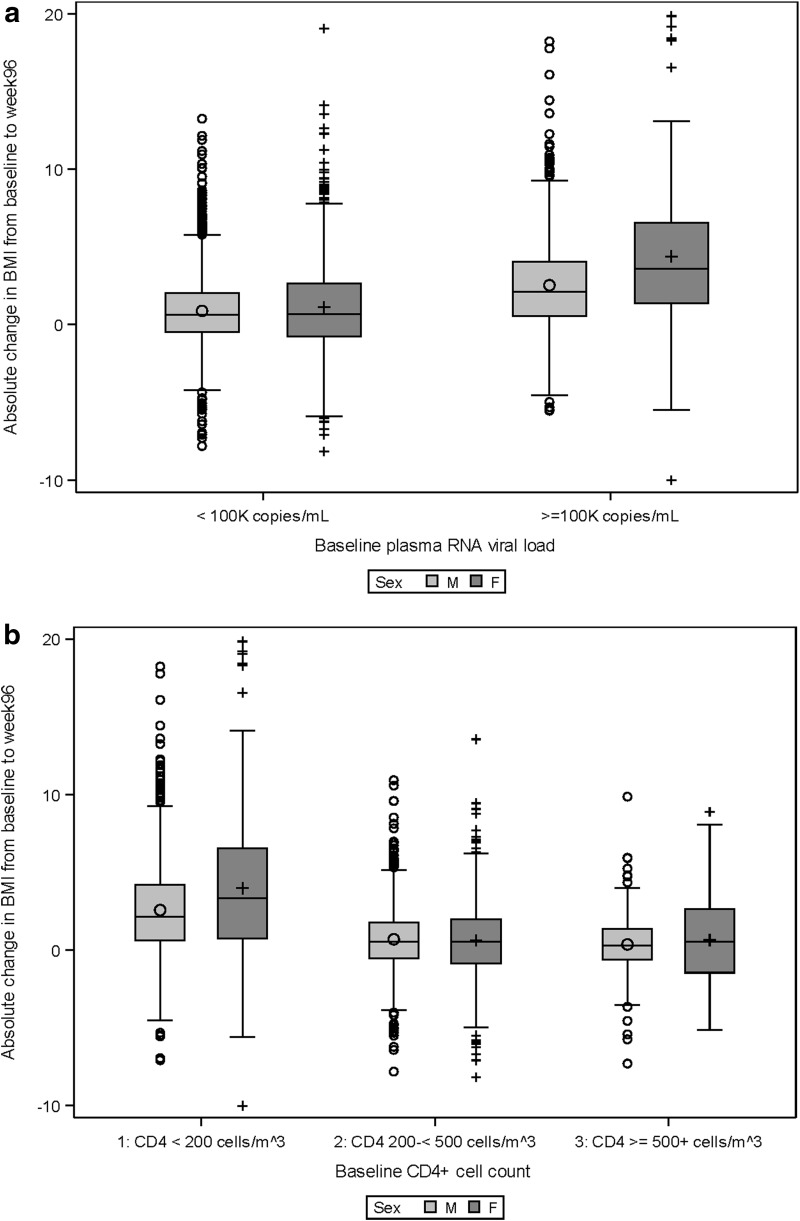
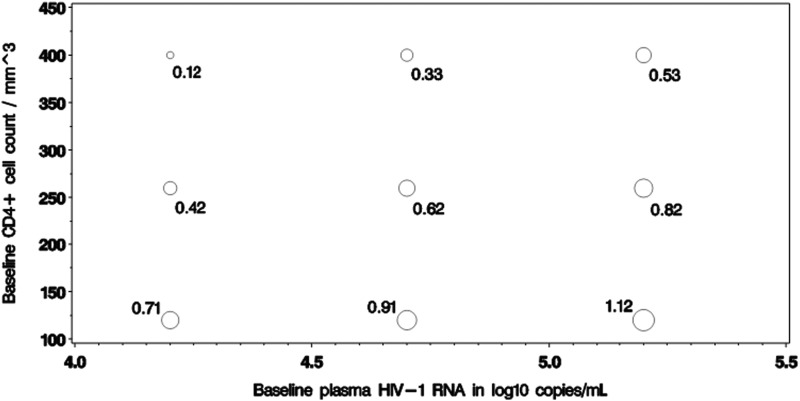
We observed significant two-way statistical interactions between sex and each of pre-ART CD4+ count and HIV-1 viral load such that for subgroups with higher viral load and lower CD4+ count at baseline, the estimated BMI changes in women are even larger than the average estimated difference. Figure 1a and b illustrate the distribution of observed BMI changes over time by sex, and separately by baseline RNA subgroup and CD4 cell count subgroup. Figure 2 illustrates the statistical interactions by showing the estimated sex differences for absolute BMI change to week 96 at various baseline CD4+ and viral load levels. For instance, at CD4 cell count of 260 and HIV-1 viral load of 4.7 log10 copies/mL (i.e., at the observed means for these laboratory values within the study sample), the estimated sex difference was 0.62 kg/m2, which was similar to the overall sex effect of 0.59 kg/m2 estimated in the main effects only multivariable model. However, for those with more advanced HIV disease characteristics—for example, with CD4 cell count of 120 and HIV viral load of 5.2, women gain an excess of 1.12 kg/m2 in BMI more than men over the 96-week follow-up period. For those with less advanced HIV disease characteristics, the estimated sex difference is smaller.
FIG. 1.
(a) Observed BMI changes to week 96 by sex and RNA subgroups. (b) Observed BMI changes to week 96 by sex and CD4 subgroups. BMI, body mass index.
FIG. 2.
Model-based estimates of sex difference (women–men) in BMI changes to week 96 (in kg/m2), at different CD4 cell counts and plasma HIV-1 viral loads.
Sensitivity analysis
A sensitivity analysis was performed among participants who were virologically suppressed at weeks 48 and 96. Overall, 3091 (81%) of 3801 participants were virologically suppressed at both week 48 and 96. This subgroup was representative of the entire BMI analysis sample. The mean observed difference in absolute BMI changes between women and men in this virologically suppressed subgroup was 0.52 kg/m2 (95% CI 0.20–0.85).
Subgroup analysis
A subgroup analysis was performed within the A5257 study to adjust for SES defined by highest education attained, binge alcohol drinking, illicit drug use, smoking status, and history of metabolic syndrome. Participants of the subgroup analysis comprised 43% of the overall BMI analysis and the observed BMI sex difference was 0.59 (95% CI 0.19–0.99) within this subgroup. Overall, the subgroup was fairly representative to the full sample, but differed in the following aspects: (1) type of ART (TDF/FTC + ATV/r was more commonly used than TDF/FTC + DRV/r and TDF/FTC + RAL); (2) higher baseline CD4 cell count and lower baseline HIV-1 viral load; (3) larger proportion of women (24%); and (4) more non-white participants (66%). Both the unadjusted and adjusted sex difference estimate for BMI was 0.59 kg/m2 (for factors included in main effects model). Importantly, this difference in BMI changes by sex was not modified by SES, alcohol or illicit drug use, and metabolic syndrome.
A significant statistical interaction between smoking status and sex was observed (p = 0.03). Among non-smokers, the adjusted sex difference on BMI change to week 96 was estimated as 0.86 kg/m2. This sex difference estimate was attenuated among both former smokers (0.52 kg/m2) and current smokers (−0.04 kg/m2). Current smokers of either sex (38% of participants) gained, on average, the smallest amount of any subgroup defined by sex and smoking status (observed data): there was a mean of 0.83 kg/m2 weight gain among currently smoking men, and mean of 0.93 kg/m2 weight gain among currently smoking women.
Discussion/Conclusions
In this pooled analysis of 3801 individuals initiating ART in randomized clinical trials, we found that HIV-1-infected women experienced a significantly greater increase in BMI following ART initiation than men. This effect was independent of age and ART regimen, and remained after controlling for pre-ART CD4 count and HIV-1 viral load. A large part of the BMI increase occurred in the first 48 weeks following initiation of ART, and we found significant interactions between sex and baseline CD4+ count and HIV-1 viral load such that the estimated BMI increases in women with higher baseline viral loads and CD4+ counts were even greater. From subgroup analysis where additional characteristics were available, we found that the estimated sex difference was not modified by SES, alcohol or illicit drug use, or the metabolic syndrome, but current or former smoking status mitigated the estimated sex difference on BMI change.
Although similar proportions of women and men moved to a worse BMI category, we found that among participants moving to a worse BMI category, women gained more weight than men. Evaluating changes in both BMI category change and absolute BMI change by sex was an essential exercise because sex differences could have been missed or underestimated if either of these endpoints had been evaluated in isolation.
There are conflicting reports in the literature on the relationship between sex and changes in BMI following the initiation of ART.15–18 Our study benefits from the large number of participants and the comprehensive and uniform data that were gathered on participants in the setting of randomized clinical trials within a single HIV treatment network. We found that even after controlling for multiple potential confounders such as age, baseline CD4+ count, baseline HIV-1 viral load, race/ethnicity, study, and study treatment group, there was a robust relationship between female sex and greater increase in BMI after ART initiation. Each ART regimen was evaluated individually rather than by class (e.g., protease inhibitors [PIs] vs. non-PIs), given prior studies have not shown differences in body composition changes in PI-based regimens compared to non-PI-based regimens.29 The underlying reason for the relationship between female sex and greater BMI gain is not known, but deserves further exploration.
A state of chronic inflammation and immune activation has been described in HIV30 and immune activation has been shown to be most robust in those with low CD4+ counts and high HIV-1 viral loads,31 the subgroup in which we observed the greatest difference in BMI changes. This effect is seen in both resource-rich and resource-poor settings.32 Adipose tissue is increasingly recognized as an important metabolically active tissue and a source of bioactive peptides that participate in inflammation and immunity. A complex interplay of factors mediated by nuclear factor kappa-light-chain-enhancer of activated B cell signaling results in disordered inflammatory regulation.33 Adipose tissue has been demonstrated to produce over 50 cytokines34 from adipocytes, macrophages, or other cells of the monocyte lineage.35 Adipocytes induce secretion of inflammatory cytokines, including those interleukins associated with increased mortality and noninfectious morbidities in HIV-infected individuals, such as interleukin-6.36,37 Leptin, a key proinflammatory adipokine, is associated with an inflammatory state in the setting of obesity and its synthesis is increased by female sex hormones.38 We hypothesize that different states of immune activation may underlie the sex difference in BMI gain that we observed in this study. Indeed, prior studies have found that several markers of immune activation are higher in HIV-infected women than in men,39 and some markers of inflammation have been associated with greater gains in fat after ART.40,41
There are several limitations to our study that deserve consideration. One of the ART regimens used in one of the studies, lopinavir/ritonavir, is no longer in common use in the United States. Inclusion of participants randomized to lopinavir/ritonavir and efavirenz may be viewed as a limitation, but the overall results were similar in the A5257 subgroup analysis, and this study included only modern ART regimens. Although we did not have complete data on covariates such as tobacco use, alcohol/substance abuse, physical activity, diet, or SES, we were able to perform a subgroup analysis on A5257 participants to adjust for some of these important variables (SES as defined by highest education attained, alcohol and illicit drug use, smoking, and history of metabolic syndrome). We chose to use education level as a surrogate for SES because this is the only covariate that was available. We recognize that this analysis could have benefited from a more sensitive analysis of SES if additional indicators had been available. Given the randomized nature of the three studies, it is unlikely that the unmeasured covariates would have biased our estimates. Finally, since this was a post hoc analysis with multiple comparisons, marginally significant associations should be interpreted with caution.
One additional point to consider is that, although BMI has been used in many studies and is linked to outcomes such as diabetes and other metabolic diseases,42–44 the results must be interpreted with the understanding that BMI does not allow us to differentiate between fat and lean body mass. While BMI typically correlates with the proportion of total body fat, a normal BMI may represent excess adiposity in persons with low muscle mass. Women in general have lower total muscle mass than men and BMI may therefore underestimate the sex differences in changes in adiposity following the initiation of ART. In addition, current research suggests that the location and type of fat may be important in the inflammatory response and the resultant adverse outcomes. Visceral obesity has been implicated in cardiovascular morbidity45 and further work with this cohort will investigate sex differences in the changes in regional body fat distribution after ART.
In conclusion, we found that in the setting of randomized clinical trials, HIV-1-infected women experienced a significantly greater increase in BMI following ART initiation than men. The fact that these sex differences exist, among persons who are already overweight before starting ART, suggests a problem of clinical significance to women living with HIV. Future work will explore the impact of immune activation on the observed sex differences.
Acknowledgments
The authors would like to thank the study coordinators and participants at each of the study sites for their contributions to this work. S.H.B. has received research funding to her institution from Gilead Sciences. G.A.M. has served as a consultant for Gilead and Merck. This article was written by C.G. in her capacity as an NIH employee, but the views expressed in this article do not necessarily represent those of the NIH. This work was supported by grants from the National Institutes of Health UM1AI068634 and UM1AI068636.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: A collaborative analysis of 14 cohort studies. Lancet 2008;372:293–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lohse N, Hansen AB, Pedersen G, et al. . Survival of persons with and without HIV infection in Denmark, 1995–2005. Ann Intern Med 2007;146:87–95 [DOI] [PubMed] [Google Scholar]
- 3.Palella FJ, Jr., Delaney KM, Moorman AC, et al. . Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV outpatient study investigators. N Engl J Med 1998;338:853–860 [DOI] [PubMed] [Google Scholar]
- 4.Sackoff JE, Hanna DB, Pfeiffer MR, Torian LV. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med 2006;145:397–406 [DOI] [PubMed] [Google Scholar]
- 5.Friis-Moller N, Sabin CA, Weber R, et al. . Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med 2003;349:1993–2003 [DOI] [PubMed] [Google Scholar]
- 6.Long JL, Engels EA, Moore RD, Gebo KA. Incidence and outcomes of malignancy in the HAART era in an urban cohort of HIV-infected individuals. AIDS 2008;22:489–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bedimo RJ, McGinnis KA, Dunlap M, Rodriguez-Barradas MC, Justice AC. Incidence of non-AIDS-defining malignancies in HIV-infected versus noninfected patients in the HAART era: Impact of immunosuppression. J Acquir Immune Defic Syndr 2009;52:203–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel P, Hanson DL, Sullivan PS, et al. . Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med 2008;148:728–736 [DOI] [PubMed] [Google Scholar]
- 9.Marin B, Thiebaut R, Bucher HC, et al. . Non-AIDS-defining deaths and immunodeficiency in the era of combination antiretroviral therapy. AIDS 2009;23:1743–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein D, Hurley LB, Quesenberry CP, Jr., Sidney S. Do protease inhibitors increase the risk for coronary heart disease in patients with HIV-1 infection? J Acquir Immune Defic Syndr 2002;30:471–477 [DOI] [PubMed] [Google Scholar]
- 11.Hsue PY, Giri K, Erickson S, et al. . Clinical features of acute coronary syndromes in patients with human immunodeficiency virus infection. Circulation 2004;109:316–319 [DOI] [PubMed] [Google Scholar]
- 12.Mary-Krause M, Cotte L, Simon A, Partisani M, Costagliola D. Increased risk of myocardial infarction with duration of protease inhibitor therapy in HIV-infected men. AIDS 2003;17:2479–2486 [DOI] [PubMed] [Google Scholar]
- 13.Grinspoon SK, Grunfeld C, Kotler DP, et al. . State of the science conference: Initiative to decrease cardiovascular risk and increase quality of care for patients living with HIV/AIDS: Executive summary. Circulation 2008;118:198–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson-Paul AM, Wei SC, Mattson CL, et al. . Obesity among HIV-infected adults receiving medical care in the United States: Data from the cross-sectional medical monitoring project and national health and nutrition examination survey. Medicine 2015;94:e1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crum-Cianflone N, Roediger MP, Eberly L, et al. . Increasing rates of obesity among HIV-infected persons during the HIV epidemic. PLoS One 2010;5:e10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mulligan K, Harris DR, Monte D, et al. . Obesity and dyslipidemia in behaviorally HIV-infected young women: Adolescent trials network study 021. Clin Infect Dis 2010;50:106–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor BS, Liang Y, Garduno LS, et al. . High risk of obesity and weight gain for HIV-infected uninsured minorities. J Acquir Immune Defic Syndr 2014;65:e33–e40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tate T, Willig AL, Willig JH, et al. . HIV infection and obesity: Where did all the wasting go? Antivir Ther 2012;17:1281–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Achhra AC, Mocroft A, Reiss P, et al. . Short-term weight gain after antiretroviral therapy initiation and subsequent risk of cardiovascular disease and diabetes: The D:A:D study. HIV Med 2016;17:255–268 [DOI] [PubMed] [Google Scholar]
- 20.Amorosa V, Synnestvedt M, Gross R, et al. . A tale of 2 epidemics: The intersection between obesity and HIV infection in Philadelphia. J Acquir Immune Defic Syndr 2005;39:557–561 [PubMed] [Google Scholar]
- 21.Messina J, McCall J, Barron A. Overweight and obesity status in an urban Canadian HIV outpatient population. J Assoc Nurs AIDS Care 2014;25:652–656 [DOI] [PubMed] [Google Scholar]
- 22.Hasse B, Iff M, Ledergerber B, et al. . Obesity trends and body mass index changes after starting antiretroviral treatment: The Swiss HIV cohort study. Open Forum Infect Dis 2014;1:ofu040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma A, Bynum SA, Schneider MF, et al. . Changes in body mass index following HAART initiation among HIV-infected women in the women's interagency HIV study. J AIDS Clin Res 2014;5:1000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koethe JR, Jenkins CA, Lau B, et al. . Rising obesity prevalence and weight gain among adults starting antiretroviral therapy in the United States and Canada. AIDS Res Hum Retrovirus 2015;32:50–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lakey W, Yang LY, Yancy W, Chow SC, Hicks C. Short communication: From wasting to obesity: Initial antiretroviral therapy and weight gain in HIV-infected persons. AIDS Res Hum Retrovirus 2013;29:435–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riddler SA, Haubrich R, DiRienzo AG, et al. . Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med 2008;358:2095–2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daar ES, Tierney C, Fischl MA, et al. . Atazanavir plus ritonavir or efavirenz as part of a 3-drug regimen for initial treatment of HIV-1. Ann Intern Med 2011;154:445–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lennox JL, Landovitz RJ, Ribaudo HJ, et al. . Efficacy and tolerability of 3 nonnucleoside reverse transcriptase inhibitor-sparing antiretroviral regimens for treatment-naive volunteers infected with HIV-1: A randomized, controlled equivalence trial. Ann Intern Med 2014;161:461–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McComsey GA, Moser C, Currier J, et al. . Body composition changes after initiation of raltegravir or protease inhibitors: ACTG A5260s. Clin Infect Dis 2016;62:853–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunt PW. HIV and inflammation: Mechanisms and consequences. Curr HIV/AIDS Rep 2012;9:139–147 [DOI] [PubMed] [Google Scholar]
- 31.Sousa AE, Carneiro J, Meier-Schellersheim M, Grossman Z, Victorino RM. CD4 T cell depletion is linked directly to immune activation in the pathogenesis of HIV-1 and HIV-2 but only indirectly to the viral load. J Immunol 2002;169:3400–3406 [DOI] [PubMed] [Google Scholar]
- 32.Mave V, Erlandson KM, Gupte N, et al. . Inflammation and change in body weight with antiretroviral therapy initiation in a multinational cohort of HIV-infected adults. J Infect Dis 2016;214:65–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanyal A, Naumann J, Hoffmann LS, et al. . Interplay between obesity-induced inflammation and cGMP signaling in white adipose tissue. Cell Rep 2017;18:225–236 [DOI] [PubMed] [Google Scholar]
- 34.Lago F, Dieguez C, Gomez-Reino J, Gualillo O. The emerging role of adipokines as mediators of inflammation and immune responses. Cytokine Growth Factor Rev 2007;18:313–325 [DOI] [PubMed] [Google Scholar]
- 35.Goyal A, Nimmakayala K, Zonszein J. Is there a paradox in obesity? Cardiol Rev 2014;22:163–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nordell AD, McKenna M, Borges AH, et al. . Severity of cardiovascular disease outcomes among patients with HIV is related to markers of inflammation and coagulation. J Am Heart Assoc 2014;3:e000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grund B, Baker JV, Deeks SG, et al. . Relevance of interleukin-6 and D-dimer for serious non-AIDS morbidity and death among HIV-positive adults on suppressive antiretroviral therapy. PLoS One 2016;11:e0155100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abella V, Scotece M, Conde J, et al. . Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat Rev Rheumatol. 2017;13:100–109 [DOI] [PubMed] [Google Scholar]
- 39.Borges AH, O'Connor JL, Phillips AN, et al. . Factors associated with D-dimer levels in HIV-infected individuals. PLoS One 2014;9:e90978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erlandson KM, Kitch D, Tierney C, et al. . Weight and lean body mass change with antiretroviral initiation and impact on bone mineral density. AIDS 2013;27:2069–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelesidis T, Tran TT, Stein JH, et al. . Changes in inflammation and immune activation with atazanavir-, raltegravir-, darunavir-based initial antiviral therapy: ACTG 5260s. Clin Infect Dis 2015;61:651–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Butt AA, McGinnis K, Rodriguez-Barradas MC, et al. . HIV infection and the risk of diabetes mellitus. AIDS 2009;23:1227–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Capeau J, Bouteloup V, Katlama C, et al. . Ten-year diabetes incidence in 1046 HIV-infected patients started on a combination antiretroviral treatment. AIDS 2012;26:303–314 [DOI] [PubMed] [Google Scholar]
- 44.Herrin M, Tate JP, Akgun KM, et al. . Weight gain and incident diabetes among HIV-infected veterans initiating antiretroviral therapy compared with uninfected individuals. J Acquir Immune Defic Syndr 2016;73:228–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Autieri MV. Adipose inflammation at the heart of vascular disease. Clin Sci (Lond) 2016;130:2101–2104 [DOI] [PMC free article] [PubMed] [Google Scholar]