Abstract
Aims
To evaluate the prognostic value of quantitative myocardial perfusion imaging with positron emission tomography (PET) for adverse cardiovascular outcomes in patients with known or suspected coronary artery disease (CAD).
Methods and results
A search in MEDLINE and Embase was conducted for studies that evaluated (i) myocardial perfusion in absolute terms with PET, (ii) prognostic value for the development of major adverse cardiovascular events (MACE), cardiac death, and/or all-cause mortality, and (iii) patients with known or suspected CAD. Studies were divided according to the radiotracer utilized and their included population (patients with and without previous infarction). Comprehensive description and a selected instance of pooling were performed. Eight studies (n = 6804) were analysed and documented clear variability in population, quantitative PET variables operationalization [stress myocardial blood flow (sMBF) and flow reserve (MFR)], statistical covariate structure, follow-up, and radiotracer utilized. MFR was independently associated with MACE in eight studies [range of adjusted hazard ratios (HRs): 1.19–2.93]. The pooling instance demonstrated that MFR significantly associates with the development of MACEs (HR: 1.92 [1.29, 2.84]; P = 0.001). sMBF was only associated with MACE in two studies that evaluated it, and only one study documented sMBF as a better predictor than MFR.
Conclusion
This systematic review demonstrates the prognostic value of quantitative myocardial perfusion evaluated with PET, in the form of MFR and sMBF, for the development of major adverse cardiovascular outcomes in populations with known or suspected CAD. In the qualitative comparison, MFR seems to outperform sMBF as an independent prognostic factor. Evidence is still lacking for assessing quantitative PET for the occurrence of cardiac death and all-cause mortality. There is clear heterogeneity in predictor operationalization and study performances.
Keywords: quantitative positron emission tomography, myocardial perfusion, myocardial blood flow, myocardial flow reserve, coronary artery disease, prognostic value, cardiovascular events
Introduction
Non-invasive imaging has rapidly developed to offer assessment of coronary atherosclerosis and myocardial ischaemia with tools such as computed tomography (CT), single photon emission tomography (SPECT), echocardiography, magnetic resonance (MR), and positron emission tomography (PET). Recently, the diagnostic capacities of these techniques have been compared, showing that the PET performs best in the detection of obstructive coronary artery disease (CAD).1
Traditionally, PET and SPECT have evaluated cardiac perfusion defects through a standardized 5-point semi-quantitative analysis. Yet, the greatest advantage of PET myocardial perfusion imaging lies in its capability to fully quantify myocardial blood flow (MBF) in absolute terms (i.e. mL/g/min), which has proved to be superior to relative uptake evaluation.2 MBF is measured during rest (rMBF) and stress (sMBF), which is achieved through pharmacological vasodilatory hyperaemia. With this measurements, the sMBF/rMBF ratio can be calculated, which is known as the coronary perfusion reserve, myocardial perfusion reserve, coronary flow reserve, or myocardial flow reserve (MFR).3 Both MFR and sMBF have demonstrated utility in the identification of significant CAD and performance superiority of the latter over the former has been suggested.4
Recently, a number of publications have explored the added prognostic value that full quantitative PET perfusion can offer.5,6 However, this data has not yet been systematically analysed,7 and sources of heterogeneity are suspected. Moreover, whether MFR or sMBF might be better suited for prognostic evaluation is still uncertain.
Hence, the purpose of this systematic review and meta-analysis is to evaluate the available literature and investigate the prognostic value of absolute MFR and sMBF quantification with PET for the occurrence of major adverse cardiovascular events (MACE) and death in patients with known or suspected CAD. Additionally, we describe how the value of MFR and sMBF compares to each other in the currently available publications.
Methods
This review was conducted in accordance to the PRISMA statement and registered in the International Prospective Register of Systematic Reviews (PROSPERO2016: CRD42016033938).
Information sources and search
A search in electronic databases was conducted including MEDLINE and Embase for studies published in English language until August 2017. The search terms included MeSH and free-words to identify relevant records. The complete search can be consulted in the Supplementary data online, Table S1.
Study screening and selection
Two independent reviewers (L.J., R.S.) screened the search results to identify studies suited for full-text evaluation using the following criteria: The studies should (i) have evaluated MFR and/or sMBF in absolute terms with PET, (ii) have evaluated its prognostic value for MACE, cardiac death, and/or all-cause mortality, and (iii) have been conducted in patients studied for CAD (known or suspected)-related ischaemia. Reviews, editorials, abstracts, animal studies, conference presentations, or studies on diagnostic performance were excluded. Studies conducted in patients with hypertrophic cardiomyopathies or heart transplantation were not included.
Data extraction and quality assessment
Data extraction was conducted according to the following subheadings: study characteristics, population description, predictor measurement (i.e. detailed description of the technique employed), index test (i.e. MFR and sMBF measurement and operationalization) and outcome measure (events description), and quantitative results (multivariate modelling and estimators). Data on the (comparative) performance of both quantitative perfusion estimates (MFR and sMBF) were also extracted when available. Discrepancies or uncertainties were resolved by consensus (R.T.). If study data were used in multiple publications (i.e. articles that referred a similar number of patients or from the same inclusion period and the same medical centre evaluated through the same imaging protocol), only the results from that with the largest number of patients in the quantitative analysis were included.
We utilized the modified Quality in Prognostic Studies (QUIPS) appraisal tool considering study participation, attrition, prognostic factor measurement, outcome measurement, confounding account, and statistical analysis.8 First, the risk of bias was determined for each domain (as low, unclear, or high-risk). Then, the overall risk for each study was judged. Study quality was not considered restrictive for inclusion, but it was comprehensively evaluated.
Index predictors’ summary measures and outcome evaluation
We evaluated the hazard ratios (HRs) and accompanying 95% confidence intervals (CIs) and P-values reported for the index predictors (MFR and sMBF) from the multivariate survival analyses reported in the included studies. Particularly, we obtained first the independent HRs of MFR, and then, the independent HRs of sMBF (if present) when accounting for MFR in the reported models in order to evaluate the comparative prognostic value of both perfusion estimates. The HR ranges were described for the outcomes of interest as follows: First, we examined the development of MACE. We documented the combination of endpoints that constituted the definition of MACE as established by the authors of every original paper9 including events such as: cardiac death, MI, acute coronary syndromes, percutaneous transluminal coronary angioplasty, CABG, heart failure, stroke, and peripheral vascular disease. Then, we analysed the occurrence of cardiac death alone. This included only those studies that specifically reported a HR for the specific outcome. Finally, we analysed the reported development of all-cause mortality by grouping reports documenting such event category.
There are no current criteria established for the evaluation of prognostic variables in systematic reviews. Nevertheless, we considered that both the MFR and sMBF fulfilled a recently proposed criterion as an independent prognostic factor (i.e. evaluated in at least three independent studies with an included summed total of >1000 patients).10
Based on clinical relevance, we highlighted the division of included studies according to the utilized perfusion tracer (13N-ammonia, 82Rb, or 15O-water) and the predominance of patients with previous myocardial infarction (MI).
Due to expected variation in MFR and sMBF handling and statistical covariate structure, we only calculated a selected pooled HR for the occurrence of any of the outcomes (MACE, cardiac death, and all-cause mortality) if the following conditions were met: (i) the studies were conducted in a similar population (either with or without a majority of patients with previous MI), (ii) the studies utilized the same cut-off value for or the same operationalization of the index predictor, and (iii) the studies performed a survival analysis that did not raise a concern for ‘overfitting’ (i.e. that the number of covariates in the model did not exceed a ratio of 1 predictor per 10 outcome events). A random-effects model was used to pool the HRs, and the inverse variance method, using the log HR and its standard error, was used to assign the weights. The amount of variance between the studies was estimated using the DerSimonian and Laird method.11
Statistical heterogeneity between included studies was assessed using the I2 statistic.12 Statistical significance was set at P < 0.05. Analyses were performed using SPSS v.21, R, and RevMan 5.
Results
Search results and study selection
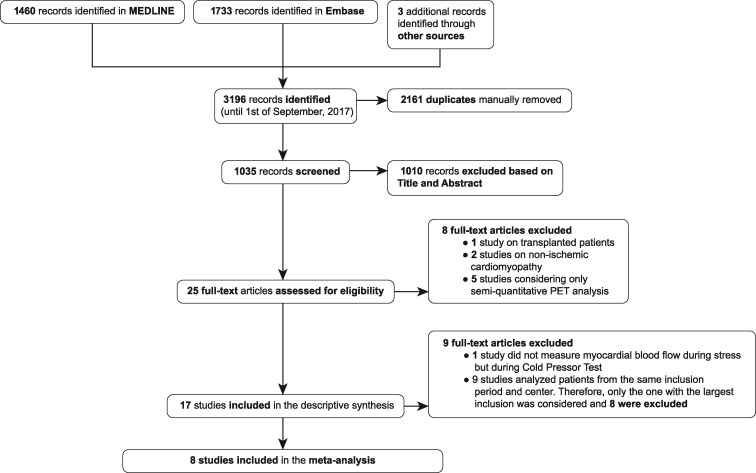
The results of the database search and study selection process are summarized in Figure 1. Our search identified 3196 potentially relevant studies. Twenty-five full-text articles were reviewed from which 17 studies were eligible, and eight were ultimately included in the quantitative description and analysis13–20 (see Supplementary data online, Table S2 for individual study characteristics).
Figure 1.
study flow chart.
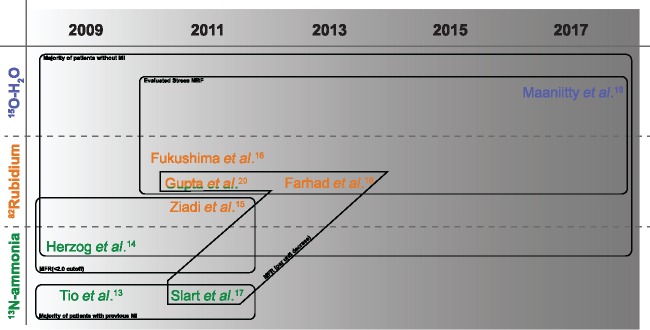
Two publications studied a population with a majority of subjects with evidence of a previous MI and were performed with 13N-ammonia,13,17 while six others studied mainly subject without previous MI, three using 82Rb,15,16,18 one using 13N-ammonia,14 one using 82Rb and 13N-ammonia,20 and one using 15O-water.19
Only four studies evaluated the prognostic value of sMBF and described its comparison to MFR (three using 82Rb16,18,20 and one using 15O-water19). Figure 2 depicts the areas of commonality between the included studies for this and other relevant aspects discussed ahead.
Figure 2.
common methodological features of included studies.
Characteristics and methodological aspects of the studies
The setting and population were clearly described in the included studies providing a summed total of 6804 patients. Five studies included mostly patients without a previous MI,14,16,18–20 which represent an interesting target population highlighted in current guideline recommendations on the role of PET myocardial perfusion imaging (i.e. patients with an intermediate pre-test likelihood of CAD). Sample characteristics and prevalence of risk factors are shown in Table 1.
Table 1.
Demographic descriptive statistics of the included studies
| Study | Perfusion tracer | Number of patients | Men (%) | Age (SD or IQR) | HTN (%) | DM (%) | Dyslip. (%) | Previous MI (%) | Previous Revasc. |
|---|---|---|---|---|---|---|---|---|---|
| Tio et al.13 | Ammonia | 344 | 78.8 | 66 (11) | 29 | 13 | 57 | 71 | 74 |
| Herzog et al.14 | Ammonia | 229 | 69.0 | 60 (12) | 60 | 18 | 59 | 0 | 53 |
| Ziadi et al.15 | 82Rb | 677 | 61.4 | 64 (12) | 68 | 29 | 69 | 40 | 45 |
| Fukushima et al.16 | 82Rb | 224 | 38.4 | 58 (13) | 63 | 34 | 45 | 12 | 0 |
| Slart et al.17 | Ammonia | 119 | 80.7 | 67 (11) | 35 | 15 | 45 | 81 | 81 |
| Farhad et al.18 | 82Rb | 318 | 63.5 | 65 (10) | 65 | 34 | 56 | 20 | 0 |
| Maaniitty et al.19 | 15O-H2O | 864 | 56.5 | 64 (9) | 67 | 20 | 70 | 0 | 0 |
| Gupta et al.20 | 82Rb/Ammonia | 4029 | 49.5 | 66 (18) | 83 | 36 | 68 | 28 | 36 |
DM, diabetes mellitus; Dyslip, dyslipidaemia; HTN, arterial hypertension; IQR, interquartile range; MI, myocardial infarction; N/R, not reported; Revasc, revascularization; SD, standard deviation.
Gupta et al.20 provided the largest sample with 4029 included patients who underwent PET imaging (86% of patients in this study were imaged using 82Rb and 14% with 13N-ammonia). Conversely, no single study with 13N-ammonia has included a comparable sample. Notably, the second study in sample size ranking (Ziadi et al.15) included roughly six times less patients. Virtually all studies included predominantly male patients, except for Fukushima et al.16 (38%) and Gupta et al.20 (50%).
The most severe clinical profile of patients was included in the studies by Tio et al.13 and Slart et al.,17 which precisely constitute the studies with a majority of patients with a previous MI (71 and 81%, respectively). Although these two studies were performed in the same centre in the Netherlands, the reported samples were independent from each other (the former considering patients without and the latter with PET-driven revascularization). Beyond the operational separation made between studies with patients with or without a majority of previous MI, there were differences in the demographic characteristics and CV risk profile of the populations recruited. Regarding age, the described and included studies were generally conducted in middle aged and elderly patients with moderate spread in the range of means (58–67 years of age). Further, arterial hypertension, Type 2 diabetes mellitus, dyslipidaemia, and smoking habit varied importantly between studies.
Three radiotracers were described for the assessment of myocardial perfusion with consequent differences in the implemented kinetic models, stressor agents, analysis, and corrections made for the resting cardiac work index [rate-pressure product (RPP)] (technical details of the selected studies are shown in Supplementary data online, Table S3).
The follow-up average range was 12–117 months for the analysis of MACE development, 66–88 months for the analysis of cardiac death, and 43–117 months for the analysis of all-cause mortality. The study by Gupta et al.20 had the longest follow-up (117 months).
The publication by Maaniitty et al.19 was the only study on the prognostic value of quantitative cardiac PET using 15O-water. Interestingly, it focused on the value of sMBF alone. Conversely, the studies by Farhad, Fukushima, and more recently, Gupta addressed the comparative value of MFR and sMBF with alternating results (see Discussion).
Although all the included studies reported performing a multivariate stepwise proportional hazard (Cox) regression that incorporated MFR, sMBF or both in the last step of the analysis, issues were found concerning the operationalization index predictors and model construction (as described recently by El Aidi et al.10). The analysis characteristics as well as number of events and reported pooled HRs, for MACE, cardiac death, and all-cause mortality are shown in Table 2.
Table 2.
Statistical analysis characteristics
| Study | Predictor of interest | Follow-up in months (SD/IQR or range) | Primary outcome (No. of events) | Primary HR [95% CI] | P-value | Types of included events |
Secondary outcome (No. of events) | Secondary HR [95% CI] | P-value | Types of included events |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cardiovascular death | MI | ACS | PTCA | CABG | Heart Failure | Stroke | Peripheral VD | Cardiovascular death | MI | ACS | PTCA | CABG | Heart Failure | Stroke | Peripheral VD | |||||||||
| Tio et al.13 | Per SD decrease in MFR | 85 (1–138) | Cardiac death (60) | 4.11 [2.98, 5.67] | <0.001 | • | MACE (183) | 1.44 [1.14, 1.84] | 0.003 | • | • | • | • | • | • | |||||||||
| Herzog et al.14 | MFR <2.0 | 66 (25.2) | Cardiac death (29) | 2.86 [1.24, 6.59] | <0.050 | • | MACE (76) | 1.6 [1.0, 2.57] | <0.05 | • | • | • | ||||||||||||
| Ziadi et al.15 | MFR <2.0 | 12.9 (1.4) | Hard cardiac events (27) | 3.3 [1.13, 9.5] | 0.029 | • | • | MACE (71) | 2.4 [1.4, 4.4] | 0.003 | • | • | • | • | • | • | ||||||||
| Fukushima et al.16 | MFR <2.11 | 12 (9.2) | MACE-hard and soft (33) | 2.93 [1.3, 6.65] | 0.009 | • | • | • | • | • | ||||||||||||||
| sMBF | N/R | 0.210 | ||||||||||||||||||||||
| Slart et al.17 | Per unit decrease in MFR | 88 (1–134) | Cardiac death (22) | 1.27 [1.12, 1.43] | <0.001 | • | MACE (57) | 1.19 [1.05, 1.33] | 0.004 | • | • | • | • | • | • | |||||||||
| Farhad et al.18 | Per unit decrease in MFR | 20.8 (5.2) | MACE (35) | 2.38 [N/R] | 0.006 | • | • | • | • | • | • | |||||||||||||
| sMBFa | 2.44 [1.49, 4.00] | 0.007 | ||||||||||||||||||||||
| Maaniitty et al.19 | sMBF ≤2.4 | 43.2 (32.4–57.6) | All-cause mortality (18) | 3.03 [N/R] | 0.098 | MACE (31) | 3.62 [1.08, 12.15] | 0.040 | • | • | ||||||||||||||
| Gupta et al.20 | Per unit decrease in MFR | 117 (N/R) | Cardiovascular death (392) | 1.83 [1.47, 2.27] | <0.001 | • | • | • | All-cause mortality (1005) | 1.72 [1.48, 2.01] | <0.001 | |||||||||||||
| Per unit decrease in sMBFa | 1.03 [0.84, 1.27] | 0.800 | 1.00 [0.89, 1.13] | 0.900 | ||||||||||||||||||||
N/R, not reported; VD, vascular disease; •, included.
sMBF was statistically tested against MFR in the multivariate survival model.
Four studies dichotomized MFR or sMBF according to a particular cut-off point either derived from literature or the variable distribution in their sample. Two of these described the same cut-off value (<2.0): one performed with 82Rb15 and the other with 13N-ammonia.14 The third study made use of a different cut-off (<2.11) and utilized 82Rb,16 while the fourth19 considered a cut-off of ≤2.4 only for sMBF. With regard to the other included studies, two operationalized MFR per unit decrease, one using 82Rb18 and the other, 13N-ammonia.17 Only, Tio et al.13 handled MFR as predictor per standard deviation decrease (see Figure 2 for a schematic depiction of these differences).
Another heterogeneity source was found in the amount of covariates included in the reported multivariate analysis across studies, which ranged from 2 in Fukushima et al.16 to 16 in Gupta et al.20 (for a complete depiction of the covariate structure and variable selection methods see Supplementary data online, Table S4). Importantly, in a relevant proportion of the included studies (4 of 8) the number of events per variable included in the multivariable survival analysis was <10, which may have led to an overestimation of the reported HRs due to ‘overfitting’.21 From these, two were performed with 13N-ammonia,14,17 one with 82Rb,18 and one with 15O-water.19
Risk of bias within studies
Figure 3 summarizes the risk of bias assessment using the QUIPS tool. The risk of bias was considered low overall. The only domain that showed a sustained uncertain risk of bias was the ‘prognostic factor measurement’ due to the differences in population characteristics and tracer utilized (see Supplementary data online, Figure S5 for the individual evaluation of the studies).
Figure 3.
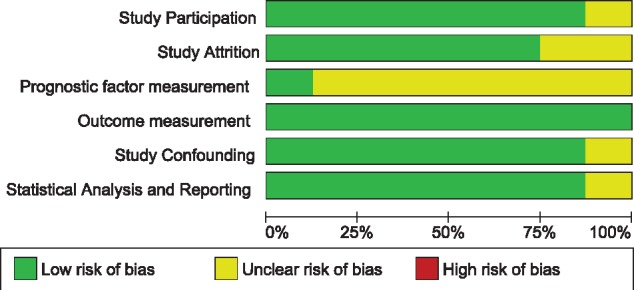
risk of bias.
Prognostic value of quantitative PET for MACE
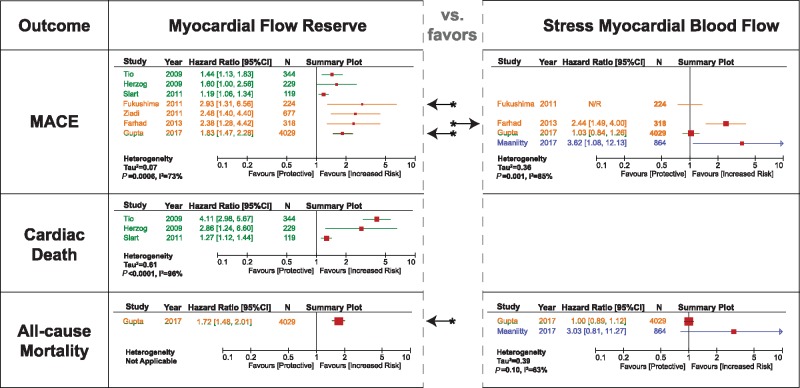
All eight studies analysed and reported HRs for the development of MACE [seven studies analysed MFR (5940 patients) and four analysed sMBF (4973 patients)]. The types of included events are shown in Table 2 (a total of 878 events were documented), and a comprehensive view of reported estimates is presented in Figure 4.
Figure 4.
summary of multivariate HRs. The colors correspond to the tracer utilized in the study (see Figure 2).
MFR demonstrated to be an independent predictor in each of the involved studies, with multivariable HRs ranging between 1.19 and 2.93. Only the lower CI described by Herzog et al.14 reached but did not cross the null effect boundary. The studies performed with 13N-ammonia reported lower HRs with narrower CIs than the ones performed with 82Rb (mean HR: 1.41 vs. 2.41, respectively). Among these 13N-ammonia studies, Tio et al.13 and Slart et al.17 included a majority of patients with previous MI, while Herzog et al.14 still reported 53% of this prevalence.
Conversely, sMBF only proved to be a significant predictor in two of four studies (HRs = 2.44–3.62) (see Figure 4 upper right panel). In the other two, Gupta et al.20 documented a non-significant HR for sMBF (1.03), while Fukushima et al.16 did not report the corresponding HR (yet disclosed as inferior and no longer significant when compared with MFR).
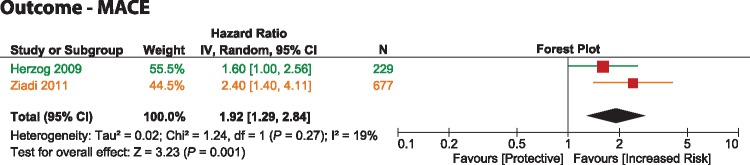
The only viable statistical pooling (as specified by the criteria mentioned under Methods) was performed for MFR with the studies by Herzog et al.14 and Ziadi et al.15 given that both included a similar population (without previous MI), both utilized the same cut-off value for MFR (<2.0) and neither raised a concern for statistical ‘overfitting’. This meta-analysis showed that a reduced MFR associated significantly with the occurrence of MACEs (pooled-HR = 1.92 [1.29–2.84]; P = 0.001) with evidence of minimal statistical heterogeneity (I2 = 19%).
Prognostic value of quantitative PET for cardiac death
Three studies13,14,17 (all using 13N-ammonia) analysed and reported HRs occurrence of cardiac death (692 patients) with 111 events documented. However, according to the cited criteria for evaluating an independent prognostic factor,10 there was not enough evidence in order to establish the prognostic value of MFR, while sMBF was not evaluated for this outcome. Still, the multivariable HRs ranged between 1.27 and 4.11. The HR reported in the study by Slart et al.,17 performed with 13N-ammonia in patients with previous MI, greatly diverged from the other three studies considered (see Figure 4 middle left panel), one of which was the study performed in the same centre by Tio et al.13 On the other hand, Herzog et al.14 documented considerably wider CIs than Tio et al.13 and Slart et al.17 Both the studies by Slart et al.17 and Herzog et al.14 raised concern for ‘overfitting’. As such, there was no viable statistical pooling to be performed as considered by our criteria.
Prognostic value of quantitative PET for all-cause mortality
Only two included studies (one performed with 82Rb and 13N-ammonia in a 9:1 ratio20 and one with 15O-water19) estimated HRs for the development of all-cause mortality (4893 patients with 1023 events). Once more, according to the criteria for prognostic factor evaluation, there was not enough evidence in order to establish the prognostic value of MFR or sMBF for this outcome (<3 studies). Interestingly, despite the large sample size analysed by Gupta et al.,20 the multivariable HR reported for MFR was statistically significant (1.72), while the one documented for sMBF was not (see Figure 4 inferior panels). Additionally, Maaniitty et al.19 directly reported a non-significant HR for sMBF.
Qualitative prognostic performance of MFR vs. sMBF
Overall, from the three included studies that compared the prognostic significance of MFR to that of sMBF, Fukushima et al.16 and Gupta et al.20 reported a better independent performance of MFR, and only Farhad et al.18 documented a marginally better performance of sMBF.
Discussion
This systematic review has confirmed that quantitative myocardial perfusion as evaluated by PET MFR is recurrently associated with an increased risk of MACE in individual studies, while consistent evidence of the prognostic value of sMBF is currently insufficient.
Interestingly, the degree to which MFR reflects this risk was found to be substantially heterogeneous across different patient populations. Furthermore, we documented relevant sources of variability in the methodological aspects of the papers involved, namely: the operationalization of the index predictors (MFR and sMBF), the covariate structure of the survival models reported (ranging from 2 to 16 covariates), and the follow-up times and the radiotracer utilized (82Rb, 13N-ammonia, and 15O-water). Because of these factors, full pooling of the reported HRs was not performed as it would generate virtually uninterpretable estimates.
Notably, we found great inconsistencies in MFR and sMBF operationalization as well as in the covariate structures utilized in the reported Cox models. This is explained by the prevalent lack of standardization in the statistical approach to evaluate the prognostic value of potential useful variables. Also, at the time some of the studies were performed, reports supporting particular diagnostic cut-off values were emerging. Still, prognostic cut-off values are generally constructed in a different fashion, according to the observed risk of events. Along this thread of thought, some reports have suggested MFR has ‘incremental’ value over left ventricular ejection fraction (LVEF) (four studies included LVEF as a covariate), which constitutes a powerful predictor of risk in patients with CAD. Interestingly, two of the studies included described some measure of incremental value based on the ‘net reclassification index’ (Ziadi et al.15 and Gupta et al.20). Still, reclassification evaluation was not widely applied in the studies included. Hence, our review could not categorically estimate the effect of the improvement in predictive performance. We would like to encourage application of novel reclassification metrics in the future.
When distinction was made between studies that included a majority of patients with previous MI, and arguably, a more severe clinical profile, it was shown that the HRs for the occurrence of MACE were lower than the ones in studies that analysed patients without previous MI (a clinical profile more commonly referred to PET imaging in diagnostic practice). It would seem, consequently, that the prognostic value of MFR is lower for high-risk populations.
There was a notable discrepancy between the reported HRs in Slart et al.17 and Tio et al.13 for the occurrence of cardiac death but not for the development of MACE. These two studies were performed in the same institution and using the same radiotracer. However, the first studied a population that did, and the second, that did not undergo PET-driven revascularization. Additionally, MFR operationalization was different between both studies. This could suggest that the principal influence of revascularization procedures per se is an improvement in the long-term risk of cardiac death rather than in other cardiovascular events. Further research in particular outcomes commonly clustered into the concept of MACE is, therefore, warranted.
The results of the single instance in which pooling of the reported HRs was conducted (shown in Figure 5) demonstrated a MFR < 2.0 roughly translates a two-fold increase in the risk of developing MACE. This result is to be considered with caution because although the two studies involved (Herzog et al.14 and Ziadi et al.15) met the requirements to minimize methodological heterogeneity, they were performed with a different tracer. On the other hand, we believe it is likely that this pooled estimate may represent an adequate approximation to the ‘real’ overall HR, and it would be of interest to explore its validation through further clinical research.
Figure 5.
forest plot. Predictor-MFR, outcome-MACE. The colors correspond to the tracer utilized in the study (see Figure 2).
Another main insight emerging from these results is the clear paucity and inconsistency of evidence regarding the prognostic role and performance of sMBF. Recent data have suggested that sMBF has a better diagnostic performance than MFR for the detection of significant CAD, and it has been suspected that sMBF may consequently convey a better prognostic value. In the present systematic review, four studies considered sMBF in their analyses16,18–20 and three of these16,18,20 compared it directly to MFR. From these studies, only Farhad et al.18 reported that the comparison favoured sMBF as a stronger independent prognostic factor. Interestingly, in the large scale comparison performed by Gupta et al.,20 MFR but not sMBF proved to be a significant predictor for the risk of all-cause mortality. On the other hand, the study by Maaniitty et al.19 only assessed sMBF due to the implemented protocol in their centre (sequential CT and stress PET). Yet, their study also demonstrated that even with very wide CIs, sMBF was only a significant predictor of MACE and not of all-cause mortality. Albeit sMBF can be obtained in rapid stress-only protocols, and it may overcome difficulties posed by groups of patients with a higher resting flow (which can artificially hamper MFR), these results suggest that MFR may convey a more robust prognostic value than sMBF. As such,
sMBF may be more suitable only in CAD diagnosis when there is no evidence of a previous infarction. As such, sMBF may be more suitable only in CAD diagnosis when there is no evidence of a previous infarction.
The clinical role of quantitative PET perfusion imaging has been already established based on its ability to provide accurate diagnosis of myocardial ischaemia in patients ranging from subclinical disease to overt symptomatic and worsening clinical pictures, with an improved performance over relative perfusion analysis. PET-measured MFR can be used to assess the ischaemic burden of atherosclerotic lesions in CAD and microvascular dysfunction. At the same time, MFR can provide a notion of the inherent risk associated with the status of the vasodilatory capacity of the coronary tree. This implies that PET-measured MFR could provide a target for the evaluation of emerging therapies that might modify patients’ short- and long-term risk profile. To present date, selected publications have only suggested its potential role in the improvement of risk stratification.
Reduction in MFR and sMBF may arise either from flow-limiting atherosclerotic lesions in epicardial coronary arteries or from vasodilatory dysfunction of small-calibre arterioles in the myocardium (because of hampering in endothelial reactivity). A clear link has been established between obstructive coronary stenosis and an increased risk of MI, although recent studies suggest that plaque characteristics and disease activity are also likely to play a role.22 In addition, small vessel disease closely relates to the presence of comorbidities (such as Type 2 diabetes mellitus and hypertension) which can also alter resting MBF and MFR. These comorbidities are themselves associated with an increased risk for progressive myocardial dysfunction and other adverse events. The combination of both these factors is therefore likely to account for the elevated risk for both fatal and non-fatal adverse outcomes associated with reductions in MFR and possibly sMBF. Still, our results support the notion that MFR is better suited for evaluating disease and risk characterization.
This report represents the first comprehensive systematic review characterizing the prognostic significance of MFR and sMBF. Due to constrains of the data and emerging criteria for the standardized evaluation of prognostic factors in biomedical sciences,10 we determined that quantitative myocardial perfusion with PET constitutes a statistically proven independent predictor for the risk of MACE and that there is currently not enough evidence in order to establish its prognostic value for cardiac death and all-cause mortality. A particular mention of the study by Gupta et al. should be made since it provided the largest sample (4029) and the longest follow-up recorded. However, the considered criteria for evaluating prognostic factors propose that evidence is insufficient when the variable has been tested in a cumulative sample of <1000 patients and/or <3 studies. This case probably constitutes a grey area in prognostic systematic reviews as we believe that their estimates should be reasonably considered as they factor strongly into the available evidence.
There were also clear technical differences documented. Although such factors may pose sources of variation in the estimates of MFR and sMBF, the reproducibility of PET quantitative perfusion has been shown to be considered good to excellent when the kinetic models for perfusion quantification estimation are equal.23 Importantly, we underline the necessity for further standardization at every level of analysis, which was recently highlighted in published PET guidelines.24
Although the reported analysis varied greatly, quality of the studies was overall good. This supports further analysis on individual patient data. As proposed elsewhere for cardiac MR,25 a PET registry would be an optimal approach to overcome complication inherent to report-based analyses.
Finally, our results may encourage practitioners to assume a more active position regarding tailored patient treatment. We believe our report could support a shift in the interest deposited in PET-derived quantitative perfusion measurements from considering them only as relevant markers of risk to potentially utilizing them as relevant trial endpoints. Finally, individual outcome specific research into the efficacy for risk modification and cost–benefit analyses may represent the best guide to develop PET quantitative myocardial perfusion regular clinical use.
Conclusion
This systematic review demonstrates the prognostic value of quantitative myocardial perfusion evaluated with PET, in the form of MFR and sMBF, for the development of major adverse cardiovascular outcomes in populations with known or suspected CAD. In the qualitative comparison, MFR seems to outperform sMBF as an independent prognostic factor. Evidence is still lacking for evaluating the prognostic value of quantitative PET for the occurrence of cardiac death and all-cause mortality. There is clear heterogeneity in predictor operationalization and study performances and underlines the need for further standardization to maximize the clinical benefit of the technique.
Conflict of interest: R.V. declares grants from the Dutch Organization for Scientific Research, outside of the submitted work. The rest of the authors have no relevant disclosures.
Supplementary Material
References
- 1. Jaarsma C, Leiner T, Bekkers SC, Crijns HJ, Wildberger JE, Nagel E. et al. Diagnostic performance of noninvasive myocardial perfusion imaging using single-photon emission computed tomography, cardiac magnetic resonance, and positron emission tomography imaging for the detection of obstructive coronary artery disease: a meta-analysis. J Am Coll Cardiol 2012;59:1719–28. [DOI] [PubMed] [Google Scholar]
- 2. Gould KL, Johnson NP, Bateman TM, Beanlands RS, Bengel FM, Bober R. et al. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. J Am Coll Cardiol 2013;62:1639–53. [DOI] [PubMed] [Google Scholar]
- 3. Schelbert HR. Anatomy and physiology of coronary blood flow. J Nucl Cardiol 2010;17:545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Joutsiniemi E, Saraste A, Pietilä M, Mäki M, Kajander S, Ukkonen H. et al. Absolute flow or myocardial flow reserve for the detection of significant coronary artery disease? Eur Heart J Cardiovasc Imaging 2014;15:659–65. [DOI] [PubMed] [Google Scholar]
- 5. Slomka PJ, Berman DS, Germano G.. New cardiac cameras: single-photon emission CT and PET. Semin Nucl Med 2014;44:232–51. [DOI] [PubMed] [Google Scholar]
- 6. Sciagrà R, Passeri A, Bucerius J, Verberne HJ, Slart RHJA, Lindner O. et al. Clinical use of quantitative cardiac perfusion PET: rationale, modalities and possible indications. Position paper of the Cardiovascular Committee of the European Association of Nuclear Medicine (EANM). Eur J Nucl Med Mol Imaging 2016;43:1530–45. [DOI] [PubMed] [Google Scholar]
- 7. Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MSV. et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation 2009;119:2408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hayden JA, Côté P, Bombardier C.. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med 2006;144:427–37. [DOI] [PubMed] [Google Scholar]
- 9. Kip KE, Hollabaugh K, Marroquin OC, Williams DO.. The problem with composite end points in cardiovascular studies: the story of major adverse cardiac events and percutaneous coronary intervention. J Am Coll Cardiol 2008;51:701–7. [DOI] [PubMed] [Google Scholar]
- 10. El Aidi H, Adams A, Moons KGM, Den Ruijter HM, Mali WPTM, Doevendans PA. et al. Cardiac magnetic resonance imaging findings and the risk of cardiovascular events in patients with recent myocardial infarction or suspected or known coronary artery disease: a systematic review of prognostic studies. J Am Coll Cardiol 2014;63:1031–45. [DOI] [PubMed] [Google Scholar]
- 11. DerSimonian R, Laird N.. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 12. Higgins JPT, Thompson SG.. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- 13. Tio RA, Dabeshlim A, Siebelink H-MJ, de Sutter J, Hillege HL, Zeebregts CJ. et al. Comparison between the prognostic value of left ventricular function and myocardial perfusion reserve in patients with ischemic heart disease. J Nucl Med 2009;50:214–9. [DOI] [PubMed] [Google Scholar]
- 14. Herzog BA, Husmann L, Valenta I, Gaemperli O, Siegrist PT, Tay FM. et al. Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Am Coll Cardiol 2009;54:150–6. [DOI] [PubMed] [Google Scholar]
- 15. Ziadi MC, DeKemp RA, Williams KA, Guo A, Chow BJW, Renaud JM. et al. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol 2011;58:740–8. [DOI] [PubMed] [Google Scholar]
- 16. Fukushima K, Javadi MS, Higuchi T, Lautamäki R, Merrill J, Nekolla SG. et al. Prediction of short-term cardiovascular events using quantification of global myocardial flow reserve in patients referred for clinical 82Rb PET perfusion imaging. J Nucl Med 2011;52:726–32. [DOI] [PubMed] [Google Scholar]
- 17. Slart RHJA, Zeebregts CJ, Hillege HL, de Sutter J, Dierckx RAJO, van Veldhuisen DJ. et al. Myocardial perfusion reserve after a PET-driven revascularization procedure: a strong prognostic factor. J Nucl Med 2011;52:873–9. [DOI] [PubMed] [Google Scholar]
- 18. Farhad H, Dunet V, Bachelard K, Allenbach G, Kaufmann PA, Prior JO.. Added prognostic value of myocardial blood flow quantitation in rubidium-82 positron emission tomography imaging. Eur Heart J Cardiovasc Imaging 2013;14:1203–10. [DOI] [PubMed] [Google Scholar]
- 19. Maaniitty T, Stenström I, Bax JJ, Uusitalo V, Ukkonen H, Kajander S. et al. Prognostic value of coronary CT angiography with selective PET perfusion imaging in coronary artery disease. JACC Cardiovasc Imaging 2017;10:1361–70. [DOI] [PubMed] [Google Scholar]
- 20. Gupta A, Taqueti VR, van de Hoef TP, Bajaj NS, Bravo PE, Murthy VL. et al. Integrated non-invasive physiological assessment of coronary circulatory function and impact on cardiovascular mortality in patients with stable coronary artery disease. Circulation 2017;136:2325–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR.. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996;49:1373–9. [DOI] [PubMed] [Google Scholar]
- 22. Joshi NV, Vesey AT, Williams MC, Shah ASV, Calvert PA, Craighead FHM. et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet 2014;383:705–13. [DOI] [PubMed] [Google Scholar]
- 23. Nesterov SV, Deshayes E, Sciagrà R, Settimo L, Declerck JM, Pan X-B. et al. Quantification of myocardial blood flow in absolute terms using (82)Rb PET imaging: the RUBY-10 Study. JACC Cardiovasc Imaging 2014;71119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dilsizian V, Bacharach SL, Beanlands RS, Bergmann SR, Delbeke D, Dorbala S. et al. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol 2016;23:1187–226. [DOI] [PubMed] [Google Scholar]
- 25. Bruder O, Schneider S, Nothnagel D, Dill T, Hombach V, Schulz-Menger J. et al. EuroCMR (European Cardiovascular Magnetic Resonance) registry: results of the German pilot phase. J Am Coll Cardiol 2009;54:1457–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.