Abstract
Most patients with acute low back pain (LBP), with or without radiculopathy, have substantial improvements in pain and function in the first 4 weeks, and they do not require routine imaging. Imaging is considered in those patients who have had up to 6 weeks of medical management and physical therapy that resulted in little or no improvement in their LBP. It is also considered for those patients presenting with suspicion for serious underlying conditions, such as cauda equina syndrome, malignancy, fracture and infection. In western country primary care settings, the prevalence has been suggested to be 0.7% for metastatic cancer, 0.01% for spinal infection and 0.04% for cauda equina syndrome. Of the small proportion of patients with any of these conditions, almost all have an identifiable risk factor. Osteoporotic vertebral compression fractures (4%) and inflammatory spine disease (<5%) may cause LBP, but these conditions typically carry lower diagnostic urgency. Imaging is an important driver of LBP care costs, not only because of the direct costs of the test procedures but also because of the downstream effects. Unnecessary imaging can lead to additional tests, follow-up, referrals and may result in an invasive procedure of limited or questionable benefit. Imaging should be delayed for 6 weeks in patients with nonspecific LBP without reasonable suspicion for serious disease.
The translational potential of this article: Diagnostic imaging studies should be performed only in patients who have severe or progressive neurologic deficits or are suspected of having a serious or specific underlying condition. Radiologists can play a critical role in decision support related to appropriateness of imaging requests, and accurately reporting the potential clinical significance or insignificance of imaging findings.
Keywords: Imaging, Low back pain, Natural history, Radicular pain, Radiculopathy, Spine
Introduction
Low back pain (LBP) is defined by the location of pain, typically between the lower rib margins and the buttock creases. It is commonly accompanied by pain in one or both legs, and some people with LBP have associated neurological symptoms in the lower limbs. LBP has a high prevalence, affecting up to two-thirds of adults at some point in their lifetime. According to the Institute for Clinical Systems Improvement, the duration of 0–6 weeks is defined as acute LBP, 6–12 weeks as subacute LBP and >12 weeks as chronic LBP [1]. The economic impact of chronic LBP stems from prolonged loss of function, resulting in loss of work productivity, treatment costs and disability payments. Back pain treatment is costly and frequently includes overuse of treatments that are unsupported by clinical guidelines.
LBP is a symptom not a disease and can result from several different known or unknown abnormalities or diseases [2]. For most patients presenting with LBP, the specific nociceptive source cannot be identified, and those affected are classified as having “nonspecific LBP”. The initial evaluation, including a history and physical examination, of patients with LBP should attempt to place patients into one of the following categories: (1) nonspecific LBP; (2) LBP associated with radiculopathy or spinal stenosis; (3) LBP referred from a nonspinal source or (4) LBP associated with other specific spinal causes (Table 1). The medical history should include questions about osteoporosis, osteoarthritis and cancer as well as a review of any prior imaging studies. Review of symptoms should focus on unexplained fevers, weight loss, morning stiffness, gynaecologic symptoms, and urinary and gastrointestinal problems. The physical examination should include the straight leg raise and a focused neuromuscular examination. Testing deep tendon reflexes, strength and sensation can help identify which nerve roots are involved [3].
Table 1.
Differential causes of low back pain.
| Nonspecific or idiopathic (70%) | Mechanical low back or leg pain (27%) | Nonmechanical spinal conditions (about 1%) | Visceral disease (2%) |
|---|---|---|---|
| Lumbar strain, sprain | Degenerative disks and facets (10%) | Neoplasia (0.7%) | Disease of pelvic organs |
| Herniated disc (4%) | Multiple myeloma | Prostatitis | |
| Spinal stenosis (3%) | Metastatic carcinoma | Endometriosis | |
| Osteoporotic compression fracturea (4%) | Lymphoma and leukaemia | Chronic pelvic inflammatory disease | |
| Spondylolisthesis (2%) | Spinal cord tumours | Renal disease | |
| Traumatic fracturea (<1%) | Retroperitoneal tumours | Nephrolithiasis | |
| Congenital disease (<1%) | Primary vertebral tumours | Pyelonephritisa | |
| Severe kyphosis | Infectiona (0.01%) | Perinephric abscessa | |
| Severe scoliosis | Osteomyelitis | Aortic aneurysm | |
| Transitional vertebrae | Septic diskitis | Gastrointestinal disease | |
| Spondylolysis | Paraspinous abscess | Pancreatitis | |
| Diskogenic low back pain | Epidural abscess | Cholecystitis | |
| Presumed instability | Shingles | Penetrating ulcer | |
| Inflammatory arthritis (often associated with human leucocyte antigen-B27) (0.3%) | |||
| Ankylosing spondylitis | |||
| Psoriatic spondylitis | |||
| Reiter's syndrome | |||
| Inflammatory bowel disease | |||
| Scheuermann's disease (osteochondrosis) | |||
| Paget's disease of bone |
Modified from: Deyo RA, Weinstein JN. Low back pain. N Engl J Med. 2001; 344:363–370.
Indicates conditions more likely to present as acute low back pain.
Overuse of imaging for LBP is common in clinical practice [4]. Though overuse of imaging for LBP has long been noted as a problem, yet the use of imaging [particularly computed tomography (CT)/magnetic resonance imaging (MRI)] continues to increase. Despite numerous published guidelines for the management of LBP, one US study [5] shows a substantial inappropriate increase in advanced diagnostic imaging for LBP during the 12-year period from January 1999 to December 2010. Some of the key challenges to implementing good practice for LBP imaging include short consultation times, clinicians' misconceptions about clinical guidelines, fear of litigation in the event of missed rare serious pathologies and a desire to maintain harmonious relationships with patients [6]. It has been shown that implementation of recommended guidelines needs regular repetition or to be continuous to effectively change the practice for LBP [7]. To be effective, efforts to reduce imaging overuse should be multifactorial and address clinician behaviours, patient expectations and education and financial incentives [8]. The examples from USA and UK showed that good supports can change clinical practice, such as the use of a special radiograph requisition form that allowed only guideline-appropriate indications, which led to a 36.8% reduction in lumbar spine imaging [9], and the addition of short educational messages to all reports of lumbar spine MRIs reduced imaging rates by 22.5% [10]. This review describes the recent guidelines of imaging for LBP and updates the available evidences on relevance of degenerative spine abnormalities for LBP.
Current position of the American College of Physicians, American Pain Society, American College of Radiology and European guidelines on imaging for LBP
Most cases of uncomplicated LBP are assumed to result from muscle sprains and strains, ligamentous injuries and spinal degenerative changes. Lumbar imaging abnormalities are common in persons without LBP and are only loosely associated with back symptoms [11]. The presence of imaging abnormalities does not mean that the abnormalities are responsible for symptoms [12]. No evidence suggests that selecting therapies on the basis of the presence of the most common imaging findings improves outcomes compared with a generalised approach [13]. A prospective study found that among patients with lumbar imaging abnormalities before the onset of LBP, 84% had unchanged or improved findings after symptoms developed [14].
Most acute episodes of LBP are self-limiting, and imaging has limited utility because most patients with LBP have nonspecific findings on imaging studies [15]. The American College of Physicians and American Pain Society LBP guideline [13], as well as the appropriateness criteria of the American College of Radiology [16], recommend selective imaging for patients in whom imaging examination is clinically indicated. Nearly all other guidelines, such as the national guidelines of European countries [17], [18], [19], [20] and the guideline on chiropractic management of LBP [21], made similar recommendations. Those deemed to be interventional candidates, with LBP lasting for >6 weeks having completed conservative management with persistent radiculopathic symptoms, may seek imaging. Diagnostic imaging is indicated for patients with LBP if they have severe progressive neurologic deficits or signs or symptoms that suggest a serious or specific underlying condition. Serious underlying conditions associated with LBP include cancer, infection and cauda equina syndrome. About 0.7% of patients with LBP in primary care settings have metastatic cancer, 0.01% have spinal infection and 0.04% have cauda equina syndrome [22], [23]. Osteoporotic vertebral compression fractures (4%) and inflammatory spine diseases (<5%) may also cause LBP, but these conditions typically carry lower diagnostic urgency [23], [24]. Cancer that has metastasised to spine is rarely curable. Of the small proportion of patients with any of these conditions, almost all will have identifiable risks factor. In a retrospective study of 963 patients with acute LBP [25], the eight patients with tumours or fractures all had clinical risk factors. A prospective study found no cases of cancer in 1170 patients aged younger than 50 years with acute LBP and no history of cancer, weight loss, other sign of systemic illness or lack of improvement [26]. Similarly, four trials that enrolled 399 patients without risk factors found no missed serious conditions [27].
In the past, risk factors, historical features and physical examination findings associated with serious diseases are widely referred to as “red flags”, but the accuracy, or risk-benefit ratio, of many previously recognised “red flag” signs and symptoms has been questioned [28]. One study shows 80% of people with acute LBP have at least one red flag despite less than 1% having a serious disorder [29]. A systematic review shows that most of the recommended individual “red flags” are uninformative and do not substantially change post-test probabilities of a serious abnormality [30]. The very low specificity of most “red flags” contributes to unnecessary specialist referrals and imaging [30], [31]. The “red flag” with the highest post-test probability for detection of spinal malignancy is the history of malignancy, and it has been noted that a “history of malignancy” and “strong clinical suspicion” are the only red flags with empirical evidence of acceptably high diagnostic accuracy [32], [33].
Previous guidelines suggested that imaging be performed in adults aged older than 50 years who present with LBP. However, no statistically significant difference has been found in the primary outcome after 1 year for older adults who underwent spinal imaging within 6 weeks after an initial visit for care for LBP versus similar patients who did not undergo early imaging [34]; currently, age older than 50 years is not included as an independent “red flag”.
One study of 1172 new presentations of acute (<2 weeks) episodes of LBP in primary care in Australia found specific causes of back pain in 0.9% of participants, with fracture being by far the most common (eight of 11 cases), followed by inflammatory disorders (two of 11 cases) [29]. However, a review from Uganda of 204 patients referred to a hospital orthopaedic clinic with a primary complaint of LBP showed that 4% of patients had serious spinal abnormalities due to tuberculosis, 3.5% had vertebral compression fractures, 1% brucellosis and 1% had malignancy [35]. These differences in the patterns of specific pathological causes could reflect the ongoing burden of infectious diseases and their manifestations as LBP in low-income countries. Therefore, evidences from high-income countries may or may not generalise to low-income countries [2].
Cauda equina syndrome is caused by severe compression of the cauda equina due to massive midline disc herniation, tumour or epidural abscess [36]. Although not strictly a cause of LBP, cauda equina compression can have catastrophic consequences. It is very rare, and it has been stated that most primary care clinicians will not see a true case in a working lifetime [37]. Cauda equina syndrome is a surgical emergency characterised by the sudden onset of axial or radicular pain, leg weakness, bowel and/or bladder dysfunction and loss of perineal sensation, which is also referred to as saddle anaesthesia. The cardinal clinical features are urinary retention and overflow incontinence [38].
Most patients with LBP present with a benign condition
Most patients with acute LBP, with or without radiculopathy, have substantial improvements in pain and function in the first 4 weeks [39], [40]. Most cases of radiculopathy are self-limiting and symptoms resolve over the course of weeks to months. A recent systematic review provides strong evidence that most episodes of LBP improve substantially within 6 weeks, and by 12 months, average pain levels are low [41].
In 1970, Hakelius reported a study that followed the clinical course of patients with lumbosacral radiculopathy. Of the 38 patients with a clinical presentation consistent with radiculopathy and a disc herniation demonstrated on myelography, 88% were symptom-free after 6 months [42]. In 1989, Saal and Saal followed 58 patients with a diagnosis of radiculopathy and had minimal treatment [43]. At the end of the 31-week follow-up period, 92% reported a good to excellent outcome and 92% had returned to work. Another study by Weber et al [44], [45] focused on the short-term evolution of lumbosacral radiculopathy in 208 patients. These patients were placed on bed rest for 1 week, and then allowed to gradually resume activity. None of the patients underwent physical therapy. After 4 weeks, 70% of patients had marked reduction in pain. A recurrence of symptoms occurs in approximately 20% of patients [44], [45]. The favourable prognosis of radiculopathy based on the natural history supports a conservative approach for the initial weeks to months for most patients.
Most cases of disc herniation reabsorb or regress by 8 weeks after symptom onset (Fig 1) [46], [47]. The spontaneous regression of a herniated disc in the lumbar spine by myelography was reported as early as in 1945 [48]. This phenomenon was confirmed with many follow-up studies in the lumbar and cervical spine [49]. In 1990, Saal et al [50] published a study of 12 patients with documented lumbar herniations on CT. These patients were rescanned at an average of 25 months, and the following findings were documented: 46% of subjects had 75%–100% resorption, 36% had 50%–75% decrease in herniation size and 11% had 0%–50% regression. Interestingly, Saal et al [50] reported that complete resorption was most frequently seen in the patients who had the largest herniations. On the other hand, they did not find a significant correlation between clinical and morphologic improvement. Bozzao et al [51] reported similar results regarding morphology of lumbar herniations on MRI of 69 patients in approximately 2-year interval: 48% of patients had greater than 70% reduction in size, 15% had a 30%–50% reduction in size, 29% had no change and 8% had an increase in size. Overall, 64% of the 69 patients had a reduction in herniation size, and the largest degree of resorption was seen in those with medium and large herniations. Cowan et al [52] performed repeat CT scans on 106 patients 1 year after being diagnosed with lumbosacral radiculopathy. Disc herniations that decreased or fully resolved were seen in 76% of patients.
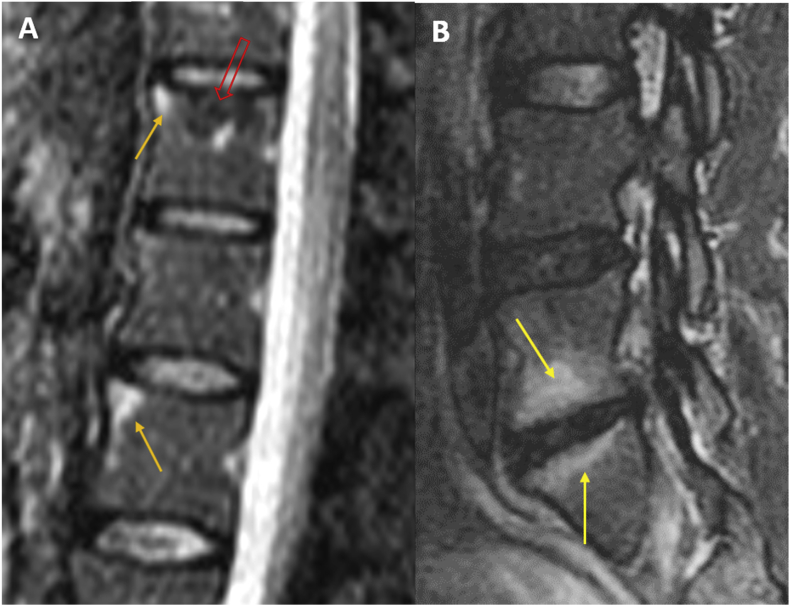
Figure 1.
Reabsorption of a left para-median disk herniation (A,B, with intense LBP and left radiculopathy) in a 4 months interval (C,D, with moderate LBP, radiculopathy disappeared). Arrow in (A) and (B) indicate the herniated disc, which became smaller after 4 months as indicated by arrow in (C) and (D). LBP = low back pain.
However, although most episodes of acute LBP will resolve, a substantial proportion of patients do develop chronic or recurrent pain [41], [53], [54], [55]. A large study [56] that followed 973 people with acute axial LBP found that 28% had not fully recovered 12 months after their initial consultation. A 2017 systematic review found that around 33% of people will have a recurrence within 1 year of recovering from a previous episode [57]; however, research does not provide robust estimates of the risk of LBP recurrence.
Common spine degenerative imaging findings in patients with LBP
Imaging features of degenerative spine disease are common in asymptomatic individuals and increase with age. Disc height loss and disk bulge are moderately prevalent among young individuals, and their prevalence increases by approximately 1% per year [58]. Disc protrusion and annular fissures are moderately prevalent across all age categories, but they do not seem to substantially increase with age [59]. Disc bulge, disc degeneration and spondylolysis have associations with LBP in adult patients aged 50 years or younger; however, the association between these degenerative findings and pain cannot be interpreted as a direct cause [59], [60]. In addition to inducing osteoporosis, menopause is also a trigger of accelerated spine degeneration in women [61], [62], and this is associated with increased prevalence of LBP [63]. Degenerative findings on MR imaging are not necessarily associated with the presence or the degree of LBP [27], [64], [65].
Among common spine degenerative imaging findings, Modic Type 1 changes and intense extensive zygapophyseal oedematous changes are more likely to correlate with LBP [66], [67], [68], [69], [70], [71]. Modic changes have been shown in 20–50% of the people with LBP, although they are also present in 10–25% of the asymptomatic patients [72], [73]. Type 1 Modic changes show an oedema pattern in MRI and may associate with LBP [74], [75]. For its cause, studies have attributed Modic Type 1 vertebral endplate changes to traumatic injury to the vertebral endplate, localised action of proinflammatory mediators or low-grade bacterial infection. In a retrospective study of 2457 discs of symptomatic patients using provocative discography as a reference examination, the positive predictive value for LBP of Type 1 Modic changes was 81% [66].
Asymptomatic Modic changes tend to be focal and localised in the upper anterosuperior endplate of the mid lumbar spine with preserved disc height; while in symptomatic patients, the lower back is most commonly affected, and the changes appear in the endplates adjacent to a degenerated disc. Chung et al [73] reported that marrow changes adjacent to vertebral end plates were found in asymptomatic subjects and involved predominantly the anterior aspects of the superior end plates of the mid-lumbar spine. The changes were focal; punctuate foci of abnormal signal rather than large confluent areas. This concurs with the two basic patterns of degenerative disc disease: spondylosis deformans and intervertebral osteochondrosis (Fig 2). The former involves essentially the annulus fibrosus and epiphyseal rings while the disc height remains normal and is considered a consequence of the natural ageing of the disc without significant clinical manifestations. Intervertebral osteochondrosis involves the disc, endplates and subchondral bone leading to scarring and loss of disc height and is considered a pathologic process that may lead to pain. Extensive Type I Modic changes at L5-S1 level are especially associated with LBP symptoms at this level [76]. Posteriorly oriented Modic changes are more painful than anteriorly oriented Modic changes. Vertically taller Modic changes are more strongly associated with LBP [77].
Figure 2.
Fat-suppressed T2-weighted magnetic resonance imaging (STIR) shows spondylosis deformans in (A, yellow arrows) and Type-1 Modic change in (B, yellow arrows). Red arrow in (A) indicates a haemangioma. The patient in B was with low back pain. STIR = short tau inversion recovery.
Some longitudinal studies have also found Modic changes can regress, but the course of regression remains unclear [78], [79]. Jensen et al [78] found small Modic changes only observed in the endplate were more likely to regress compared with those extended further into the vertebra. Mitra et al [80] showed a correlation between the favourable evolution of LBP and the transformation of Type 1 into Type 2 Modic changes. In a 1-year follow-up prospective MRI study of chronic LBP patients with large Modic 1-type change, it has been shown that the intensity of LBP decreased in most patients during 1 year, but increased or persisted in 36% [81]. Modic 1 change and associated bony endplate lesions and disc height decrease are signs for predicting long-lasting LBP [81].
There are evidences supporting a possible infectious aetiology for some cases of LBP [82]. Dudli et al reported that a low-grade infection by Propionibacterium acnes might promote the development of Modic changes [83]. Albert et al reported that in 162 patients with chronic LBP and endplate signal changes indicative of Modic Type 1 after a recent (<24 months) disc herniation found significant reductions in both back and leg pain in those treated with oral antibiotics for 100 days [84]. Further confirmative studies are required [85].
Vertebral endplate signal abnormalities may also be present in other conditions that should be differentiated from Modic changes based on clinical and imaging findings. These include the Anderson and Romanus lesions in seronegative spondyloarthropathies, the subligamentous oedema in diffuse idiopathic skeletal hyperostosis, haemodialysis spondyloarthropathy, neuropathic spine and infectious spondylodiscitis [70], [86].
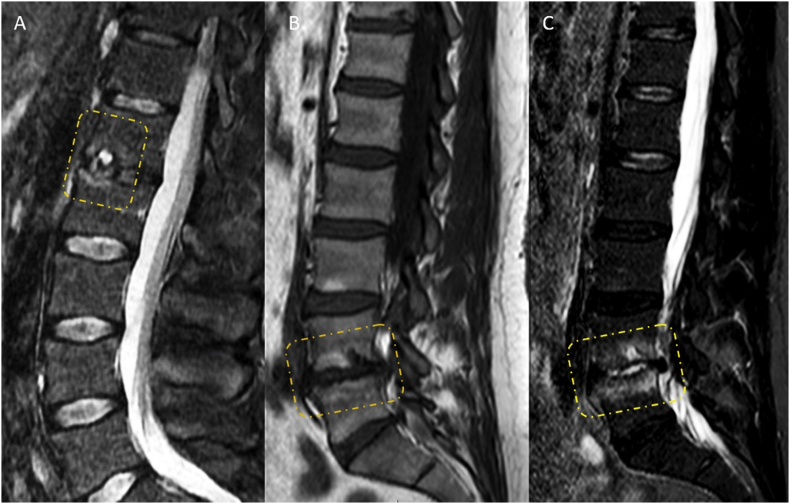
Schmorl's nodes are a common phenomenon in the adult population and are often asymptomatic. However, bone oedema peripheral to Schmorl's nodes is correlated with LBP (Fig. 3) [87], [88].
Figure 3.
Schmorl's nodes with T2-weighted magnetic resonance imaging showing oedematous change. (A) T2-weighted image shows painful Schmorl's node at L1. (B and C) T1-weighted image and fat-suppressed T2-weighted image (STIR) show Type 1 Modic change at L5/S1, and oedematous Schmorl's node. Both patients were with back pain.
Disc herniation in conjunction with local inflammation is the most common cause of radicular pain and radiculopathy. However, disc herniations can also be seen on imaging in the asymptomatic population, and they often resolve or disappear over time independent of resolution of pain. Patients with radiculopathy were more likely to have an extrusion and nerve root compression, but there was no correlation between the severity of disease seen on MR images and patient function and pain [65]. Size and type of disc herniation and location and presence of nerve root compression, which were important in terms of morphologic alteration, are not related to patient outcome [65]. Advance imaging with CT or MRI can visualise nerve root impingement. Most studies have demonstrated a strong association between severe nerve root compression and pain distal to the knee [23]. Even for patients with evidence of radiculopathy, conservative care for 6 weeks without imaging is appropriate [23].
The association between disc annular fissures and LBP remains controversial [69]. The systematic review of Brinjikji et al [58], [59] found that in the adult population aged 50 years or younger, disc annular fissures and disc high-intensity zones had no association with LBP. Mitra et al did not show concordance between the development of disc annular fissures and the development of LBP [89].
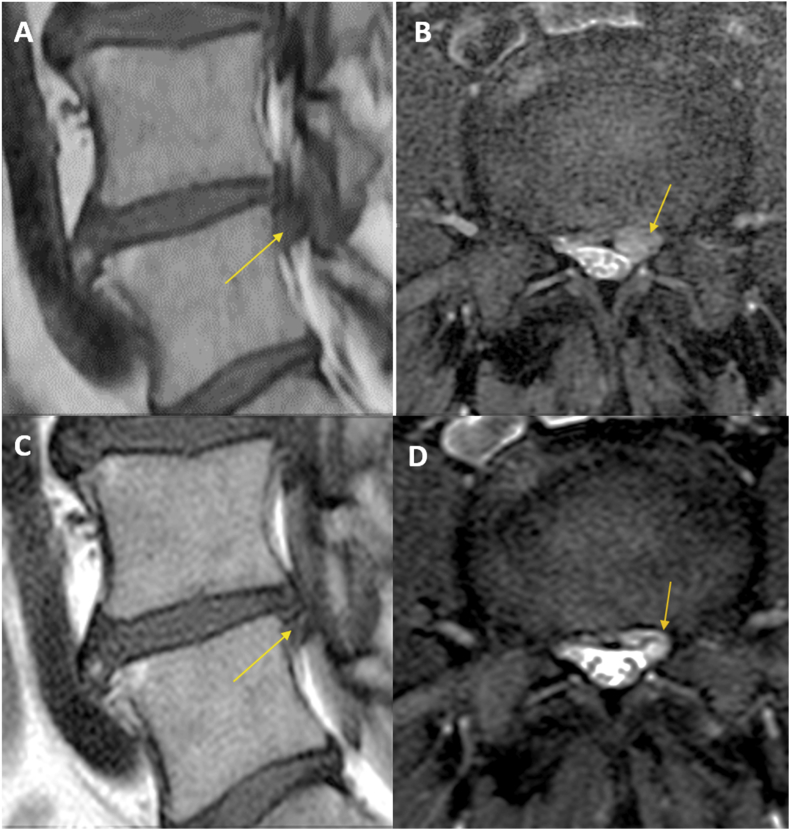
Recent reviews reported that clinical identification of individuals whose facet joints are contributing to their pain is not possible [60], [90]. Nevertheless, studies using MRI found that the presence or increased intra-articular fluid and facet oedema are associated with instability of the involved segment and with the presence of symptoms (Fig 4) [71], [91], [92]. These abnormalities may help to identify the targets for percutaneous imaging-guided therapies [93]. T2-weighted high intensity of the pedicle observed in fractures of isthmus may signal the cause of the pain [94], [95].
Figure 4.
Painful spondylolysis in an 11-year-old boy with low back pain. Magnetic resonance imaging shows the lysis and oedema. Arrow in (A) indicates the spondylolysis location. Cycle in (B) and arrows in (C, D) indicates oedema adjacent to spondylolysis.
Lumbar spinal stenosis is usually caused by narrowing of the spinal canal or foramina due to a combination of degenerative changes such as facet osteoarthritis, ligamentum flavum hypertrophy and bulging discs. Antero-posterior spinal canal diameter less than 12 mm in size is considered strongly suggestive of stenosis [96]; however, the cross-sectional area of the thecal dural sac is considered more suited in the diagnosis of spinal canal stenosis. The dural sac cross-sectional area values <76 mm2 are considered as severe stenosis, while 76–100 mm2 are considered as moderate stenosis [97], [98]. For foraminal stenosis, Grade 0 refers to the absence of foraminal stenosis. Grade 1 refers to mild foraminal stenosis showing perineural fat obliteration surrounding the nerve root in the two opposing directions (vertical or transverse). It involves a contact with the superior and inferior portions of the nerve root or anterior and posterior portions of the nerve root. No evidence of morphologic change in the nerve root is shown. Grade 2 refers to moderate foraminal stenosis showing perineural fat obliteration surrounding the nerve root in the four directions without morphologic change in both vertical and transverse directions. Grade 3 refers to severe foraminal stenosis showing nerve root collapse or morphologic change [99]. As with disc herniations, stenosis is common in asymptomatic persons (4%–28%) [100], [101]. The diagnosis of the clinical syndrome of lumbar spinal stenosis requires both the presence of characteristic symptoms and signs as well as imaging confirmation of narrowing of the lumbar spinal canal or foramina [102].
Symptoms of lumbar spinal stenosis are thought to result from venous congestion or ischaemia of the nerve roots due to compression [103]. Most patients with conservatively treated spinal stenosis will report either stable or improved symptoms [103]. In a partially randomised study, Amundsen et al [104] found that 57% of a nonrandomised cohort (n = 50) with mild symptoms obtained a good outcome at 4-year follow-up, whereas 44% of 18 randomised nonsurgically treated patients had a good outcome at 4 years. At a mean follow-up period of 11.1 years, Minamide et al [105] found that similar proportions of 34 patients with lumbar spinal stenosis treated conservatively experienced improvement, no change or worsening of symptoms. A cohort study evaluating 56 patients with symptomatic mild-to-moderate lumbar spinal stenosis symptoms who were treated conservatively found that 34 patients (60.7%) had a stable or improved clinical status at a median follow-up period of 88 months [106].
Degenerative spondylolisthesis is a disorder that causes the slip of one vertebral body over the one below due to degenerative changes, and it can be associated with central canal stenosis (Fig 5) [62]. Spondylolytic spondylolisthesis is lysis of the isthmus or pars interarticularis, being congenital or acquired (such as due to trauma). Both degenerative and spondylolytic spondylolisthesis are commonly seen as incidental findings in asymptomatic patients [62], [107]. The imaging features include the essential finding of degenerative spondylolisthesis on a lateral view of forward (or backward) displacement of L4 on L5 or, less commonly, L5 on S1 or L3 on L4 in the presence of an intact neural arch. In the cases of spondylolytic spondylolisthesis, the spinous process does not shift with the vertebral body and, contrary to degenerative spondylolisthesis, the central canal widens, while foramina are usually narrowed [108]. Retrolisthesis, the posterior shifting of a cephalad over caudal vertebra, can be secondary to the loss of disk material caused by intervertebral osteochondrosis or an acute herniation of the nucleus pulposus [109]. Most spondylolisthesis, being degenerative or spondylolytic, do not have a clinical symptom; even severe spondylolisthesis subjects may be asymptomatic [110], [111]. Occasionally, spondylolisthesis can indicate spinal instability (segmental hypermobility), which may require surgical treatment [111], [112].
Figure 5.
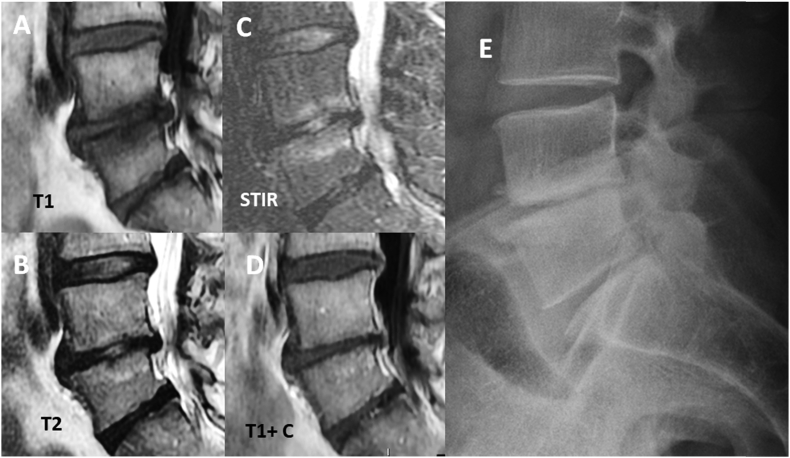
A chronic low back pain patient with Type-1 Modic change and L4 degenerative retrolisthesis. (A) T1-weighted MRI; (B) T2-weighted MRI; (C) fat-suppressed T2-weighted (STIR) MRI; (D): Gadolinium contrast-enhanced T1-weighted MRI. (E) In radiography, subchondral sclerosis is also observed. MRI = magnetic resonance imaging.
Baastrup's disease (kissing spine syndrome) refers to the development of a neo-arthrosis between the spinous processes generally secondary to degenerative disc disease with alignment abnormalities and loss of height [113], [114]. The frequency of Baastrup's disease shows a decade-on-decade increase with higher occurrence at ages over 70 and no gender predilection [113], [114]. Baastrup's disease is likely a degenerative process that occurs with ageing, and caution should be taken before it is diagnosed as the cause of back pain [114], [115].
Imaging techniques may depict findings that are more probably associated with LBP and may orientate to tailored treatment in some cases. However, it should be kept in mind that it is difficult to prove that the patients' pain originated solely from the abnormality identified by imaging. Even when radiological investigations show an abnormality, the positive findings may not necessarily relate directly to the back pain.
Potential harms of imaging studies for LBP
Imaging is an important driver of LBP costs, not only because of the direct costs of the procedures but also because of the downstream effects. Imaging can lead to additional tests, follow-up, referrals and may result in an invasive procedure of limited or questionable benefit. Routine imaging does not seem to improve clinical outcomes but may expose patients to unnecessary harms [27], [116]. A meta-analysis of six randomised trials [27], which comprised 1804 patients with primarily acute or subacute LBP and no clinical or historical features that suggested a specific underlying condition, found no differences between routine lumbar imaging (radiography, MRI or CT) and usual care without routine imaging in terms of pain, function, quality of life or overall patient-rated improvement. For short-term outcomes (<3 months), trends slightly favoured usual care without routine imaging. These results can probably be generalised to patients with or without radiculopathy [27]. The conclusions of the meta-analysis did not seem to be affected by whether radiography, MRI or CT was evaluated [27].
Telling patients that they have a spine imaging abnormality can result in unintended harms related to labelling. Knowledge of clinically irrelevant imaging findings might hinder recovery by causing patients to worry more, focus excessively on minor back symptoms or avoid exercise or other recommended activities because of the fear that they could cause more structural damage. In an acute LBP trial that performed lumbar spine MRI on all patients [117], patients randomly assigned to routinely receive their results reported smaller improvements in general health than those who were blinded to their results. In another trial, patients with back pain of at least 6-week duration who had routine radiography reported more pain and worse overall health status after 3 months than those who did not have radiography and were more likely to seek follow-up care [118].
Despite the uncertainties related to interpretation of most spinal imaging abnormalities, imaging abnormalities may be viewed as targets for surgery or other interventions [119]. Findings such as disc degeneration, facet hypertrophy and disc protrusion are often interpreted as causes of LBP, triggering both medical and surgical interventions, which are sometimes unsuccessful in alleviating the patient's symptoms [14], [117], [120]. Lurie et al [116] reported that the rates of spine surgery in the USA have increased along with a concurrent rise in the use of CT and MRI. One study found that for work-related acute LBP, MRI within the first month was associated with more than an eightfold increase in risk for surgery and more than a fivefold increase in subsequent total medical costs compared with propensity matched control patients who did not have early MRI [121].
Selection of imaging modalities for LBP
If decided to perform imaging, MRI is usually preferred because it involves no radiation exposure and has better soft-tissue contrast resolution, and it is sensitive for bone marrow abnormalities. As osteoporotic fracture and vertebral metastases affect patients of same age range, MRI can play an important role in differentiating benign from malign fractures [86], [122], [123], [124]. Up to one-third of vertebral fractures in patients with known primary neoplasia may be secondary to osteoporosis [125].
Radiography is the initial imaging study of choice for assessing LBP in patients with a history of trauma and patients suspected of having possible vertebral compression fractures. Flexion and extension radiographs can be performed to evaluate lumbar spine instability [126] (Fig 6). Diagnostic criteria of spinal instability are not universally accepted; however, instability is often diagnosed comparing lateral flexion and extension radiographs when more than 3 mm of vertebral displacement is found, or when differences greater than 10° are identified in the angle between the two vertebral plateaus of the disc under study [127].
Figure 6.
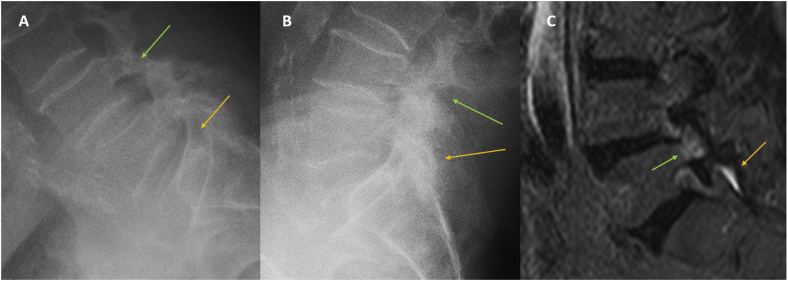
A case of chronic LBP, worse with standing and walking. Signs of instability are seen at L4-L5 (green arrow) and L5-S1 (orange arrow) in flexion (A) and extension (B) radiography. MRI shows bone edema (green arrow) and facets joint effusion (orange arrow) (C). LBP = low back pain; MRI = magnetic resonance imaging.
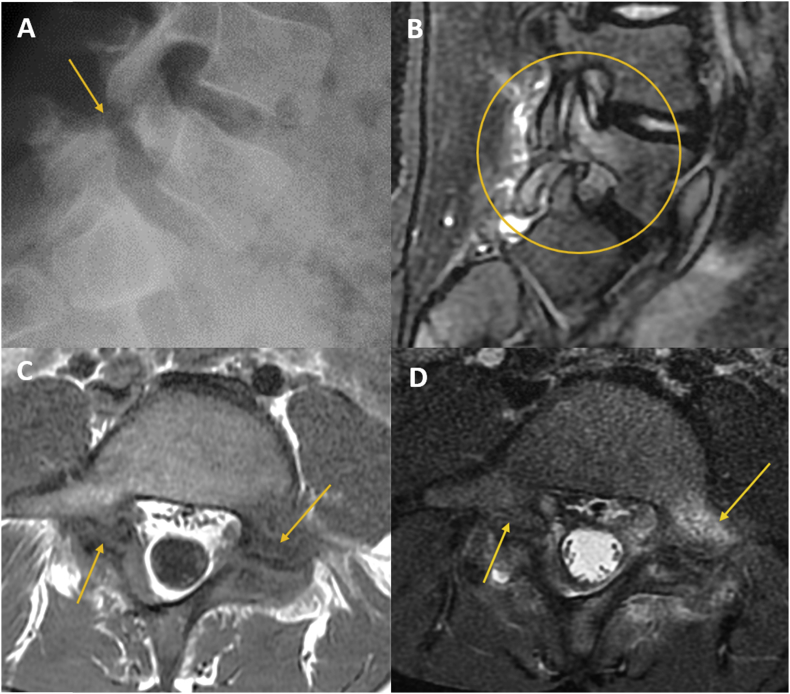
CT scans provide superior bone detail but are not as useful in depicting extradural soft-tissue pathologies, such as disc disease. CT with multiplanar reformatted sagittal and coronal planes is useful for revealing bone structural problems such as spondylolysis, pseudarthrosis, fracture, scoliosis and stenosis and for postsurgical evaluation of bone graft integrity, surgical fusion, and instrumentation. In patients who cannot undergo MRI, CT with myelography can be performed to assess the patency of the spinal canal and thecal sac and patency of the neural foramen (Fig 7). CT myelography may also be indicated for patients with extensive susceptibility artifacts related to metalic implants on MRI. Myelography has the disadvantage of requiring an invasive procedure to introduce intrathecal contrast agents.
Figure 7.
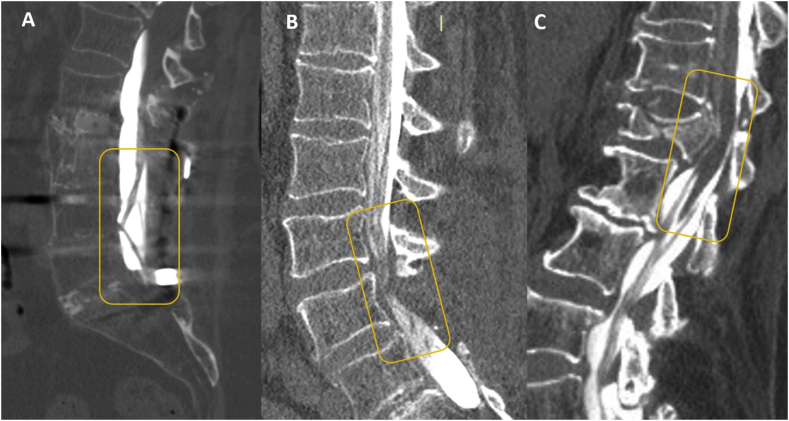
Myelography computed tomography (A) Arachnoiditis; (B) postsurgical canal stenosis (C): Post-traumatic medullary compression.
Lumbar radiography and CT contribute to cumulative radiation exposure, which could promote carcinogenesis. Lumbar spine CT is associated with an average effective radiation dose of 6–7 mSv [128], [129]. The radiation exposure from lumbar radiography is of concern in young women because of the proximity to the gonads, which are difficult to effectively shield.
The role of isotope bone scanning in patients with acute LBP has changed in recent years with the wide availability of MRI. Technetium-99m methylene diphosphonate bone scanning with single-photon emission CT is a sensitive test for detecting the presence of infection or occult fractures of the vertebrae but not for specifying the diagnosis. In a young patient with suspicion for lumbar spondylolysis, the gold standard for detection of radiographically occult active spondylolysis has been single-photon emission CT. There are disadvantages of this method, related not only to the invasive injection of a radiotracer but also to the concurrent radiation exposure. Recently, the diagnostic utility of MRI for radiographically occult spondylolysis has been demonstrated [130].
Decisions regarding repeated imaging should be based on the development of new or changed clinical features, such as new or progressive neurologic symptoms or recent trauma.
Treatment principles for LBP
Treatment should begin with nonpharmacological care, including self-management, exercise, education, physical and psychological therapies, complementary treatments such as acupuncture, massage, spinal manipulation, superficial heat, yoga and Tai Chi [17], [131], [132], [133]. Patients should be educated about the nature of LBP with or without sciatica and encouraged to continue the normal activities, but not bed rest [134].
Pharmacologic treatment is recommended for patients when nonpharmacological care did not help. The principle of pharmacologic treatment is to relief pain and while to minimise the potential harms of drugs. Oral nonsteroidal anti-inflammatory drugs are the first to be considered for LBP, with the risk of gastrointestinal, liver and cardio-renal toxicity being weighted. When nonsteroidal anti-inflammatory drug is contraindicated, not tolerated, or has been ineffective, opioids (with or without acetaminophen) are used for selected patients in short duration. Adjunct medication of muscle relaxants can be considered for short-term use [132]. The antidepressants also can be considered for chronic LBP where necessary [17]. There is still debate of using pregabalin for radicular pain [135], [136].
For those with severe functional disabilities, radicular symptoms or refractory pain, referral for epidural steroid injection or surgical evaluation can be considered. Epidural steroid injections may help patients with acute radicular symptoms. Epidural therapy at lumbar level can be done by a transforaminal, interlaminar or a caudal way. It can be combined with conservative treatment to provide pain relief and mobility improvement. While studies have found conflicting results for epidural steroid injections, the trend is toward a small improvement for up to 3 months after injection [137]. The scientific evidence is strong for epidural injections in managing chronic LBP of disc herniation in the short term (<6 months) and moderate in the long term (≥6 months) [138]. There is no evidence to support the use of epidural steroid injections in patients without radicular symptoms [139]. Injections are less effective in patients with severe spinal stenosis and those with stenotic lesions encompassing more than three lumbar levels [137], [139]. Epidural steroid injection cannot decrease the long-term risk of surgical treatment [131], [140].
Most patients with LBP without serious conditions will not benefit from surgery. However, if anatomic abnormalities consistent with the distribution of pain are identified, surgery can be considered in persons who have experienced significant functional disabilities and in those with unremitting pain, or with progressive neurological deficits, especially pain lasting longer than 6–12 months despite multiple nonsurgical treatments. In a systematic review [141] that investigated the effects of spinal decompression compared with nonoperative management of radicular pain, early surgery within 12 weeks of the onset of radicular pain was associated with faster pain relief compared with prolonged conservative treatment. However, there was no significant group difference in pain or functionality at 1- and 2-year follow-up. For symptomatic spinal stenosis, patients treated by surgery may have better pain and function than nonsurgery [142], but spine fusion seems to add little/no value than decompression alone [143], [144], which should be limited to lumbar stenosis patients accompanied with spinal instability or deformity [145].
Radiofrequency denervation of the medial branches of the dorsal ramus of the spinal nerve to the facet joint is considered by some authors as the standard treatment for facet joints pain [146]. The evidence for continuous radiofrequency denervation is superior to that of pulsed radiofrequency denervation [147], [148]
In summary, there is strong evidence that routine imaging for LBP by using radiography or CT/MRI is not associated with a clinically meaningful benefit on patient outcomes. Unnecessary imaging exposes patients to preventable harms, which may lead to additional unnecessary interventions. Diagnostic imaging studies should be performed only in selected, higher-risk patients who have severe or progressive neurologic deficits or are suspected of having a serious or specific underlying condition. A thorough history and physical examination are necessary to guide imaging decision. Radiologists can play a critical role through providing consultative expertise in decision support programs related to appropriateness of imaging requests, and accurately reporting the prevalence and potential clinical significance (or insignificance) of imaging findings.
Conflict of interest
The authors have no conflicts of interest relevant to this article.
References
- 1.Goertz M., Thorson D., Bonsell J., Institute for Clinical Systems Improvement . March 2018. Adult acute and subacute low back pain.https://www.icsi.org/guidelines__more/catalog_guidelines_and_more/catalog_guidelines/catalog_musculoskeletal_guidelines/low_back_pain/ [Google Scholar]
- 2.Hartvigsen J., Hancock M.J., Kongsted A., Lancet Low Back Pain Series Working Group What low back pain is and why we need to pay attention. Lancet. 2018;391:2356–2367. doi: 10.1016/S0140-6736(18)30480-X. [DOI] [PubMed] [Google Scholar]
- 3.Last A.R., Hulbert K. Chronic low back pain: evaluation and management. Am Fam Physician. 2009;79:1067–1074. [PubMed] [Google Scholar]
- 4.Medicare Part B Imaging Services . U.S. Government Accountability Office; Washington: 2008. Rapid spending growth and shift to physician offices indicate need for CMS to consider additional management practices. [Google Scholar]
- 5.Mafi J.N., McCarthy E.P., Davis R.B., Landon B.E. Worsening trends in the management and treatment of back pain. JAMA Intern Med. 2013;173:1573–1581. doi: 10.1001/jamainternmed.2013.8992. Erratum in: JAMA Intern Med. 2015;175:869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slade S.C., Kent P., Patel S., Bucknall T., Buchbinder R. Barriers to primary care clinician adherence to clinical guidelines for the management of low back pain: a systematic review and meta-synthesis of qualitative studies. Clin J Pain. 2015;32:800–816. doi: 10.1097/AJP.0000000000000324. [DOI] [PubMed] [Google Scholar]
- 7.Mesner S.A., Foster N.E., French S.D. Implementation interventions to improve the management of non-specific low back pain: a systematic review. BMC Musculoskelet Disord. 2016;17:258. doi: 10.1186/s12891-016-1110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chou R., Deyo R.A., Jarvik J.G. Appropriate use of lumbar imaging for evaluation of low back pain. Radiol Clin N Am. 2012;50:569–585. doi: 10.1016/j.rcl.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Baker S.R., Rabin A., Lantos G., Gallagher E.J. The effect of restricting the indications for lumbosacral spine radiography in patients with acute back symptoms. AJR Am J Roentgenol. 1987;149:535–538. doi: 10.2214/ajr.149.3.535. [DOI] [PubMed] [Google Scholar]
- 10.Eccles M., Steen N., Grimshaw J. Effect of audit and feedback, and reminder messages on primary-care radiology referrals: a randomised trial. Lancet. 2001;357:1406–1409. doi: 10.1016/S0140-6736(00)04564-5. [DOI] [PubMed] [Google Scholar]
- 11.Boden S.D., Davis D.O., Dina T.S., Patronas N.J., Wiesel S.W. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Jt Surg Am. 1990;72:403–408. [PubMed] [Google Scholar]
- 12.Jarvik J.G., Hollingworth W., Martin B. Rapid magnetic resonance imaging vs radiographs for patients with low back pain: a randomized controlled trial. J Am Med Assoc. 2003;289:2810–2818. doi: 10.1001/jama.289.21.2810. [DOI] [PubMed] [Google Scholar]
- 13.Chou R., Qaseem A., Snow V., Clinical Efficacy Assessment Subcommittee of the American College of Physicians Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147:478–491. doi: 10.7326/0003-4819-147-7-200710020-00006. [DOI] [PubMed] [Google Scholar]
- 14.Carragee E., Alamin T., Cheng I., Franklin T., van den Haak E., Hurwitz E. Are first-time episodes of serious LBP associated with new MRI findings? Spine J. 2006;6:624–635. doi: 10.1016/j.spinee.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Don A.S., Carragee E. A brief overview of evidence-informed management of chronic low back pain with surgery. Spine J. 2008;8:258–265. doi: 10.1016/j.spinee.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 16.Patel N.D., Broderick D.F., Burns J. ACR appropriateness criteria low back pain. J Am Coll Radiol. 2016 Sep;13(9):1069–1078. doi: 10.1016/j.jacr.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Oliveira C.B., Maher C.G., Pinto R.Z. Clinical practice guidelines for the management of non-specific low back pain in primary care: an updated overview. Eur Spine J. 2018 Jul 3 doi: 10.1007/s00586-018-5673-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.van Tulder M., Becker A., Bekkering T. COST B13Working group on guidelines for the management of acute low back pain in primary care. Chapter 3, European guidelines for the management of acute nonspecific low back pain in primary care. Eur Spine J. 2006;15(Suppl. 2):S169–S191. doi: 10.1007/s00586-006-1071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Airaksinen O., Brox J.I., Cedraschi C. COST B13Working Group on Guidelines for Chronic Low Back Pain. Chapter 4, European guidelines for the management of chronic nonspecific low back pain. Eur Spine J. 2006;15(Suppl. 2):S192–S300. doi: 10.1007/s00586-006-1072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pennekamp W. Radiological differential diagnosis of chronic back pain. Anasthesiol Intensivmed Notfallmed Schmerzther. 2009;44:32–38. doi: 10.1055/s-0028-1128184. [DOI] [PubMed] [Google Scholar]
- 21.Globe G., Farabaugh R.J., Hawk C. Clinical practice guideline: chiropractic care for low back pain. J Manip Physiol Ther. 2016;39:1–22. doi: 10.1016/j.jmpt.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Deyo R.A., Rainville J., Kent D.L. What can the history and physical examination tell us about low back pain? J Am Med Assoc. 1992;268:760–765. [PubMed] [Google Scholar]
- 23.Jarvik J.G., Deyo R.A. Diagnostic evaluation of low back pain with emphasis on imaging. Ann Intern Med. 2002;137:586–597. doi: 10.7326/0003-4819-137-7-200210010-00010. [DOI] [PubMed] [Google Scholar]
- 24.Underwood M.R., Dawes P. Inflammatory back pain in primary care. Br J Rheumatol. 1995;34:1074–1077. doi: 10.1093/rheumatology/34.11.1074. [DOI] [PubMed] [Google Scholar]
- 25.Suarez-Almazor M.E., Belseck E., Russell A.S., Mackel J.V. Use of lumbar radiographs for the early diagnosis of low back pain. Proposed guidelines would increase utilization. J Am Med Assoc. 1997;277:1782–1786. [PubMed] [Google Scholar]
- 26.Deyo R.A., Diehl A.K. Cancer as a cause of back pain: frequency, clinical presentation, and diagnostic strategies. J Gen Intern Med. 1988;3:230–238. doi: 10.1007/BF02596337. [DOI] [PubMed] [Google Scholar]
- 27.Chou R., Fu R., Carrino J.A. Imaging strategies for low-back pain: systematic review and meta-analysis. Lancet. 2009;373:463–472. doi: 10.1016/S0140-6736(09)60172-0. [DOI] [PubMed] [Google Scholar]
- 28.Verhagen A.P., Downie A., Popal N., Maher C., Koes B.W. Red flags presented in current low back pain guidelines: a review. Eur Spine J. 2016;25:2788–2802. doi: 10.1007/s00586-016-4684-0. [DOI] [PubMed] [Google Scholar]
- 29.Henschke N., Maher C.G., Refshauge K.M. Prevalence of and screening for serious spinal pathology in patients presenting to primary care settings with acute low back pain. Arthritis Rheum. 2009;60:3072–3080. doi: 10.1002/art.24853. [DOI] [PubMed] [Google Scholar]
- 30.Downie A., Williams C.M., Henschke N. Red flags to screen for malignancy and fracture in patients with low back pain: systematic review. BMJ. 2013;347:f7095. doi: 10.1136/bmj.f7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Underwood M., Buchbinder R. Red flags for back pain. BMJ. 2013;347:f7432. doi: 10.1136/bmj.f7432. [DOI] [PubMed] [Google Scholar]
- 32.Jenkins H.J., Downie A.S., Maher C.G., Moloney N.A., Magnussen J.S., Hancock M.J. Imaging for low back pain: is clinical use consistent with guidelines? A systematic review and meta-analysis. Spine J. 2018 May 3 doi: 10.1016/j.spinee.2018.05.004. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Verhagen A.P., Downie A., Maher C.G., Koes B.W. Most red flags for malignancy in low back pain guidelines lack empirical support: a systematic review. Pain. 2017;158:1860–1868. doi: 10.1097/j.pain.0000000000000998. [DOI] [PubMed] [Google Scholar]
- 34.Jarvik J.G., Gold L.S., Comstock B.A. Association of early imaging for back pain with clinical outcomes in older adults. JAMA. 2015;313:1143–1153. doi: 10.1001/jama.2015.1871. [DOI] [PubMed] [Google Scholar]
- 35.Galukande M., Muwazi S., Mugisa D.B. Aetiology of low back pain in Mulago Hospital, Uganda. Afr Health Sci. 2005;5:164–167. [PMC free article] [PubMed] [Google Scholar]
- 36.Gardner A., Gardner E., Morley T. Cauda equina syndrome: a review of the current clinical and medico-legal position. Eur Spine J. 2011;20(5):690–697. doi: 10.1007/s00586-010-1668-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lavy C., James A., Wilson-MacDonald J., Fairbank J. Cauda equina syndrome. BMJ. 2009;338:b936. doi: 10.1136/bmj.b936. [DOI] [PubMed] [Google Scholar]
- 38.Abrahm J.L. Assessment and treatment of patients with malignant spinal cord compression. J Support Oncol. 2004;2:88–91. [PubMed] [Google Scholar]
- 39.Pengel L.H., Herbert R.D., Maher C.G., Refshauge K.M. Acute low back pain: systematic review of its prognosis. BMJ. 2003;327:323. doi: 10.1136/bmj.327.7410.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vroomen P.C., de Krom M.C., Knottnerus J.A. Predicting the outcome of sciatica at short-term follow-up. Br J Gen Pract. 2002;52:119–123. [PMC free article] [PubMed] [Google Scholar]
- 41.da C Menezes Costa L., Maher C.G., Hancock M.J., McAuley J.H., Herbert R.D., Costa L.O. The prognosis of acute and persistent low-back pain: a meta-analysis. CMAJ. 2012;184:E613–E624. doi: 10.1503/cmaj.111271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hakelius A. Prognosis in sciatica: a clinical follow-up of surgical and non surgical treatment. Acta Orthop Scand Suppl. 1970;129:1–76. doi: 10.3109/ort.1970.41.suppl-129.01. [DOI] [PubMed] [Google Scholar]
- 43.Saal J.A., Saal J.S. Nonoperative treatment of herniated lumbar intervertebral disc with radiculopathy. Spine (Phila Pa 1976) 1989;1:431–437. doi: 10.1097/00007632-198904000-00018. [DOI] [PubMed] [Google Scholar]
- 44.Weber H., Holme I., Amilie E. The natural course of acute sciatica, with nerve root symptoms in a double blind placebo-controlled trial evaluating the effect of piroxicam. Spine (Phila Pa 1976) 1993;18:1433–1438. [PubMed] [Google Scholar]
- 45.Weber H. Lumbar disc herniation. A controlled prospective study with ten years of observation. Spine (Phila Pa 1976) 1983;8:131–140. [PubMed] [Google Scholar]
- 46.Autio R.A., Karppinen J., Niinimaki J. Determinants of spontaneous resorption of intervertebral disc herniations. Spine (Phila Pa 1976) 2006;31:1247–1252. doi: 10.1097/01.brs.0000217681.83524.4a. [DOI] [PubMed] [Google Scholar]
- 47.Chiu C.C., Chuang T.Y., Chang K.H., Wu C.H., Lin P.W., Hsu W.Y. The probability of spontaneous regression of lumbar herniated disc: a systematic review. Clin Rehabil. 2015;29:184–195. doi: 10.1177/0269215514540919. [DOI] [PubMed] [Google Scholar]
- 48.Key J.A. The conservative and operative treatment of lesions of the intervertebral discs in the low back. Surgery. 1945;17:291–303. [Google Scholar]
- 49.Teplick J.G., Haskin M.E. Spontaneous regression of herniated nucleus pulposus. AJR Am J Roentgenol. 1985;145:371–375. doi: 10.2214/ajr.145.2.371. [DOI] [PubMed] [Google Scholar]
- 50.Saal J.A., Saal J.S., Herzog R.J. The natural history of lumbar intervertebral disc extrusions treated nonoperatively. Spine (Phila Pa 1976) 1990;15:683–686. doi: 10.1097/00007632-199007000-00013. [DOI] [PubMed] [Google Scholar]
- 51.Bozzao A., Gallucci M., Masciocchi C. Lumbar disc herniation: MR imaging assessment of natural history in patients treated without surgery. Radiology. 1992;185:135–141. doi: 10.1148/radiology.185.1.1523297. [DOI] [PubMed] [Google Scholar]
- 52.Cowan N., Bush K., Katz D. The natural history of sciatica: a prospective radiological study. Clin Radiol. 1992;46:7–12. doi: 10.1016/s0009-9260(05)80025-x. [DOI] [PubMed] [Google Scholar]
- 53.Dunn K.M., Hestbaek L., Cassidy J.D. Low back pain across the life course. Best Pract Res Clin Rheum. 2013;27:591–600. doi: 10.1016/j.berh.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 54.Kongsted A., Kent P., Axen I., Downie A.S., Dunn K.M. What have we learned from ten years of trajectory research in low back pain? BMC Musculoskelet Dis. 2016;17:220. doi: 10.1186/s12891-016-1071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Itz C.J., Geurts J.W., van Kleef M., Nelemans P. Clinical course of non-specific low back pain: a systematic review of prospective cohort studies set in primary care. Eur J Pain. 2013;17:5–15. doi: 10.1002/j.1532-2149.2012.00170.x. [DOI] [PubMed] [Google Scholar]
- 56.Henschke N., Maher C.G., Refshauge K.M. Prognosis in patients with recent onset low back pain in Australian primary care: inception cohort study. BMJ. 2008;337:a171. doi: 10.1136/bmj.a171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.da Silva T., Mills K., Brown B.T., Herbert R.D., Maher C.G., Hancock M.J. Risk of recurrence of low back pain: a systematic review. J Orthop Sports Phys Ther. 2017;47:305–313. doi: 10.2519/jospt.2017.7415. [DOI] [PubMed] [Google Scholar]
- 58.Brinjikji W., Diehn F.E., Jarvik J.G. MRI findings of disc degeneration are more prevalent in adults with low back pain than in asymptomatic controls: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2015;36(12):2394–2399. doi: 10.3174/ajnr.A4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brinjikji W., Luetmer P.H., Comstock B. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. AJNR Am J Neuroradiol. 2015;36(4):811–816. doi: 10.3174/ajnr.A4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raastad J., Reiman M., Coeytaux R., Ledbetter L., Goode A.P. The association between lumbar spine radiographic features and low back pain: a systematic review and meta-analysis. Semin Arthritis Rheum. 2015;44(5):571–585. doi: 10.1016/j.semarthrit.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 61.Wang Y.X. Postmenopausal Chinese women show accelerated lumbar disc degeneration compared with Chinese men. J Orthop Transl. 2015;3:205–211. doi: 10.1016/j.jot.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y.X., Kaplar Z., Deng M., Leung J.C. Lumbar degenerative spondylolisthesis epidemiology: a systematic review with a focus on gender-specific and age-specific prevalence. J Orthop Transl. 2016;11:39–52. doi: 10.1016/j.jot.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Y.X., Wang J.Q., Kaplar Z. Increased low back pain prevalence in females than in males after menopause age: evidences based on synthetic literature review. Quant Imaging Med Surg. 2016;6:199–206. doi: 10.21037/qims.2016.04.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Steffens D., Hancock M.J., Maher C.G. Does magnetic resonance imaging predict future low back pain? A systematic review. Eur J Pain. 2014;18:755–765. doi: 10.1002/j.1532-2149.2013.00427.x. [DOI] [PubMed] [Google Scholar]
- 65.Modic M.T., Obuchowski N.A., Ross J.S. Acute low back pain and radiculopathy: MR imaging findings and their prognostic role and effect on outcome. Radiology. 2005;237:597–604. doi: 10.1148/radiol.2372041509. [DOI] [PubMed] [Google Scholar]
- 66.Thompson K.J., Dagher A.P., Eckel T.S., Clark M., Reinig J.W. Modic changes on MR images as studied with provocative diskography: clinical relevance–a retrospective study of 2457 disks. Radiology. 2009;250(3):849—55. doi: 10.1148/radiol.2503080474. [DOI] [PubMed] [Google Scholar]
- 67.Hancock M.J., Maher C.G., Latimer J. Systematic review of tests to identify the disc, SIJ or facet joint as the source of low back pain. Eur Spine J. 2007;16:1539–1550. doi: 10.1007/s00586-007-0391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weishaupt D., Zanetti M., Hodler J., Min K., Fuchs B., Pfirrmann C.W. Painful lumbar disk derangement: prelevance of endplateabnormalities at MR imaging. Radiology. 2001;218(2):420–427. doi: 10.1148/radiology.218.2.r01fe15420. [DOI] [PubMed] [Google Scholar]
- 69.Ract I., Meadeb J.M., Mercy G., Cueff F., Husson J.L., Guillin R. A review of the value of MRI signs in low back pain. Diagn Interv Imaging. 2015;96:239–249. doi: 10.1016/j.diii.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 70.Ruiz Santiago F., Castellano García M.M., Guzmán Álvarez L., Tello Moreno M. Computed tomography and magnetic resonance imaging for painful spinal column: contributions and controversies. Radiologia. 2011;53:116–133. doi: 10.1016/j.rx.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 71.Friedrich K.M., Nemec S., Peloschek P. The prevalence of lumbar facet joint edema in patients with low back pain. Skeletal Radiol. 2007;36:755–760. doi: 10.1007/s00256-007-0293-7. [DOI] [PubMed] [Google Scholar]
- 72.Fayad F., Lefevre-Colau M.M., Drape J.L. Reliability of a modified Modic classification of bone marrow changes in lumbar spine MRI. Joint Bone Spine. 2009;76:286–289. doi: 10.1016/j.jbspin.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 73.Chung C.B., Vande Berg B.C., Tavernier T. End plate marrow changes in the asymptomatic lumbosacral spine: frequency, distribution and correlation with age and degenerative changes. Skeletal Radiol. 2004;33:399–404. doi: 10.1007/s00256-004-0780-z. [DOI] [PubMed] [Google Scholar]
- 74.Jensen R.K., Leboeuf-Yde C., Wedderkopp N. Is the development of Modic changes associated with clinical symptoms? A 14-month cohort study with MRI. Eur Spine J. 2012;21:2271–2279. doi: 10.1007/s00586-012-2309-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kjaer P., Korsholm L., Bendix T., Sorensen J.S., Leboeuf-Y de C. Modic changes and their associations with clinical findings. Eur Spine J. 2006;15:1312–1319. doi: 10.1007/s00586-006-0185-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuisma M., Karppinen J., Niinimäki J. Modic changes in endplates of lumbar vertebral bodies: prevalence and association with low back and sciatic pain among middle-aged male workers. Spine (Phila Pa 1976) 2007;32(10):1116–1122. doi: 10.1097/01.brs.0000261561.12944.ff. [DOI] [PubMed] [Google Scholar]
- 77.Määttä J.H., Karppinen J., Paananen M. Refined phenotyping of modic changes: imaging biomarkers of prolonged severe low back pain and disability. Medicine (Baltim) 2016 May;95(22):e3495. doi: 10.1097/MD.0000000000003495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jensen T.S., Bendix T., Sorensen J.S. Characteristics and natural course of vertebral endplate signal (Modic) changes in the Danish general population. BMC Musculoskelet Disord. 2009;10:81. doi: 10.1186/1471-2474-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Määttä J.H., Kraatari M., Wolber L. Vertebral endplate change as a feature of intervertebral disc degeneration: a heritability study. Eur Spine J. 2014;23:1856–1862. doi: 10.1007/s00586-014-3333-8. [DOI] [PubMed] [Google Scholar]
- 80.Mitra D., Cassar-Pullicino V.N., McCall I.W. Longitudinal study of vertebral type-1 end-plate changes on MR of the lumbar spine. Eur Radiol. 2004;14(9):1574–1581. doi: 10.1007/s00330-004-2314-4. [DOI] [PubMed] [Google Scholar]
- 81.Luoma K., Vehmas T., Kerttula L., Grönblad M., Rinne E. Chronic low back pain in relation to Modic changes, bony endplate lesions, and disc degeneration in a prospective MRI study. Eur Spine J. 2016 Sep;25(9):2873–2881. doi: 10.1007/s00586-016-4715-x. [DOI] [PubMed] [Google Scholar]
- 82.Urquhart D.M., Zheng Y., Cheng A.C. Could low grade bacterial infection contribute to low back pain? A systematic review. BMC Med. 2015;13:13. doi: 10.1186/s12916-015-0267-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dudli S., Liebenberg E., Magnitsky S., Miller S., Demir-Deviren S., Lotz J.C. Propionibacterium acnes infected intervertebral discs cause vertebral bone marrow lesions consistent with Modic changes. J Orthop Res. 2016;34:1427–1455. doi: 10.1002/jor.23265. [DOI] [PubMed] [Google Scholar]
- 84.Albert H.B., Sorensen J.S., Christensen B.S., Manniche C. Antibiotic treatment in patients with chronic low back pain and vertebral bone edema (Modic type 1 changes): a double blind randomized clinical controlled trial of efficacy. Eur Spine J. 2013;22:697–707. doi: 10.1007/s00586-013-2675-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Crockett M.T., Kelly B.S., van Baarsel S., Kavanagh E.C. Modic type 1 vertebral endplate changes: injury, inflammation, or infection? AJR Am J Roentgenol. 2017;209:167–170. doi: 10.2214/AJR.16.17403. [DOI] [PubMed] [Google Scholar]
- 86.Teh J., Iman A., Watts C. Imaging of back pain. Imaging. 2005;17:171–207. [Google Scholar]
- 87.Stabler A., Bellan M., Weiss M., Gartner C., Brossmann J., Reiser M.F. MR imaging of enhancing intraosseous disk herniation (Schmorl's nodes) AJR Am J Roentgenol. 1997;168:933–938. doi: 10.2214/ajr.168.4.9124143. [DOI] [PubMed] [Google Scholar]
- 88.Takahashi K., Miyazaki T., Ohnari H., Takino T., Tomita K. Schmorl's nodes and low-back pain. Analysis of magnetic resonance imaging findings in symptomatic and asymptomatic individuals. Eur Spine J. 1995;4:56–59. doi: 10.1007/BF00298420. [DOI] [PubMed] [Google Scholar]
- 89.Mitra D., Cassar-Pullicino V.N., McCall I.W. Longitudinal study of high intensity zones on MR of lumbar intervertebral discs. Clin Radiol. 2004;59:1002–1008. doi: 10.1016/j.crad.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 90.Maas E.T., Juch J.N., Ostelo R.W. Ystematic review of patient history and physical examination to diagnose chronic low back pain originating from the facet joints. Eur J Pain. 2017;21:403–414. doi: 10.1002/ejp.963. [DOI] [PubMed] [Google Scholar]
- 91.Lakadamyali H., Tarhan N.C., Ergun T., Cakir B., Agildere A.M. STIR sequence for depiction of degenerative changes in posterior stabilizing elements in patients with lower back pain. AJR Am J Roentgenol. 2008;191:973–979. doi: 10.2214/AJR.07.2829. [DOI] [PubMed] [Google Scholar]
- 92.Rihn J.A., Lee J.Y., Khan M. Does lumbar facet fluid detected on magnetic resonance Imaging correlate with radiographic instability in patients with degenerative lumbar disease? Spine (Phila Pa 1976) 2007;32:1555–1560. doi: 10.1097/BRS.0b013e318067dc55. [DOI] [PubMed] [Google Scholar]
- 93.Manchikanti L., Benyamin R.M., Singh V. An update of comprehensive evidence-based guidelines for interventional techniques in chronic spinal pain. Part II: guidance and recommendations. Pain Physician. 2013;16:S49–S283. [PubMed] [Google Scholar]
- 94.Borg B., Modic M.T., Obuchowski N., Cheah G. Pedicle marrow signal hyperintensity on short tau inversion recovery- and t2-weighted images: prevalence and relationship to clinical symptoms. AJNR Am J Neuroradiol. 2011;32(9):1624–1631. doi: 10.3174/ajnr.A2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sakai T., Sairyo K., Mima S., Yasui N. Significance of magnetic resonance imaging signal change in the pedicle in the management of pediatric lumbar spondylolysis. Spine (Phila Pa 1976) 2010;35:E641–E645. doi: 10.1097/BRS.0b013e3181c9f2a2. [DOI] [PubMed] [Google Scholar]
- 96.Bartynski W.S., Petropoulou K.A. The MR imaging features and clinical correlates in low back pain-related syndromes. Magn Reson Imaging Clin N Am. 2007;15:137–154. doi: 10.1016/j.mric.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 97.Sirvanci M., Bhatia M., Ganiyusufoglu K.A. Degenerative lumbar spinal stenosis: correlation with Oswestry disability index and MR imaging. Eur Spine J. 2008;17:679–685. doi: 10.1007/s00586-008-0646-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Griffith J.F., Huang J., Law S.W., Xiao F., Leung J.C., Wang D. Population reference range for developmental lumbar spinal canal size. Quant Imaging Med Surg. 2016;6:671–679. doi: 10.21037/qims.2016.12.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee S., Lee J.W., Yeom J.S., Kim K.J., Kim H.J., Chung S.K. A practical MRI grading system for lumbar foraminal stenosis. AJR Am J Roentgenol. 2010;194:1095–1098. doi: 10.2214/AJR.09.2772. [DOI] [PubMed] [Google Scholar]
- 100.Porter R.W., Bewley B. A ten-year prospective study of vertebral canal size as a predictor of back pain. Spine (Phila Pa 1976) 1994;19:173–175. doi: 10.1097/00007632-199401001-00010. [DOI] [PubMed] [Google Scholar]
- 101.Wilmink J.T. CT morphology of intrathecal lumbosacral nerve-root compression. AJNR Am J Neuroradiol. 1989;10:233–248. [PMC free article] [PubMed] [Google Scholar]
- 102.Tomkins-Lane C., Melloh M., Lurie J. Consensus on the clinical diagnosis of lumbar spinal stenosis: results of an international Delphi study. Spine (Phila Pa 1976) 2016;41:1239–1246. doi: 10.1097/BRS.0000000000001476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chad D.A. Lumbar spinal stenosis. Neurol Clin. 2007;25:407–418. doi: 10.1016/j.ncl.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 104.Amundsen T., Weber H., Nordal H.J., Magnaes B., Abdelnoor M., Lilleâs F. Lumbar spinal stenosis: conservative or surgical management? A prospective 10-year study. Spine (Phila Pa 1976) 2000;25:1424–1435. doi: 10.1097/00007632-200006010-00016. discussion 1435-1426. [DOI] [PubMed] [Google Scholar]
- 105.Minamide A., Yoshida M., Maio K. The natural clinical course of lumbar spinal stenosis: a longitudinal cohort study over a minimum of 10 years. J Orthop Sci. 2013;18:693–698. doi: 10.1007/s00776-013-0435-9. [DOI] [PubMed] [Google Scholar]
- 106.Micankova Adamova B., Vohanka S., Dusek L., Jarkovsky J., Bednarik J. Prediction of long-term clinical outcome in patients with lumbar spinal stenosis. Eur Spine J. 2012;21:2611–2619. doi: 10.1007/s00586-012-2424-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.He L.C., Wang Y.X., Gong J.S., Griffith J.F., Zeng X.J., Kwok A.W. Prevalence and risk factors of lumbar spondylolisthesis in elderly Chinese men and women. Eur Radiol. 2014;24(2):441–448. doi: 10.1007/s00330-013-3041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ly J.Q. Systematic approach to interpretation of the lumbar spine MR imaging examination. Magn Reson Imaging Clin N Am. 2007;15:155–166. doi: 10.1016/j.mric.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 109.Resnick D. Degenerative diseases of the vertebral column. Radiology. 1985;156:3–14. doi: 10.1148/radiology.156.1.3923556. [DOI] [PubMed] [Google Scholar]
- 110.Wáng Y.X., Deng M., Griffith J.F., Kwok A.W., Leung J.C., Ahuja A.T. Lumbar spondylolisthesis progression and de novo spondylolisthesis in elderly Chinese men and women: a year-4 follow-up study. Spine (Phila Pa 1976) 2016;41(13):1096–1103. doi: 10.1097/BRS.0000000000001507. [DOI] [PubMed] [Google Scholar]
- 111.North American Spine Society . North American Spine Society; Burr Ridge, USA: 2008. Clinical guidelines for multidisciplinary spine care. Diagnosis and treatment of degenerative lumbar spondylolisthesis. [Google Scholar]
- 112.Káplár Z., Wáng Y.X. South Korean degenerative spondylolisthesis patients had surgical treatment at earlier age than Japanese, American, and European patients: a published literature observation. Quant Imaging Med Surg. 2016;6:785–790. doi: 10.21037/qims.2016.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pinto P.S., Boutin R.D., Resnick D. Spinous process fractures associated with Baastrup disease. Clin Imaging. 2004;28:219–222. doi: 10.1016/S0899-7071(03)00156-6. [DOI] [PubMed] [Google Scholar]
- 114.Kwong Y., Rao N., Latief K. MDCT findings in Baastrup disease: disease of normal feature of the aging spine. AJR Am J Roentgenol. 2011;196:1156–1159. doi: 10.2214/AJR.10.5719. [DOI] [PubMed] [Google Scholar]
- 115.Beks J.W. Kissing spines: fact or fancy? Acta Neurochir (Wien) 1989;100:134–135. doi: 10.1007/BF01403600. [DOI] [PubMed] [Google Scholar]
- 116.Lurie J.D., Birkmeyer N.J., Weinstein J.N. Rates of advanced spinal imaging and spine surgery. Spine (Phila Pa 1976) 2003;28:616–620. doi: 10.1097/01.BRS.0000049927.37696.DC. [DOI] [PubMed] [Google Scholar]
- 117.Ash L.M., Modic M.T., Obuchowski N.A., Ross J.S., Brant-Zawadzki M.N., Grooff P.N. Effects of diagnostic information, per se, on patient outcomes in acute radiculopathy and low back pain. AJNR Am J Neuroradiol. 2008;29(6):1098–1103. doi: 10.3174/ajnr.A0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kendrick D., Fielding K., Bentley E., Kerslake R., Miller P., Pringle M. Radiography of the lumbar spine in primary care patients with low back pain: randomised controlled trial. BMJ. 2001;322:400–405. doi: 10.1136/bmj.322.7283.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rhodes L.A., McPhillips-Tangum C.A., Markham C., Klenk R. The power of the visible: the meaning of diagnostic tests in chronic back pain. Soc Sci Med. 1999;48:1189–1203. doi: 10.1016/s0277-9536(98)00418-3. [DOI] [PubMed] [Google Scholar]
- 120.Graves J.M., Fulton-Kehoe D., Jarvik J.G., Franklin G.M. Health care utilization and costs associated with adherence to clinical practice guidelines for early magnetic resonance imaging among workers with acute occupational low back pain. Health Serv Res. 2014;49:645–665. doi: 10.1111/1475-6773.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Webster B.S., Cifuentes M. Relationship of early magnetic resonance imaging for work-related acute low back pain with disability and medical utilization outcomes. J Occup Environ Med. 2010;52:900–907. doi: 10.1097/JOM.0b013e3181ef7e53. [DOI] [PubMed] [Google Scholar]
- 122.Wáng Y.X.J., Santiago F.R., Deng M., Nogueira-Barbosa M.H. Identifying osteoporotic vertebral endplate and cortex fractures. Quant Imaging Med Surg. 2017;7:555–591. doi: 10.21037/qims.2017.10.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ruiz Santiago F., Tomás Muñoz P., Moya Sánchez E. Classifying thoracolumbar fractures: role of quantitative imaging. Quant Imaging Med Surg. 2016;6:772–784. doi: 10.21037/qims.2016.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jung H.S., Jee W.H., McCauley T.R. Discrimination of metastatic acute osteoporotic compression spinal fractures with MR imaging. Radiographics. 2003;23:179–187. doi: 10.1148/rg.231025043. [DOI] [PubMed] [Google Scholar]
- 125.Tann S.B., Kozak J.A., Mawad M.E. The limitations of magnetic resonance imaging in the diagnosis of pathologic vertebral fractures. Spine (Phila Pa 1976) 1991;16(8):919–923. doi: 10.1097/00007632-199108000-00009. [DOI] [PubMed] [Google Scholar]
- 126.Sanada S. Functional dynamic radiography with computer analysis-for physiological chest imaging and kinematic joint imaging. Quant Imaging Med Surg. 2017;7:698–706. doi: 10.21037/qims.2017.12.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Leone A., Guglielmi G., Cassar-Pullicino V.N., Bonomo L. Lumbar intervertebral instability: a review. Radiology. 2007;245:62–77. doi: 10.1148/radiol.2451051359. [DOI] [PubMed] [Google Scholar]
- 128.Fazel R., Krumholz H.M., Wang Y. Exposure to low-dose ionizing radiation from medical imaging procedures. N Engl J Med. 2009;361:849–857. doi: 10.1056/NEJMoa0901249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Crownover B.K., Bepko J.L. Appropriate and safe use of diagnostic imaging. Am Fam Physician. 2013;87:494–501. [PubMed] [Google Scholar]
- 130.Kobayashi A., Kobayashi T., Kato K., Higuchi H., Takagishi K. Diagnosis of radiographically occult lumbar spondylolysis in young athletes by magnetic resonance imaging. Am J Sports Med. 2013;41:169–176. doi: 10.1177/0363546512464946. [DOI] [PubMed] [Google Scholar]
- 131.Stochkendahl M.J., Kjaer P., Hartvigsen J. National clinical guidelines for non-surgical treatment of patients with recent onset low back pain or lumbar radiculopathy. Eur Spine J. 2018;27:60–75. doi: 10.1007/s00586-017-5099-2. [DOI] [PubMed] [Google Scholar]
- 132.Bernstein I.A., Malik Q., Carville S. Low back pain and sciatica: summary of NICE guidance. BMJ. 2017;356:i6748. doi: 10.1136/bmj.i6748. [DOI] [PubMed] [Google Scholar]
- 133.Qaseem A., Wilt T.J., McLean R.M., Forciea M.A., Clinical Guidelines Committee of the American College of Physicians Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514–530. doi: 10.7326/M16-2367. [DOI] [PubMed] [Google Scholar]
- 134.Foster N.E., Anema J.R., Cherkin D. Prevention and treatment of low back pain: evidence, challenges, and promising directions. Lancet. 2018 doi: 10.1016/S0140-6736(18)30489-6. [DOI] [PubMed] [Google Scholar]
- 135.Mathieson S., Maher C.G., McLachlan A.J. Trial of pregabalin for acute and chronic sciatica. N Engl J Med. 2017;376:1111–1120. doi: 10.1056/NEJMoa1614292. [DOI] [PubMed] [Google Scholar]
- 136.Sekiguchi M., Kikuchi S. Efficacy of pregabalin in patients with sciatica: a randomized, double-blind, placebo controlled trial. AME Med J. 2017;2(2):83. [Google Scholar]
- 137.Armon C., Argoff C.E., Samuels J., Backonja M.M., For the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology Assessment: use of epidural steroid injections to treat radicular lumbosacral pain. Neurology. 2007;68:723–729. doi: 10.1212/01.wnl.0000256734.34238.e7. [DOI] [PubMed] [Google Scholar]
- 138.Manchikanti L., Benyamin R.M., Falco J.J.E., Kaye A.D., Hirsch J.A. Do epidural injections provide short- and long-term relief for lumbar disc herniation? A systematic review. Clin Orthop Relat Res. 2015;473:1940–1956. doi: 10.1007/s11999-014-3490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.DePalma M.J., Slipman C.W. Evidence-informed management of chronic low back pain with epidural steroid injections. Spine J. 2008;8:45–55. doi: 10.1016/j.spinee.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 140.Chou R., Hashimoto R., Friedly J. Epidural corticosteroid injections for radiculopathy and spinal stenosis: a systematic review and meta-analysis. Ann Intern Med. 2015;163:373–381. doi: 10.7326/M15-0934. [DOI] [PubMed] [Google Scholar]
- 141.Jacobs W.C., van Tulder M., Arts M. Surgery versus conservative management of sciatica due to a lumbar herniated disc: a systematic review. Eur Spine J. 2011;20:513–522. doi: 10.1007/s00586-010-1603-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Weinstein J.N., Tosteson T.D., Lurie J.D. Surgical versus nonoperative treatment for lumbar spinal stenosis four-year results of the Spine Patient Outcomes Research Trial. Spine (Phila Pa 1976) 2010;35:1329–1338. doi: 10.1097/BRS.0b013e3181e0f04d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Forsth P., Olafsson G., Carlsson T. A randomized, controlled trial of fusion surgery for lumbar spinal stenosis. N Engl J Med. 2016;374:1413–1423. doi: 10.1056/NEJMoa1513721. [DOI] [PubMed] [Google Scholar]
- 144.Ghogawala Z., Dziura J., Butler W.E. Laminectomy plus fusion versus laminectomy alone for lumbar spondylolisthesis. N Engl J Med. 2016;374:1424–1434. doi: 10.1056/NEJMoa1508788. [DOI] [PubMed] [Google Scholar]
- 145.Wu A.M., Tong T.J., Wang X.Y. A rethink of fusion surgery for lumbar spinal stenosis. J Evid Base Med. 2016;9:166–169. doi: 10.1111/jebm.12215. [DOI] [PubMed] [Google Scholar]
- 146.Van Kleef M., Vanelderen P., Cohen S.P., Lataster A., Van Zundert J., Mekhail N. Pain originating from lumbar facet joints. Pain Pract. 2010;10:459–469. doi: 10.1111/j.1533-2500.2010.00393.x. [DOI] [PubMed] [Google Scholar]
- 147.Cohen S.P., Williams K.A., Kurihara C., Nguyen C., Shields C., Kim P., Griffith S.R., Larkin T.M., Crooks M., Williams N., Morlando B., Strassels S.A. Multicenter, randomized comparative cost effectiveness study comparing 0, 1, and 2 diagnostic medial branch (facet joint nerve) block treatment paradigms before lumbar facet radiofrequency denervation. Anesthesiology. 2010;113:395–405. doi: 10.1097/ALN.0b013e3181e33ae5. [DOI] [PubMed] [Google Scholar]
- 148.Tekin I., Mirzai H., Ok G., Erbuyun K., Vatansever D. A comparison of conventional and pulsed radiofrequency denervation in the treatment of chronic facet joint pain. Clin J Pain. 2007;23:524–529. doi: 10.1097/AJP.0b013e318074c99c. [DOI] [PubMed] [Google Scholar]