Highlights
-
•
Endometrial hyperplasia/carcinoma regression rates with LNG-IUS were examined by BMI.
-
•
Morbidly obese patients with EH/EHA/EC are more likely to progress.
-
•
Despite addition of oral progesterone to LNG-IUS, morbid obesity increases the odds of progression.
1. Introduction
Approximately 2.8% of women will be diagnosed with endometrial carcinoma (EC) during their lifetime, based on 2012–2014 data (National Cancer Institute, 2016). Due to early diagnosis and treatment, EC is only the eighth leading cause of cancer deaths, with an 81.7% five year survival rate.
There are many known risk factors in the development of EC, such as obesity, exogenous estrogen, nulliparity, history of diabetes, and hypertension, (Barakat, 2013). Obesity is a significant risk factor that is widely prevalent today. Obese patients are at an increased risk of morbidity as a result of their cancer as compared to normal weight women with the same pathologic carcinoma (Reeves et al., 2011).
Obese women are also at an increased risk for the development of endometrial hyperplasia, the precursor to EC. Untreated hyperplasia without atypia (EH) has a 1.6% risk of progression to EC, while untreated atypical hyperplasia (EHA) has a 20–30% risk of progression to EC (Barakat, 2013; Lacey et al., 2010). Given the high rate of progression in patients with EHA, the standard of care for women is a hysterectomy (American College of Obstetricians and Gynecologists, 2015). However, a hysterectomy is not always a feasible option for women with high risk peri-operative morbidity due to underlying medical conditions and morbid obesity. For this reason, there is a growing need for alternative conservative therapies in these patients.
Levonorgestrel-releasing intrauterine system (LNG-IUS) as well as oral progestin have been used to effectively treat EH/EHA with regression rates varying from 66% to 92% (Hubbs et al., 2013; Gallos et al., 2013; Wildemeersch et al., 2007). However, the efficacy of LNG-IUS therapy has not been looked at specifically in the obese population. As the prevalence of non-surgical candidates secondary to obesity increases, the need for more appropriate and feasible treatment of EH/EHA/EC is necessary. We aim to provide an overall characterization of the patients being offered LNG-IUS therapy at a single institution with corresponding response rates. Further, we wish to provide insight into the response rates stratified by body mass index (BMI).
2. Methods
A retrospective analysis was performed consisting of cases from two tertiary care hospitals. The records of all patients diagnosed with EH/EHA/EC and had a LNG-IUS placed between January 1, 2009 and January 1, 2017 with repeat endometrial sample were included. Patients were identified using ICD-9 and ICD-10 codes for endometrial pathology (182.0, 621.2, 621.3, 179, 233.2, 239.5, N85.0, C55, and C54) and CPT codes (58300) for LNG-IUS placement.
After review of 172 patients identified, 88 patients were excluded for incorrect diagnosis in EMR (n = 34), paraguard IUD placement (n = 8), pathology resolved prior to LNG-IUS placement (n = 25) and no follow up or insufficient data (n = 21). Data was obtained including patient demographics, diagnosis, receipt of concurrent oral progesterone, and repeat sampling results. Patients were grouped by BMI using WHO classification: normal (BMI <25 kg/m2) and overweight (BMI 25-30 kg/m2), class I and II obesity (BMI 30-40 kg/m2), and class III obesity (>40 kg/m2). The first endometrial sampling post LNG-IUS insertion was compared to the patient's initial diagnosis. Progression was defined as the repeat sampling biopsy showing: 1) a more severe form of hyperplasia, 2) progression EC (if prior diagnosis was EH/EGA) or 3) progression to a higher grade of EC. Persistent diagnosis was defined as repeat sampling biopsy matching the initial diagnosis. Regression was defined as repeat sampling biopsy showing: 1) a less severe form of hyperplasia, 2) transition from EC down to EH/EHA or 3) normal endometrium. Persistent disease and regression were combined into a “no-progression” category for statistical analysis as these outcomes are considered adequate in patients that are unable to undergo or decline surgical management and are using a LNG-IUS for treatment or a means to allow time to medically optimize patients.
Continuous variables were summarized using means, medians, SDs, and IQRs where appropriate, while frequencies were used to describe categorical variables. Univariate associations of continuous variables and BMI groups were assessed using one-way ANOVA or Kruskall-Wallis tests. Pearson Chi-square and Fisher's exact tests were used to test associations between BMI groups and categorical variables. Differences were considered significant when p ≤ 0.05. Linear regression was used to assess the relationship between BMI and follow up results. Multivariable logistic regression was used to assess risk of progression and adjust for covariates of interest. Statistical analysis was performed using Stata 14.2 software.
3. Results
Sixty women were identified who met the inclusion criteria. Demographic characterization of the cohort is presented in Table 1. The average age of the cohort was 47.4 ± 12.7 years. The majority of patients were Caucasian (63.3%), non-smokers (66.7%), and 48.3% (n = 29) were nulliparous. Notably, the mean BMI of the patients being treated with LNG-IUS was 42.5 kg/m2 ± 13.5 kg/m2 (254.9 lb. ± 80.9 lb). Twelve patients (20%) in the study population had a BMI < 30 kg/m2, 17 patients (28.3%) had a BMI between 30 kg/m2 and 40 kg/m2, and 31 patients (51.7%) had a BMI > 40 kg/m2. The most prevalent medical comorbidities included hypertension (45%) and diabetes (26.7%).
Table 1.
Demographics. Baseline demographics of all patients using the LNG-IUS for treatment of EH/EHA/EC stratified by BMI.
| Cohort |
BMI <30 |
BMI 30–40 |
BMI >40 |
p-value | |
|---|---|---|---|---|---|
| n = 60 n (%) | n = 12 n (%) | n = 17 n (%) | n = 31 n (%) | ||
| Age (years) | 47.4(±12.7) | 46.8 (±10.0) | 46.4 (±14.4) | 48.1(±13.0) | 0.899 |
| BMI (kg/m2) | 42.5(±13.5) | 25.9 (±2.4) | 34.6(±2.6) | 53.2(±9.3) | <0.001 |
| Nulliparous | 29 (48.3) | 5 (41.7) | 9 (52.9) | 15 (48.4) | 0.998 |
| Endometrial strip (mm) | 10 (7–14) | 8.5(4–13) | 8 (5.5–16) | 10(8–15) | 0.456 |
| Smoking history | |||||
| None | 40 (66.7) | 7(58.3) | 12(70.6) | 21(67.7) | 0.923 |
| History | 17 (28.3) | 4(33.3) | 4(23.5) | 9(29.0) | |
| Current | 3(5) | 1(8.3) | 1(5.8) | 1(3.2) | |
| Race | |||||
| White | 38 (63.3) | 9 (75.0) | 10 (58.8) | 19 (61.3) | 0.476 |
| Black | 14(23.3) | 1 (8.3) | 4(23.5) | 9(29.0) | |
| Asian | 4(6.7) | 2 (16.7) | 1 (5.88) | 1(3.23) | |
| Other | 4(6.7) | 0 | 2 (11.7) | 2(6.45) | |
| Comorbidities | |||||
| HTN | 27 (45) | 1 (8.3) | 7 (41.2) | 19 (61.3) | 0.006 |
| DM | 16 (26.7) | 2 (16.7) | 6 (35.3) | 8 (25.8) | 0.543 |
| Arthritis | 8 (13.3) | 1 (8.3) | 2 (11.8) | 5 (16.1) | 0.786 |
| Depression | 13 (21.7) | 4 (33.3) | 2 (11.8) | 7 (22.6) | 0.388 |
| HLD | 11 (18.3) | 1 (8.3) | 4(23.5) | 6 (19.4) | 0.581 |
| Thyroid disease | 8 (13.3) | 2 (16.7) | 1 (5.9) | 5 (16.1) | 0.578 |
| Sleep apnea | 5 (8.3) | 0 (0) | 0 (0) | 5 (16.1) | 0.079 |
| Asthma | 7 (11.7) | 0 (0) | 2(11.8) | 5 (16.1) | 0.348 |
| Pathology | |||||
| EH | 24(40) | 8 (66.7) | 7(41.2) | 9(29.0) | 0.057 |
| EHA | 18(30) | 1 (8.3) | 3(17.7) | 14(45.2) | |
| EC | 18(30) | 3(25.0) | 7(41.2) | 8(25.8) | |
| IUD reason | |||||
| Desires expectant management | 17 (28.3) | 7(58.3) | 4(23.5) | 6(19.4) | 0.032 |
| Desires fertility | 17(28.3) | 4(33.3) | 6(35.3) | 7(22.6) | |
| Not surgical candidate | 26(43.3) | 1(8.3) | 7(41.2) | 18(58.1) | |
| Concurrent Progesterone based therapy | 22(36.7) | 4(33.3) | 6(35.3) | 12(38.7) | 0.941 |
| Initial follow up EMB time | 4.1 months (3.2–6.3) | 5.8 months (4.5–6.7) | 4.1 months (3.0–6.3) | 3.6 months (3.2–5.9) | 0.112 |
The most common indication for LNG-IUS treatment was poor surgical candidate (n = 26), followed by a desire for preservation of fertility (n = 17) and a desire for conservative medical treatment (n = 17). Twenty-four patients were diagnosed with EH (40%). The median time from LNG-IUS insertion to repeat endometrial biopsy was 4.1 months (IQR 3.2–6.4).
Repeat endometrial biopsy resulted in regression in 41 patients (68.3%). Twelve patients (20%) had persistent disease on repeat sampling, and 7 patients (11.7%) experienced progression. Of the patients with the initial diagnosis of EH (n = 24), 83.3 (n = 20) experienced regression while 91.7% (n = 22) had regression or persistent disease. The patients with initial diagnosis of EHA (n = 18) experienced lower rates of regression (66.6%, n = 12), but maintained high rates of regression or persistent disease in 83.3% (n = 15). Similarly those with the initial diagnosis of EC (n = 18) had 50% regression rate (n = 9) but regression or persistent disease in 88.9%(n = 16). Initial diagnosis was not statistically associated with follow-up diagnosis (p = 0.123) (Table 2).
Table 2.
Follow up endometrial sampling stratified by initial diagnosis (p = 0.129).
| Initial diagnosis |
|||
|---|---|---|---|
| Follow up result | EH |
EHA |
EC |
| n = 24 n (%) | n = 18 n (%) | n = 18 n (%) | |
| Regression | 20 (83.3) | 12 (66.6) | 9 (50) |
| Persistent | 2 (8.3) | 3 (16.6) | 7 (38.9) |
| Progression | 2 (8.3) | 3 (16.6) | 2 (11.1) |
Abbreviations: EH, endometrial hyperplasia; EHA, endometrial hyperplasia with atypia; EC, endometrial carcinoma.
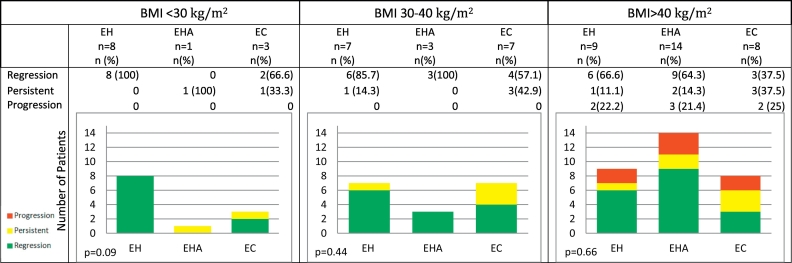
Treatment response was then stratified by BMI. Of the BMI < 30 kg/m2 cohort, 10 patients (83.3%) had regression and the remaining patients had persistent disease (n = 2). No patients experienced progression of their disease. Within the BMI 30 kg/m2 to 40 kg/m2 cohort, 13 patients (76.5%) experienced regression and the remaining 4 patients (23.5%) had persistent disease. Again, no patient experienced progression. In the BMI > 40 kg/m2 cohort, 18 patients (58.1%) experienced regression of their diagnosis while 6 patients (19.4%) had persistent disease. Contrary to other cohorts, 7 patients (22.6%) had progression of disease. Even when stratified by BMI, initial diagnosis was not associated with follow-up diagnosis (p = 0.09, 0.44, 0.66) (Table 3). However, progression was statistically associated with BMI given only the BMI >40 kg/m2 group had progression of disease (p = 0.03). Using linear regression, the mean BMI for those that progressed was 53.1 ± 9.8 kg/m2 compared to 41.1 ± 3.6 kg/m2 in those that did not progress (p = 0.026).
Table 3.
Follow up endometrial sampling stratified by BMI (p = 0.03). Graphical representation of results of repeat endometrial sampling following the use of LNG-IUS stratified by BMI and initial endometrial sampling.
Abbreviations: EH, endometrial hyperplasia; EHA, endometrial hyperplasia with atypia; EC, endometrial carcinoma.
As expected, higher BMI was associated with worse initial pathology. The mean BMI of those with EH was 38.2 ± 5.4 kg/m2 while the mean BMI of those with EHA or EC was 45.3 ± 4.4 kg/m2 (p = 0.044). Adjusting for initial diagnosis, progression remained significantly associated with a higher BMI of 48.4 ± 10.3 kg/m2 compared to 43.8 ± 4.5 kg/m2 (p = 0.034) in non-progressors.
Additionally, concurrent progesterone was given to 36.7% of patients and distributed equally among BMI groups. Adjusting for concurrent progesterone, mean BMI in those that progressed remained statistically higher than the mean BMI in those that did not progress (55.5 ± 13.1 kg/m2 vs. 39.1 ± 6.2 kg/m2, p = 0.021).
4. Discussion
The preservation of fertility among women with EH/EHA/EC has been extensively studied. This includes using progesterone and/or LNG-IUS for treatment until desired fertility has been attained and hysterectomy can be performed. Response rates have varied between 58% and 100% (Gotlieb et al., 2003; Gallos et al., 2012).
Although the majority of the literature available has reported response rates following LNG-IUS without addressing stratification of BMI, a small amount of literature has looked at the treatment of EH/EHA/EC specifically in the obese population. Currently in the United States, 75.1% of adults have a BMI >25 kg/m2, 35.7% have a BMI >30 kg/m2 and 6.3% have a BMI >40 kg/m2 (Jensen et al., 2014). Our series mimics these attributes with the average BMI being 42.5 ± 13.5 kg/m2. As weight increases, not only are medical comorbidities more prevalent (Arem & Irwin, 2013), but surgical morbidity and mortality increase as well (Walker et al., 2009). Patients often have diabetes, cardiac disease, and other processes that affect intra-operative difficulty and post-operative recovery. Further, Calle et al. have shown that women with a BMI of at least 40 kg/m2 were 6.25 (CI 3.75-10.42) times more likely to die from uterine cancer (Calle et al., 2003). Several studies have shown an increased risk of EH/EHA/EC in the obese population, as well as their increased risk of morbidity and mortality from standard treatment (Calle et al., 2003; Renehan et al., 2008). Our series shows that higher BMI was associated with a higher likelihood of EHA/EC compared to EH and further, higher BMI was associated with an increased risk of progression.
In this series, the use of LNG-IUS therapy in place of hysterectomy was performed predominantly because of poor surgical candidacy. Given the importance in lack of progression during medical optimization prior to surgery or during treatment in those that cannot undergo surgery, no progression compared to progression was compared. We found that the use of LNG-IUS resulted in no progression in 88.3% of all patients. Stratifying by BMI, no progression was found in 100% of patients with BMI < 30 kg/m2 and BMI 30–40 kg/m2 groups. Alternatively, in the BMI > 40 kg/m2 group, 77.4% experienced no progression. Progression occurred only in the BMI > 40 kg/m2 group. This data suggests that LNG-IUS therapy may not be as effective at preventing progression with increasing BMI, particularly in patients with class III obesity.
The use of exogenous progesterone compared to LNG-IUS in the treatment of EH/EHA/EC has also been studied with varying regression rates (Hubbs et al., 2013; Gallos et al., 2013; Kim et al., 2011). Some data suggests that LNG-IUS achieves higher regression rates compared to exogenous progesterone (66% compared to 92%) (Gallos et al., 2013). Few studies have looked at concurrent progesterone and LNG-IUS. In our study, 36.2% of patients received concurrent progesterone and was distributed equally in each BMI sub-category. Adjusting for concurrent progesterone, BMI remained associated with progression, therefore suggesting that combined systemic plus local progesterone may remain less effective in patients with higher BMI.
We acknowledge that there are limitations to this study, however, it was meant predominantly as exploratory using a small number of retrospectively collected cases. Even though from a single institution, there are multiple centers with different practice patterns. Further, this enabled consistent pathology reporting between initial and follow-up specimens. We also acknowledge that the median re-sampling time for this cohort was 4 months (IQR 3.2-6.4) and that there is evidence to suggest that optimal response time may be 6–12 months. Therefore, these patients may still respond if they were to be re-sampled further from placement of LNG-IUS. In 2009, there was not an accepted standard for the length of time between LNG-IUS and follow-up endometrial biopsy and these results reflect this deviation from today's standard.
In the future, we will await the results of the current clinical trial studying the result of oral progesterone versus oral progesterone and LNG-IUG. Further, a randomized clinical trial of LNG-IUS versus LNG-IUS with oral progesterone would be most beneficial at further elucidating the role of LNG-IUS therapy specifically in obese patients. Alternately, given more progression in the most obese patients, additional therapies or therapy combinations should be investigated. It may also be pertinent to study serial repeat endometrial sampling in this subgroup to further elicit the role of BMI on disease progression.
In conclusion, this study was meant to be a descriptive case series analysis of the role of LNG-IUS in obese women with EH/EHA/EC. The results reveal that in patients with BMIs 40+ kg/m2, treatment outcomes using LNG-IUS were more varied, and were more likely to experience disease progression compared to patients with lower BMI. We further suggest that regardless of initial diagnosis or concurrent progesterone therapy with LNG-IUS, higher BMI is associated with progression of disease.
Author contribution
Ashley Graul was the primary researcher who was responsible for the final manuscript, data collection oversight, initial data analysis and submission with the oversight of Sarah Kim as the Principal Investigator. Elise Wilson contributed to a majority of the data collection as well as initial drafts of the manuscript. Emily Ko contributed to the development of the clinical question as well as manuscript revisions and critique. Ashley Haggerty was also involved in assisting in data collection technique as well as manuscript revision and critique. Helen Reed contributed to initial data collection. Nathanael Koelper is a statistician who confirmed appropriate statistical analysis was used. All authors have approved the final manuscript.
Disclosures
All authors listed have approved the final manuscript.
Dr. Graul reports a T32 grant from NIH Reproductive Epidemiology during the conduct of the study. There is no conflict of interest for all other authors in this study.
References
- American College of Obstetricians and Gynecologists Endometrial cancer. Obstet. Gynecol. 2015;125(4):1006–1026. [Google Scholar]
- Arem H., Irwin M.L. Obesity and endometrial cancer survival: a systematic review. Int. J. Obes. 2013;37(5):634–639. doi: 10.1038/ijo.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakat R.R. 6th ed. Wolters Kluwer Health/Lippincott Williams & Wilkins; 2013. Principles and Practice of Gynecologic Oncology. (847 p) [Google Scholar]
- Calle E.E., Rodriguez C., Walker-Thurmond K., Thun M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. (Apr 24) [DOI] [PubMed] [Google Scholar]
- Gallos I.D., Yap J., Rajkhowa M., Luesley D.M., Coomarasamy A., Gupta J.K. Regression, relapse, and live birth rates with fertility-sparing therapy for endometrial cancer and atypical complex endometrial hyperplasia: a systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2012;207(4):266.e1–266.e12. doi: 10.1016/j.ajog.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Gallos I.D., Ganesan R., Gupta J.K. Prediction of regression and relapse of endometrial hyperplasia with conservative therapy. Obstet. Gynecol. 2013 Jun;121(6):1165–1171. doi: 10.1097/AOG.0b013e31828cb563. [DOI] [PubMed] [Google Scholar]
- Gotlieb W.H., Beiner M.E., Shalmon B., Korach Y., Segal Y., Zmira N. Outcome of fertility-sparing treatment with progestins in young patients with endometrial cancer. Obstet. Gynecol. 2003 Oct;102(4):718–725. doi: 10.1016/s0029-7844(03)00667-7. [DOI] [PubMed] [Google Scholar]
- Hubbs J.L., Saig R.M., Abaid L.N., Bae-Jump V.L., Gehrig P.A. Systemic and local hormone therapy for endometrial hyperplasia and early adenocarcinoma. Obstet. Gynecol. 2013 Jun;121(6):1172–1180. doi: 10.1097/AOG.0b013e31828d6186. [DOI] [PubMed] [Google Scholar]
- Jensen M.D., Ryan D.H., Apovian C.M., Ard J.D., Comuzzie A.G., Donato K.A. 2013 AHA/ACC/TOS Guideline for the management of overweight and obesity in adults. Circulation. 2014;129(25 suppl 2):S102–S138. doi: 10.1161/01.cir.0000437739.71477.ee. (Jun 24) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.K., Yoon B.S., Park H., Seong S.J., Chung H.H., Kim J.W. Conservative treatment with medroxyprogesterone acetate plus levonorgestrel intrauterine system for early-stage endometrial cancer in young women: pilot study. Int. J. Gynecol. Cancer. 2011;21(4):673–677. doi: 10.1111/IGC.0b013e3181fd9a06. (May) [DOI] [PubMed] [Google Scholar]
- Lacey J.V., Sherman M.E., Rush B.B., Ronnett B.M., Ioffe O.B., Duggan M.A. Absolute risk of endometrial carcinoma during 20-year follow-up among women with endometrial hyperplasia. J. Clin. Oncol. 2010;28(5):788–792. doi: 10.1200/JCO.2009.24.1315. (Feb 10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute SEER Cancer Stat Facts: Endometrial Cancer. 2016. https://seer.cancer.gov/statfacts/html/corp.html [Internet], Available from:
- Reeves K.W., Carter G.C., Rodabough R.J., Lane D., McNeeley S.G., Stefanick M.L. Obesity in relation to endometrial cancer risk and disease characteristics in the Women's Health Initiative. Gynecol. Oncol. 2011 May 1;121(2):376–382. doi: 10.1016/j.ygyno.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renehan A.G., Tyson M., Egger M., Heller R.F., Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. doi: 10.1016/S0140-6736(08)60269-X. (Feb 16) [DOI] [PubMed] [Google Scholar]
- Walker JL, Piedmonte MR, Spirtos NM, Eisenkop SM, Schlaerth JB, Mannel RS, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: gynecologic oncology group study LAP2. J. Clin. Oncol. 2009 [cited 2018 Aug 27];27(32):5331–6. (Nov 10), [Internet]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19805679. [DOI] [PMC free article] [PubMed]
- Wildemeersch D., Janssens D., Pylyser K., De Wever N., Verbeeck G., Dhont M. Management of patients with non-atypical and atypical endometrial hyperplasia with a levonorgestrel-releasing intrauterine system: long-term follow-up. Maturitas. 2007 Jun 20;57(2):210–213. doi: 10.1016/j.maturitas.2006.12.004. [DOI] [PubMed] [Google Scholar]