Abstract
The biological functions of 1α,25-dihydroxyvitamin D3 are regulated by nuclear receptor vitamin D receptor (VDR). The expression level of VDR is high in intestine. VDR is an essential regulator of intestinal cell proliferation, barrier function, and immunity. Vitamin D/VDR plays a protective role in inflammatory bowel diseases (IBDs), both ulcerative colitis and Crohn’s disease. Emerging evidence demonstrates low VDR expression and dysfunction of vitamin D/VDR signaling in patients with IBD. Here, we summarize the progress made in vitamin D/VDR signaling in genetic regulation, immunity, and the microbiome in IBD. We cover the mechanisms of intestinal VDR in regulating inflammation through inhibiting the NF-ĸB pathway and activating autophagy. Recent studies suggest that the association of VDR single nucleotide polymorphisms with immune and intestinal pathology may be sex dependent. We emphasize the tissue specificity of VDR and its sex- and time-dependent effects. Furthermore, we discuss potential clinical application and future direction of vitamin D/VDR in preventing and treating IBD.
Keywords: immunity, infection, myeloid, nuclear receptor, Salmonella
INTRODUCTION
Vitamin D receptor (VDR) is an ancient nuclear receptor that has been highly conserved in birds, fish, and mammals.1 Based on epidemiological studies, we know that vitamin D (VD) deficiency is implicated in various diseases.2 VD absorbed from the diet or produced in the skin after exposure to sunlight is then converted to 25(OH) VD by the liver and to the active vitamin D metabolite 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3) by the kidney.3 VDR regulates the major biological functions of 1,25(OH)2D3. VDR is highly expressed in the small intestine and colon (The Human Protein Atlas, https://www.proteinatlas.org/ENSG00000111424-VDR/tissue; 20 April 2018, date last accessed).4,5 Intestinal VDR plays critical roles in immunity, proliferation, differentiation, permeability, and host-microbial interactions.6
It is acknowledged that genetic factors, environmental triggers, immune responses, and intestinal bacteria contribute to the pathogenesis of Crohn’s disease (CD) and ulcerative colitis (UC).7–9 Low vitamin D status has been observed in inflammatory bowel disease (IBD) patients.10–12 Due to insufficient sunlight in regions farther from the equator, a north-south gradient in CD rate indicates that vitamin D deficiency may be an environmental trigger contributing to IBD pathogenesis.11 Evidence strongly supports a protective effect of vitamin D and VDR in IBD. Low VDR expression and dysfunction of vitamin D/VDR signaling has been reported in both CD and UC patients.13,14 We show that a low level of intestinal epithelial VDR is accompanied by a reduction of Atg16l1, an IBD risk gene and regulator of autophagy, which leads to dysbiosis and impaired autophagic responses.13
Here, we will summarize the recent research advancement in human Vdr gene variation, vitamin D/VDR signaling in transcriptional regulation, immunity, and the microbiome in IBD. The mechanisms of VDR in regulating inflammation are also discussed. The vitamin D/VDR pathway is critical in maintaining intestinal homeostasis and regulating microbiota-host interactions. Further, we will discuss the potential clinical applications of vitamin D/VDR in IBD.
We performed an electronic literature search of papers written in English in the MEDLINE database via PubMed. Searches included combinations of the following terms: vitamin D, vitamin D receptor, microbiome/microbiota, inflammatory bowel disease, inflammation, autophagy, nuclear factor kappa B. Papers without clear relevance to the role of VDR signaling in inflammation or microbiota regulation were excluded.
HUMAN VDR GENE VARIATION IN IBD
The human Vdr gene encodes 6 domains of VDR protein. The Vdr gene is on chromosome 12q, comprised of promoter and regulatory regions (1a–1f) and exons 2–9. When 1,25(OH)2D3, the active form of vitamin D, binds to VDR protein, VDR translocates to the nucleus and functions as a transcription factor.15 The VDR target genes include the autophagy regulator ATG16L1,13 barrier protein claudin 2,16 scaffold protein Axin1,17 and antimicrobial peptides cathelicidin18 and defensin-β2.19
There are numerous single nucleotide polymorphisms (SNPs) in the Vdr gene. Susceptibility to IBD is associated with polymorphisms of Vdr.20–22 Five are of particular interest in IBD research: the nonsynonymous FokI and synonymous BsmI, ApaI, TaqI, and Tru9I. These SNPs, each named for the restriction endonuclease site it disrupts, are associated with risk of colon cancer.15, 23 Studies on the role of these SNPs in IBD susceptibility have been contradictory.24 Recent studies suggest that the association of Vdr SNPs with immune and intestinal pathology may be sex dependent. The Taq1 SNP is more frequent in male UC patients.25 Another study found that the haplotype “baT,” which contains the ancestral alleles for BsmI, ApaI, and TaqI, protects against adenomatous polyps only in females, whereas the FokI SNP increases risk particularly in males.26 There is also sex disparity in the association of FokI with lumbar pathology. The Ff allele of Fok1 is protective in females whereas ff is required for protection in males.27 The sex-specific effects of Vdr SNPs are also observed in other immune-related diseases, including multiple sclerosis28 and type I diabetes.29
RELEVANCE OF INTESTINAL VDR IN IBD
Although VDR is expressed in numerous tissues, intestinal epithelial expression is of particular importance in protecting against IBD. The expression level of VDR is critical in IBD pathology and treatment. Intestinal VDR expression decreases in both UC and CD patients,14 which reduces the anti-inflammatory effect of the vitamin D analogue. Tumor necrosis factor–alpha (TNF-α), a pro-inflammatory cytokine associated with IBD, is known to reduce the expression level of intestinal VDR.30 Constitutive RAS signaling, which is commonly observed in colon cancer, downregulates VDR expression and thus inhibits vitamin D signaling.31 However, the heterogeneity of VDR and its variable expression in physiological and pathological conditions are overlooked when we assess the efficacy of vitamin D or vitamin D analogue for IBD treatment.
In experimental models of colitis, Vdr whole-body knockout mice are known to develop severe colitis.32 This can be ameliorated by introducing transgenic expression of VDR in intestinal epithelial cells. Additionally, wild-type mice with transgenic overexpression VDR in the epithelium are less susceptible to chemical-induced models of colitis.14 This protection is associated with decreased epithelial production of pro-inflammatory cytokines. Our study has demonstrated that the bacterial product butyrate or probiotics protect against colitis by increasing the expression of VDR at the mRNA and protein levels, thus activating VDR signaling in epithelial cells.13, 33
Conditional knockout of intestinal epithelial Vdr alters the microbiota, which promotes more severe inflammation in chemical-induced models of colitis. These conditional knockouts have impaired Paneth cell function marked by deficient autophagy and decreased production of antimicrobial peptides (AMPs).13 Dietary vitamin D deficiency also impairs AMP production, resulting in dysbiosis and worsened high-fat diet–induced metabolic disease.34 VDR signaling in Paneth cells may be critical in preventing dysbiosis and maintaining mucosal health.
The intestinal barrier is critical in maintaining intestinal homeostasis. Vitamin D treatment has been found to promote barrier function in in vitro and in vivo studies.35–37 Loss of VDR expression in the intestine leads to decreased production of the barrier protein claudin 216 in cells without any stimulation. VDR also promotes barrier integrity by preventing intestinal epithelial apoptosis.38 These studies suggest that VDR signaling may protect against IBD by promoting intestinal barrier function. Further study is warranted to determine if intestinal VDR influences the expression of additional barrier proteins.
Interleukin 10 (IL-10) knockout mice have lower intestinal VDR expression and spontaneously develop microbiota-dependent colitis, which is more severe in Vdr/IL-10 double knockouts.39 Transgenic expression of human VDR in the mouse epithelium ameliorates IL-10 knockout colitis,38 whereas 1,25(OH)2D3 treatment does not.40 VDR expression reduces epithelial pro-inflammatory cytokine production and immune cell infiltration into the mucosa,38 indicating that epithelial VDR signaling is critical in modulating the immune response in IBD. Thus, VDR could be both a cause (eg, gene variation and conditional knockout) and a consequence (reduced intestinal VDR protein) of chronic inflammation.
VITAMIN D/VDR REGULATES IMMUNITY
VDR Regulates Both Innate and Adaptive Immune Cells
As described above, epithelial VDR expression alone is able to ameliorate genetic and chemical models of colitis. However, VDR expression in both innate and adaptive immune cells also affects the inflammatory response. Deletion of VDR in macrophages and granulocytes mildly affects colitis, but greatly enhances pro-inflammatory cytokine expression in the colon. These data indicate an essential role of VDR for innate immune cells in intestinal inflammation.41 Stimulation of macrophages with 1,25(OH2)D3 abrogates their pro-inflammatory and T-cell-recruiting cytokine production.42
VDR signaling promotes anti-inflammatory cytokine secretion and promotes tolerogenic dendritic cell and regulatory T-cell differentiation to reduce inflammation.43, 44 The severe inflammation observed in Vdr/IL-10 double knockout mice is attributed to an augmented pro-inflammatory T-cell response.38 VDR signaling in T cells influences differentiation. Treatment with 1,25(OH)2D3 prevents pathogenic Th17 differentiation, along with decreased expression of the pro-inflammatory cytokines interferon γ (IFNγ) and IL-17.45
Mechanisms: VDR Signaling, Autophagy, and NF-ĸB Activation
The VDR target gene Atg16l113 is an IBD risk gene that regulates intestinal homeostasis. ATG16L1 is a key autophagy protein that mediates autophagosome formation. The Atg16l1-T300A mutation increases degradation of the ATG16L1 protein and is associated with increased risk of CD. In intestinal inflammation, the T300A risk allele results in impaired bacterial clearance and dysbiosis.46 Our study has demonstrated that lack of VDR in the intestinal epithelium leads to dysbiosis and susceptibility to colitis via reducing ATG16L1 expression and dampening inflammatory responses.13, 33 Furthermore, loss of intestinal VDR shifts the microbiota to a pro-inflammatory profile. These data are consistant with our findings in human IBD samples: Decreased VDR in the intestine is associated with reduced ATG16L1 expression.13
Impaired autophagy in myeloid cells caused by myeloid-specific knockout of Atg16l1 contributes to intestinal inflammation.47, 48 These mice exhibit increased susceptibility to dextran sulphate sodium (DSS)-induced colitis due to impaired autophagy. Both macrophages and dendritic cells deficient in ATG16L1 have impaired processing of MHC class II antigens, and thus impaired CD4+ T-cell activation.47, 48 ATG16L1 is also critical for T-cell function. Targeted deletion of ATG16L1 in CD4+ T cells results in increased Th2 expansion and impaired Treg development, leading to spontaneous intestinal inflammation.49 Overall, induction of Atg16l1 expression by VDR13 in intestinal epithelial cells may play a critical role in maintaining intestinal homeostasis.
Intestinal inflammation is largely mediated by activation of nuclear factor-kappa B (NF-ĸB). VDR is known to downregulate NF-ĸB signaling and ameliorate intestinal inflammation through its interaction with NF-ĸB and its inhibitor inhibitory kappa B kinase beta (IKKβ).50 Interaction of VDR with NF-ĸB p65 blocks nuclear translocation to lessen the inflammatory response. Loss of VDR in intestinal epithelial cells and MEF cells leads to increased NF-ĸB signaling, which promotes pro-inflammatory cytokine production.5 In macrophage cells, vitamin D activation of VDR promotes interaction of VDR with the NF-ĸB p50 subunit, which prevents LPS-induced proliferation by preventing colocalization of NF-ĸB p50 with KLF5.51 Similarly, vitamin D treatment can prevent lipopolysaccharide (LPS)-induced placental inflammation by promoting dimerization of VDR with NF-ĸB p65.52 Thus, suppression of NF-ĸB signaling by VDR ameliorates inflammation, which supports the use of vitamin D supplementation in inflammatory diseases like IBD.
The article by Singh et al.53 has broadened our understanding of the interaction of VDR and NF-ĸB and further supports a critical role of VDR in regulating inflammatory disease. By integrating disease-associated SNPs identified by genome-wide association studies (GWAS) with VDR genomic binding sites identified by Chip-Seq, the authors identified 42 SNPs associated with immune phenotypes and VDR binding. Interestingly, most SNPs were not within canonical DR3-type vitamin D response elements (VDREs) that bind the VDR-retinoid X receptor (RXR) heterodimer. Rather, the transcription factor most frequently found to bind these 42 SNPs was NF-ĸB. Activated VDR and NF-ĸB share many genomic binding sites, including 22 of the 42 disease-associated SNPs. The data suggest that VDR likely influences transcription indirectly by interacting with NF-ĸB and other non-RXR transcription factors. The identification of non-VDRE SNPs bound by VDR opens new avenues of study in assessing the mechanism of VDR signaling in disease.
VDR SIGNALING REGULATES THE GUT MICROBIOTA
The human microbiota modulates host physiology and is a critical factor in the development of IBD.54, 55 Vitamin D may benefit the intestinal microbiome56 and improve glucose homeostasis in diabetes.57 We are pioneers in studying how intestinal VDR expression influences the microbiome.5, 13 From the results of our studies utilizing mouse models of Salmonella colitis, and mono-association of the commensal Escherichia coli F18 in originally germ-free mice models, we have begun to understand that enteric bacteria activate VDR signaling.5 We have demonstrated that VDR expression protects the host from invasive pathogens and maintains intestinal homeostasis.5, 58 Our recent study59 demonstrated that Vdr gene variation in humans influences the intestinal microbiota. A meta-analysis in humans showed Parabacteroides to be the most significant taxon correlated with the Vdr gene. Vdr-/- mice showed changes in Parabacteroides abundance.59Lactobacillus was depleted, whereas Clostridium and Bacteroides were enriched in Vdr-/- mice. We have identified several important pathways (eg, cancer, detoxification, infections, signal transduction, and metabolism) affected by Vdr gene status.60
Intriguingly, lack of VDR in the intestine leads to dysbiosis.13 We have reported profound alterations in the gut microbiome profile, with increased abundance of Bacteroidaceae in intestinal epithelial Vdr conditional knockout (VdrΔIEC) mice.13 Likewise, IBD patients have altered mucosal colonization by the Bacteroidaceae.61VdrΔIEC mice had dysbiosis and abnormal Paneth cells without any induction of colitis, suggesting the genetic role of intestinal epithelial Vdr in the development of chronic inflammation. The microbiota may play a role in the vulnerability of VdrΔIEC mice to colitis. The absence of intestinal epithelial VDR confers a transmissible risk for DSS-induced colitis, based on the cohousing study. Thus, intestinal epithelial VDR contributes to microbial homeostasis and host protection against inflammation.
Vitamin D deficiency in the diet induces intestinal dysbiosis; notable changes include increased Helicobacter hepaticus and decreased Akkermansia municiphila populations in the gut.34H. hepaticus is known to induce colon cancer and colitis in mice with impaired immunity,62 whereas low A. municiphila is decreased in mouse models of colitis and a low population level is associated with ulcerative colitis.63A. municiphila is also known to improve barrier function and metabolic health.64, 65
The Vdr gene transcribes for antimicrobial peptides and autophagy-related proteins (eg, ATG16l1).13 Vitamin D deficiency reduces production of alpha defensin Defa1 and MMP7 in Paneth cells.34 Further, Wang et al. reported that human Vdr gene variation is a key host factor influencing the gut microbiome.59 Therefore, the Vdr gene may be critical in homeostasis and signaling between the microbiota and host in intestinal inflammation.
Gut bacteria can regulate the immune response in a VDR-dependent fashion. We have shown that the protective effect of probiotics against colitis is mediated by epithelial VDR signaling.33 The secondary bile acid lithocholic acid, which is produced by Clostridium bacteria in the gut lumen, inhibits the Th1 immune response and suppresses IFNγ and IL-2 production by activating VDR in T cells.66Vdr knockout mice have lower Clostridium in the gut,60 illustrating the influence of crosstalk between the microbiome and VDR signaling in immunity. Butyrate, a short-chain fatty acid produced by gut microbes, can increase epithelial VDR expression and thus decrease production of pro-inflammatory cytokines.13
Taken together, the current studies provide new insights into the mechanism of VDR regulation in bacterial-host interactions and inflammation.
VITAMIN D AND HUMAN INTESTINAL INFLAMMATION
A recent study has demonstrated that serum levels of 25-hydroxy vitamin D of 35 ng/mL or less in UC patients in clinical remission are associated with disease relapse. Patients in remission with serum levels <35 ng/mL had more basal inflammation, were more likely to relapse, and relapsed sooner than patients with levels >35 ng/mL.67 Increasing levels of vitamin D might reduce their risk for UC relapse.
A small study of patients in remission from CD found that daily oral supplementation with 10,000 International Units (IU) of 1α,25-dihidroxy vitamin D3 conferred greater protection against relapse than a smaller dose of 1000 IU/d over 12 months.68 A similar study in UC patients with serum vitamin D levels under 30 ng/mL found that daily 4000 IU supplementation over 90 days improved patient quality of life but did not significantly improve clinical UC disease activity.69
To our knowledge, there is no report on the direct role of vitamin D supplementation on the microbiome in IBD patients. A recent study shows that high-dose vitamin D supplementation in humans markedly reduces opportunistic pathogens (eg, Gammaproteobacteria) and increases phylotype richness.70 It is believed that vitamin D3 supplementation reduces inflammation in the intestinal environment, thus diminishing the competitive advantage of opportunistic pathogens (eg, Escherichia/Shigella spp. or Pseudomonas spp.). Thus, the low inflammatory environment allows beneficial bacteria to outcompete pathogens for enhanced phylotype richness. These studies have shown that vitamin D may promote “healthy” microbiota that potentially confer protection against intestinal inflammation. However, we do not know the change of human VDR protein after using vitamin D supplementation and its role in regulating the gut microbiome in health and inflammation.
CONCLUSION AND FUTURE DIRECTION
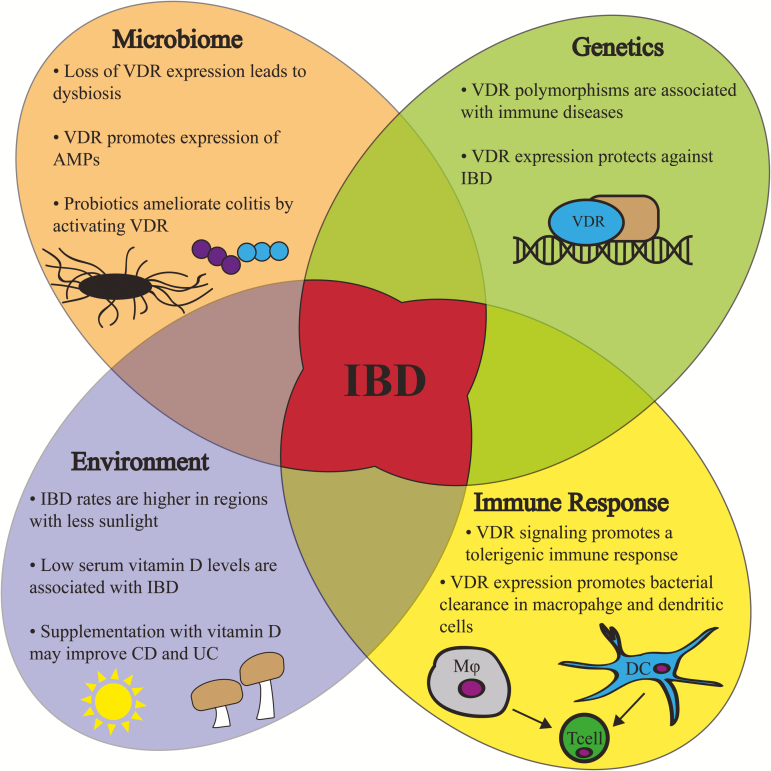
In conclusion, vitamin D/VDR signaling contributes to the genetic, environmental, immune, and microbial aspects of IBD, making it a significant protein of interest in understanding IBD pathology and in developing treatments (Fig. 1). As a transcription factor, VDR regulates the expression and signaling of target genes contributing to intestinal inflammation and dysbiosis, including Atg16l1. Genetic variation of the Vdr gene and its targets is associated with inflammation. VDR also directly influences both the host immune system and microbiome through its expression in intestinal epithelial and immune cells. Using a vitamin D supplement confers protection against relapse of IBD. Thus, intestinal VDR expression and vitamin D/VDR signaling protect against intestinal inflammation and dysbiosis.
FIGURE 1.
A working model of VDR signaling involved in the pathogenesis of IBD. Vitamin D/VDR regulates the genetic, environmental, immune, and microbial aspects in IBD, thus making VDR a significant host factor in IBD pathology and in developing treatments. Susceptibility to IBD is associated with polymorphisms in the Vdr gene. Moreover, VDR expression is significantly decreased in IBD patients. Vitamin D deficiency may be an environment trigger contributing to the pathogenesis of IBD. Vitamin D supplement may reduce the relapse of IBD. Vitamin D/VDR seems to be an important immunological regulator of IBD. Vitamin D/VDR signaling promotes microbial and mucosal homeostasis and protects against inflammatory diseases. DC, dendritic cell; Mφ, macrophage.
Microbiome studies and genetics analyses have brought new insights into an old topic on vitamin D/VDR in IBD. Future studies and clinical applications should consider the newly discovered functions of vitamin D/VDR in the intestine. The areas include:
consideration of intestinal VDR expression as a clinical biomarker for identifying patients who might benefit from currently available interventions;
development of strategies to restore the healthy host-microbiome interactions via vitamin D/VDR;
exploration of interactions between the microbiota and vitamin D/VDR that may affect the outcome of therapies;
consideration of VDR expression on the response to microbiome-targeted therapies, including fecal microbiota transplant;
exploration of new roles of VDR in other digestive diseases.
VDR signaling in the intestine and immune system promotes mucosal homeostasis and protects against inflammatory disease. The tissue-specific roles of VDR may offer a diagnostic/prognostic indicator in IBD. The current research indicates that vitamin D/VDR will likely become a potential therapeutic target for IBD.
Conflicts of interest: None of the authors has any conflict of interest to disclose.
Supported by: We would like to acknowledge the National Institute of Diabetes and Digestive and Kidney Diseases/National Institutes of Health grant R01 DK105118 and Department of Defense BC160450P1 to Jun Sun.
REFERENCES
- 1. Newmark H, Dantoft W, Ghazal P. Evolutionary origin of the interferon-immune metabolic axis:the sterol-vitamin D link. Front Immunol. 2017;8:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clark A, Mach N. Role of vitamin D in the hygiene hypothesis: the interplay between vitamin D, vitamin D receptors, gut microbiota, and immune response. Front Immunol. 2016;7:627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bilke DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21(3):319–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Uhlén M, Fagerberg L, Hallström BM et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. [DOI] [PubMed] [Google Scholar]
- 5. Wu S, Liao AP, Xia Y et al. Vitamin D receptor negatively regulates bacterial-stimulated NF-kappaB activity in intestine. Am J Pathol. 2010;177(2):686–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barbáchano A, Fernández-Barral A, Ferrer-Mayorga G et al. The endocrine vitamin D system in the gut. Mol Cell Endocrinol. 2017;453:79–87. [DOI] [PubMed] [Google Scholar]
- 7. Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology. 2017;152:313–21.e2. [DOI] [PubMed] [Google Scholar]
- 8. Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390–407. [DOI] [PubMed] [Google Scholar]
- 9. Corridoni D, Arseneau KO, Cifone MG, Cominelli F. The dual role of nod-like receptors in mucosal innate immunity and chronic intestinal inflammation. Front Immunol. 2014;5:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abreu MT, Kantorovich V, Vasiliauskas EA et al. Measurement of vitamin D levels in inflammatory bowel disease patients reveals a subset of Crohn’s disease patients with elevated 1,25-dihydroxyvitamin D and low bone mineral density. Gut. 2004;53:1129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lim WC, Hanauer SB, Li YC. Mechanisms of disease: vitamin D and inflammatory bowel disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2:308–15. [DOI] [PubMed] [Google Scholar]
- 12. Sentongo TA, Semaeo EJ, Stettler N et al. Vitamin D status in children, adolescents, and young adults with Crohn disease. Am J Clin Nutr. 2002;76:1077–81. [DOI] [PubMed] [Google Scholar]
- 13. Wu S, Zhang YG, Lu R et al. Intestinal epithelial vitamin D receptor deletion leads to defective autophagy in colitis. Gut. 2015;64:1082–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu W, Chen Y, Golan MA et al. Intestinal epithelial vitamin D receptor signaling inhibits experimental colitis. J Clin Invest. 2013;123:3983–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7:684–700. [DOI] [PubMed] [Google Scholar]
- 16. Zhang Y, Wu S, Lu R et al. Tight junction CLDN2 gene is a direct target of the vitamin D receptor. Sci Rep. 2015;5(10642):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jin D, Zhang YG, Wu S et al. Vitamin D receptor is a novel transcriptional regulator for axin1. J Steroid Biochem Mol Biol. 2017;165:430–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. Faseb J. 2005;19:1067–77. [DOI] [PubMed] [Google Scholar]
- 19. Wang T, Dabbas B, Laperriere D et al. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin β2 innate immune pathway defective in Crohn disease. J Biol Chem. 2010;285(4):2227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simmons JD, Mullighan C, Welsh KI, Jewell DP. Vitamin D receptor gene polymorphism: association with Crohn’s disease susceptibility. Gut. 2000;47:211–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Azad AK, Sadee W, Schlesinger LS. Innate immune gene polymorphisms in tuberculosis. Infect Immun. 2012;80:3343–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee YH, Song GG. Pathway analysis of a genome-wide association study of ileal Crohn’s disease. DNA Cell Biol. 2012;31:1549–54. [DOI] [PubMed] [Google Scholar]
- 23. Touvier M, Chan DS, Lau R et al. Meta-analyses of vitamin D intake, 25-hydroxyvitamin D status, vitamin D receptor polymorphisms, and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2011;20:1003–16. [DOI] [PubMed] [Google Scholar]
- 24. Ardesia M, Ferlazzo G, Fries W. Vitamin D and inflammatory bowel disease. Biomed Res Int. 2015;2015:470805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bentley RW, Keown DA, Gearry RB et al. Vitamin D receptor polymorphisms in colorectal cancer in New Zealand: an association study. N Z Med J. 2012;125:47–51. [PubMed] [Google Scholar]
- 26. Beckett EL, Le Gras K, Martin C et al. Vitamin D receptor polymorphisms relate to risk of adenomatous polyps in a sex-specific manner. Nutr Cancer. 2016;68:193–200. [DOI] [PubMed] [Google Scholar]
- 27. Colombini A, Brayda-Bruno M, Ferino L et al. Gender differences in the VDR-foki polymorphism and conventional non-genetic risk factors in association with lumbar spine pathologies in an Italian case-control study. Int J Mol Sci. 2015;16:3722–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ben-Selma W, Ben-Fredj N, Chebel S et al. Age- and gender-specific effects on VDR gene polymorphisms and risk of the development of multiple sclerosis in Tunisians: a preliminary study. Int J Immunogenet. 2015;42:174–81. [DOI] [PubMed] [Google Scholar]
- 29. Györffy B, Vásárhelyi B, Krikovszky D et al. Gender-specific association of vitamin D receptor polymorphism combinations with type 1 diabetes mellitus. Eur J Endocrinol. 2002;147:803–8. [DOI] [PubMed] [Google Scholar]
- 30. Chen Y, Du J, Zhang Z et al. Microrna-346 mediates tumor necrosis factor α-induced downregulation of gut epithelial vitamin D receptor in inflammatory bowel diseases. Inflamm Bowel Dis. 2014;20:1910–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. DeSmet ML, Fleet JC. Constitutively active RAS signaling reduces 1,25 dihydroxyvitamin D-mediated gene transcription in intestinal epithelial cells by reducing vitamin D receptor expression. J Steroid Biochem Mol Biol. 2017;173:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kong J, Zhang Z, Musch MW et al. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. 2008;294:G208–16. [DOI] [PubMed] [Google Scholar]
- 33. Wu S, Yoon S, Zhang YG et al. Vitamin D receptor pathway is required for probiotic protection in colitis. Am J Physiol Gastrointest Liver Physiol. 2015;309:G341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Su D, Nie Y, Zhu A et al. Vitamin D signaling through induction of paneth cell defensins maintains gut microbiota and improves metabolic disorders and hepatic steatosis in animal models. Front Physiol. 2016;7:498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Du J, Chen Y, Shi Y et al. 1,25-dihydroxyvitamin D protects intestinal epithelial barrier by regulating the myosin light chain kinase signaling pathway. Inflamm Bowel Dis. 2015;21:2495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shi YY, Liu TJ, Fu JH et al. Vitamin D/VDR signaling attenuates lipopolysaccharide-induced acute lung injury by maintaining the integrity of the pulmonary epithelial barrier. Mol Med Rep. 2016;13:1186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kühne H, Hause G, Grundmann SM et al. Vitamin D receptor knockout mice exhibit elongated intestinal microvilli and increased ezrin expression. Nutr Res. 2016;36:184–92. [DOI] [PubMed] [Google Scholar]
- 38. Golan MA, Liu W, Shi Y et al. Transgenic expression of vitamin D receptor in gut epithelial cells ameliorates spontaneous colitis caused by interleukin-10 deficiency. Dig Dis Sci. 2015;60:1941–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Froicu M, Zhu Y, Cantorna MT. Vitamin D receptor is required to control gastrointestinal immunity in IL-10 knockout mice. Immunology. 2006;117:310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Glenn AJ, Fielding KA, Chen J et al. Long-term vitamin D3 supplementation does not prevent colonic inflammation or modulate bone health in IL-10 knockout mice at young adulthood. Nutrients. 2014;6:3847–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leyssens C, Verlinden L, De Hertogh G et al. Impact on experimental colitis of vitamin D receptor deletion in intestinal epithelial or myeloid cells. Endocrinology. 2017;158:2354–66. [DOI] [PubMed] [Google Scholar]
- 42. Korf H, Wenes M, Stijlemans B et al. 1,25-dihydroxyvitamin D3 curtails the inflammatory and T cell stimulatory capacity of macrophages through an IL-10-dependent mechanism. Immunobiology. 2012;217:1292–300. [DOI] [PubMed] [Google Scholar]
- 43. Barragan M, Good M, Kolls JK. Regulation of dendritic cell function by vitamin D. Nutrients. 2015;7:8127–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bakdash G, van Capel TM, Mason LM et al. Vitamin D3 metabolite calcidiol primes human dendritic cells to promote the development of immunomodulatory IL-10-producing T cells. Vaccine. 2014;32:6294–302. [DOI] [PubMed] [Google Scholar]
- 45. Fawaz L, Mrad MF, Kazan JM et al. Comparative effect of 25(OH)D3 and 1,25(OH)2D3 on Th17 cell differentiation. Clin Immunol. 2016;166–167:59–71. [DOI] [PubMed] [Google Scholar]
- 46. Sadaghian Sadabad M, Regeling A, de Goffau MC et al. The ATG16L1-T300A allele impairs clearance of pathosymbionts in the inflamed ileal mucosa of Crohn’s disease patients. Gut. 2015;64:1546–52. [DOI] [PubMed] [Google Scholar]
- 47. Zhang H, Zheng L, Chen J et al. The protection role of atg16l1 in CD11C+dendritic cells in murine colitis. Immunobiology. 2017;222:831–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang H, Zheng L, McGovern DPB et al. Myeloid ATG16L1 facilitates host–bacteria interactions in maintaining intestinal homeostasis. J Immunol. 2017;198:2133–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kabat AM, Harrison OJ, Riffelmacher T et al. The autophagy gene atg16l1 differentially regulates treg and TH2 cells to control intestinal inflammation. Elife. 2016;5:e12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chen Y, Zhang J, Ge X et al. Vitamin D receptor inhibits nuclear factor kappa B activation by interacting with I kappa B kinase beta protein. J Biol Chem. 2013;288(27):19450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ma D, Zhang RN, Wen Y et al. 1, 25(OH)2D3-induced interaction of vitamin D receptor with p50 subunit of NF-κb suppresses the interaction between KLF5 and p50, contributing to inhibition of LPS-induced macrophage proliferation. Biochem Biophys Res Commun. 2017;482:366–74. [DOI] [PubMed] [Google Scholar]
- 52. Chen Y, Yu Z, Fu L et al. Vitamin D3 inhibits placental inflammation through reinforcing interaction between vitamin D receptor and nuclear factor kappa B p65 subunit. Nat Publ Gr. 2015;1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Singh PK, Van Den Berg PR, Long MD et al. Integration of VDR genome wide binding and GWAS genetic variation data reveals co-occurrence of VDR and NF- κ B binding that is linked to immune phenotypes. BMC Genomics. 2017;18(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Young VB, Kahn SA, Schmidt TM, Chang EB. Studying the enteric microbiome in inflammatory bowel diseases: getting through the growing pains and moving forward. Front Microbiol. 2011;2:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Devkota S, Wang Y, Musch MW et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in il10-/- mice. Nature. 2012;487:104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ooi JH, Li Y, Rogers CJ, Cantorna MT. Vitamin D regulates the gut microbiome and protects mice from dextran sodium sulfate-induced colitis. J Nutr. 2013;143:1679–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Barengolts E. Vitamin D and prebiotics may benefit the intestinal microbacteria and improve glucose homeostasis in prediabetes and type 2 diabetes. Endocr Pract. 2013;19:497–510. [DOI] [PubMed] [Google Scholar]
- 58. Sun J, Kong J, Duan Y et al. Increased NF-kappab activity in fibroblasts lacking the vitamin D receptor. Am J Physiol Endocrinol Metab. 2006;291:E315–22. [DOI] [PubMed] [Google Scholar]
- 59. Wang J, Thingholm LB, Skiecevičienė J et al. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat Genet. 2016;48:1396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jin D, Wu S, Zhang YG et al. Lack of vitamin D receptor causes dysbiosis and changes the functions of the murine intestinal microbiome. Clin Ther. 2015;37:996–1009.e7. [DOI] [PubMed] [Google Scholar]
- 61. Frank DN, Robertson CE, Hamm CM et al. Disease phenotype and genotype are associated with shifts in intestinal-associated microbiota in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17:179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fox JG, Ge Z, Whary MT et al. Helicobacter hepaticus infection in mice: models for understanding lower bowel inflammation and cancer. Mucosal Immunol. 2011;4:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Håkansson Å, Tormo-Badia N, Baridi A et al. Immunological alteration and changes of gut microbiota after dextran sulfate sodium (DSS) administration in mice. Clin Exp Med. 2015;15:107–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ottman N, Reunanen J, Meijerink M et al. Pili-like proteins of akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS One. 2017;12:e0173004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Everard A, Belzer C, Geurts L et al. Cross-talk between akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110:9066–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pols TWH, Puchner T, Korkmaz HI et al. Lithocholic acid controls adaptive immune responses by inhibition of th1 activation through the vitamin D receptor. PLoS One. 2017;12:e0176715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gubatan J, Mitsuhashi S, Zenlea T et al. Low serum vitamin D during remission increases risk of clinical relapse in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2017;15(2):240–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Narula N, Cooray M, Anglin R et al. Impact of high-dose vitamin D3 supplementation in patients with Crohn’s disease in remission: a pilot randomized double-blind controlled study. Dig Dis Sci. 2017;62:448–55. [DOI] [PubMed] [Google Scholar]
- 69. Mathur J, Naing S, Mills P, Limsui D. A randomized clinical trial of vitamin D3(cholecalciferol) in ulcerative colitis patients with hypovitaminosis D3. Peerj. 2017;5:e3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bashir M, Prietl B, Tauschmann M et al. Effects of high doses of vitamin D3 on mucosa-associated gut microbiome vary between regions of the human gastrointestinal tract. Eur J Nutr. 2016;55:1479–89. [DOI] [PMC free article] [PubMed] [Google Scholar]