Abstract
Portal vein thrombosis is an uncommon finding that typically arises in the context of cirrhosis. In the acute setting, it may present with abdominal pain, portal hypertension, ascites, gastrointestinal bleeding, or mesenteric ischemia. Local risk factors that predispose its formation include: cirrhosis, hepatocellular carcinoma, pancreatitis, and intraabdominal infection. Systemic factors, including hypercoagulable states and sepsis, also pose an increased risk. JAK2 V617F positive myeloproliferative disorders are associated with systemic prothrombotic states and are a less frequently identified cause of portal vein thrombosis. We present a case of acute unprovoked portal vein thrombosis diagnosed in a 59-year-old male without local disease factors. Computed tomography, magnetic resonance cholangiopancreatography, and ultrasound demonstrated the presence of portal vein thrombosis with neighboring periportal and pancreatic head edema. Peripheral blood testing detected the presence of JAK2 V617F mutation. The patient was discharged on 6-month anticoagulation therapy and outpatient follow-up.
Keywords: Portal vein thrombosis, JAK2 V617F
Introduction
Portal vein thrombosis (PVT) is defined as a partial or complete obstruction of the portal vein by a clot resulting in impeded flow [1]. It is an infrequent occurrence that has been increasingly recognized with the broader utilization of radiological imaging in clinical practice [1]. Population prevalence is estimated to be 1% with 0.6%-16% occurring in asymptomatic liver disease, 15% of patients awaiting liver transplant, and 35% in the setting of cirrhosis with hepatocellular carcinoma [2].
The portal vein is formed by the confluence of the splenic and superior mesenteric veins. It divides superiorly at the porta hepatis into left and right branches supplying the left and right hepatic lobes, respectively. PVT forms in the trunk of the portal vein and can extend into the left and right intrahepatic branches or the splenic and superior mesenteric veins. Proposed grading of PVT by Yerdel et al is based on the percentage of portal vein occlusion and extension into the superior mesenteric vein (Table 1) [1], [2], [3], [4], [5]. Mesenteric vein involvement is associated with an increased risk of intestinal infarction and mortality [2]. Over time, if portal vein obstruction persists, surrounding vessels can dilate to permit collateral flow resulting in the formation of a cavernoma [1].
Table 1.
Classifications of PVT based on anatomic extent by Yerdel et al [5].
| Classification | Occlusion of portal vein | Extension of thrombus |
|---|---|---|
| Grade 1 | Minimal or partial thrombosis of the portal vein with <50% occlusion | With or without minimal SMV involvement |
| Grade 2 | >50% and complete thrombosis of the portal vein | With or without minimal SMV involvement |
| Grade 3 | Complete thrombosis of the portal vein | Proximal SMV is occluded with patent distal SMV |
| Grade 4 | Complete thrombosis of the portal vein | Proximal and distal SMV occlusion |
Abbreviation: SMV, superior mesenteric vein.
Predisposition to PVT is based on features of Virchow's triad: hypercoagulability, endothelial injury, and stasis [1]. Local factors, such as cirrhosis and intraabdominal infections carry an increased risk of developing PVT. Reduced portal flow and abdominal inflammation leads to endothelial activation and release of prothrombotic factors [2]. Systemic factors including hypercoagulable states and sepsis predispose the formation of PVT. Underlying malignancies, most commonly hepatocellular carcinoma, can also increase the risk of thrombosis due to invasion of the vasculature or compression of the portal vein [3]. Other malignancies associated with PVT include pancreatic, biliary, islet cell, and metastatic cancer [3]. In noncirrhotic cases of PVT, also termed extrahepatic portal vein obstruction, thrombophilic risk factors are assessed particularly when a local cause cannot be identified during presentation [1] (Table 2). Patients with myeloproliferative neoplasms (MPN) carry an increased risk of developing PVT by 30%-40% [3], [6]. MPN are characterized by clonal expansion or overproduction of mature and functional cell types with increased granulocytes, increased red blood cells in polycythemia vera (PCV), or increased platelets in essential thrombocytosis (ET) [6]. V617F mutant JAK2 protein is positive in nearly all cases of PCV and in approximately 50% of cases of ET and primary myelofibrosis [6]. Thus, it is routinely tested when there is high suspicion of MPN [6], [7]. JAK2 V617F mutation exerts its action directly on bone marrow hematopoietic stem cells, resulting in an expansion of mature hematologic cells. Clonal expansion in the setting of this mutation is associated with an increased risk of thrombosis (relative risk, 2.94) and predisposes formation of PVT [6], [8].
Table 2.
Differential diagnosis for PVT associated findings with risk and frequency of underlying causes.
| Cause | Labs | Clinical findings | Imaging | PVT frequency | PVT risk |
|---|---|---|---|---|---|
| Liver cirrhosis | CBC, liver chemistry [3] | Ascites, jaundice, hepatic encephalopathy, gastrointestinal bleeding, portal hypertension, abdominal pain [2] | Irregular liver outline, portal vein dilation [2] | Incidence: 11.2%-16.6% [2], [3] |
Odds ratio: 17.1 in cirrhosis with primary hepatic cancer, 5.2 in cirrhosis without primary hepatic cancer[3] |
| Liver carcinoma | Serum AFP levels [9] | Advanced stage, major vessel involvement, low serum albumin, high serum AFP levels [3] | Filling defect with rim enhancement of vessel wall, disruption of vessel wall, expansive effect due to tumor mass [2] | Incidence: 20%-44% [3], 35% in combination with cirrhosis [2] |
|
| Liver transplant | - | Decreased caliber of portal vein, donor/recipient portal vein diameter mismatch [2] | - | Incidence: 13.8% no portosystemic shunt, 38.9% prior portosystemic shunt [3] | - |
| Pancreatitis | Serum amylase/ lipase [15] | Premature activation of digestive enzymes and inflammation, acute severe epigastric pain radiating to the back [15] | Acute pancreatitis: Diffuse enlargement of pancreas, heterogeneous enhancement, peripancreatic stranding [15] | Incidence: 23% in acute pancreatitis, 57% in pancreatic necrosis [2] |
- |
| Hypercoagulable states (factor V leiden, protein C deficiency, protein S deficiency, antithrombin III deficiency, prothrombin mutation, antiphospholipid syndrome, paroxysmal nocturnal hemoglobinuria) |
Factor V mutation, protein C & S levels, ATIII, G20210A, cardiolipin, lupus anticoagulant, anti-beta2 glycoprotein antibodies, CD55/CD59 [2] |
Acute PVT: Abdominal pain, fever, ascites, splenomegaly [2] Chronic PVT: Recurrent upper abdominal pain, portal hypertension, varices, splenomegaly, anemia, thrombocytopenia [2] | Acute PVT: hypoechogenic/ hypodense/ hypointense thrombus, absence of porto-systemic collaterals [2] Chronic PVT: absent (fibrotic) portal vein with cavernoma, portal hypertension, wall/thrombus calcifications hyperdensities on CT [2] |
- | Relative risk: 10-20 in Protein C, Protein S, and Antithrombin III deficiency; 8 in Antiphospholipid syndrome [3] |
| Myeloproliferative disorders (PCV, ET) | JAK2 V617F [2], [3], [7] | 30%-40% [2], [3] | Odds ratio: 3.0 [10] |
Few cases of PVT in the presence of JAK2 V617F mutation have been published in the literature. We report a case of a 59-year-old male who presented with acute unprovoked PVT without a history of heavy alcohol use or liver disease. Initial computed tomography (CT) scan revealed portal vein thrombosis with mild infiltrative changes along the portal vein and pancreatic head raising the possibility of pancreatitis. However, serum amylase and lipase were within normal limits decreasing suspicion for pancreatitis as the primary cause. A multidisciplinary approach was implemented to identify the etiology of the patient's condition, and appropriate therapy was administered.
Case presentation
A 59-year-old male presented to the emergency department with complaints of 10-pound weight loss, poor appetite, nausea, dry heaving, and abdominal pain. His pain, described as constant and diffuse without radiation, started 1 week earlier. The patient denied changes in bowel habits, bloody, or bilious vomiting, melena, or hematochezia. Chronic problems included gastroesophageal reflux disease, hypercholesterolemia, hypertensive disorder, degenerative disc disease, nephrolithiasis, and alpha-1-antitrypsin mutation without systemic deficiency. Family history was positive for colon cancer, multiple myeloma, and factor V Leiden mutation. The patient denied heavy alcohol use and history of smoking.
Physical examination demonstrated generalized abdominal tenderness without peritoneal signs. Complete blood count (CBC) revealed a red blood cell count of 4.59 × 106/µL (reference range: 4.3-5.9 × 109/µL), hemoglobin of 14.4 g/dL (reference range: 13.5-17.5 g/dL), hematocrit of 43.0% (reference range: 41%-53%), and platelet count of 211 × 103/µL (reference range: 150-400 × 103/µL). Other laboratory findings of note included a white blood count of 10.5 × 109/L (reference range: 4.5-11.0 × 109/L), aspartate aminotransferase of 56 U/L (reference range: 8-20 U/L), alanine aminotransferase of 105 U/L (reference range: 8-20 U/L), and total bilirubin of 1.4 mg/dL (reference range: 0.1-1 mg/dL). Serum amylase and lipase were within normal limits. Due to acute abdominal symptoms, the initial study performed was contrast CT with 300 mg intravenous iopromide (Ultravist, Bayer Pharmaceuticals, Berlin, Germany) of the abdomen and pelvis. Findings demonstrated decreased attenuation in the main portal vein extending from the main portal vein into the porta hepatis (Fig. 1). Neighboring mild inflammatory changes surrounding the head of the pancreas suggested pancreatitis, but serum amylase and lipase levels were within normal limits. There were no signs of pancreatic necrosis, peripancreatic fluid collection, or dilation of the common bile duct. Follow-up magnetic resonance cholangiopancreatography without contrast supported initial CT findings, showing thrombosis involving the main portal vein, both left and right branches, the splenic vein, and the mesenteric vein (Fig. 2). Mild increased signal around the pancreatic head and body was also observed. Portal vein ultrasound was also performed, confirming the presence of PVT (Fig. 3).
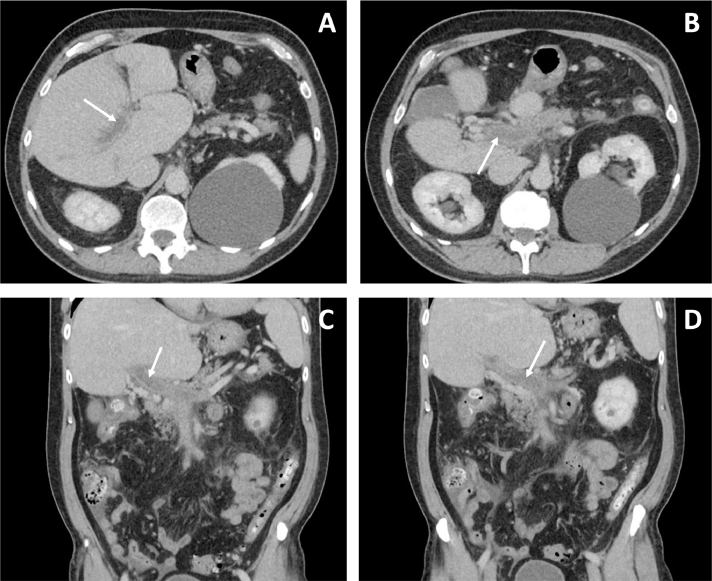
Fig. 1.
IV contrast enhanced CT images with axial (A, B) and coronal (C, D) depict decreased attenuation in the portal vein consistent with thrombosis (white arrows) with axial and coronal views. Reticulation of the neighboring periportal fat to include the peripancreatic fat indicates edema. Incidentally noted is a large left renal cyst.
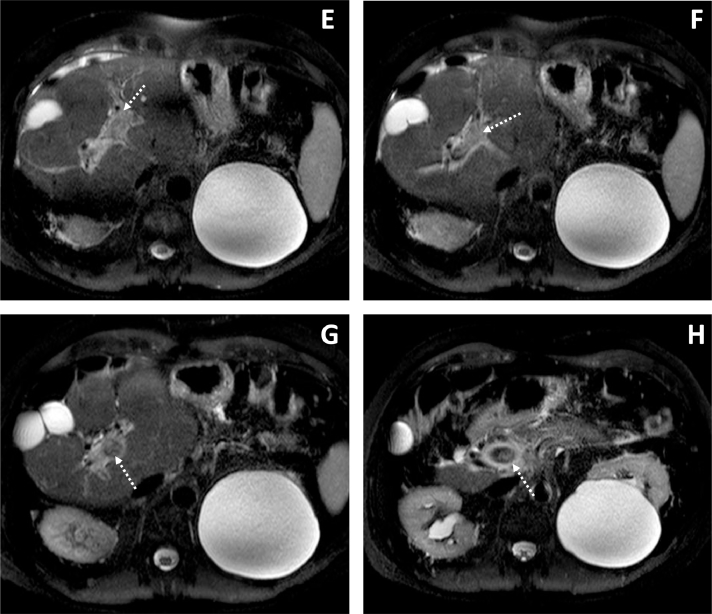
Fig. 2.
T2-weighted spectral attenuated inversion recovery (SPAIR) MRI axial images (E-H) reveal increased signal in the portal vein indicative of thrombosis (dashed arrows) and increased signal surrounding the vein consistent with edema. Incidentally noted is a large left renal cyst.
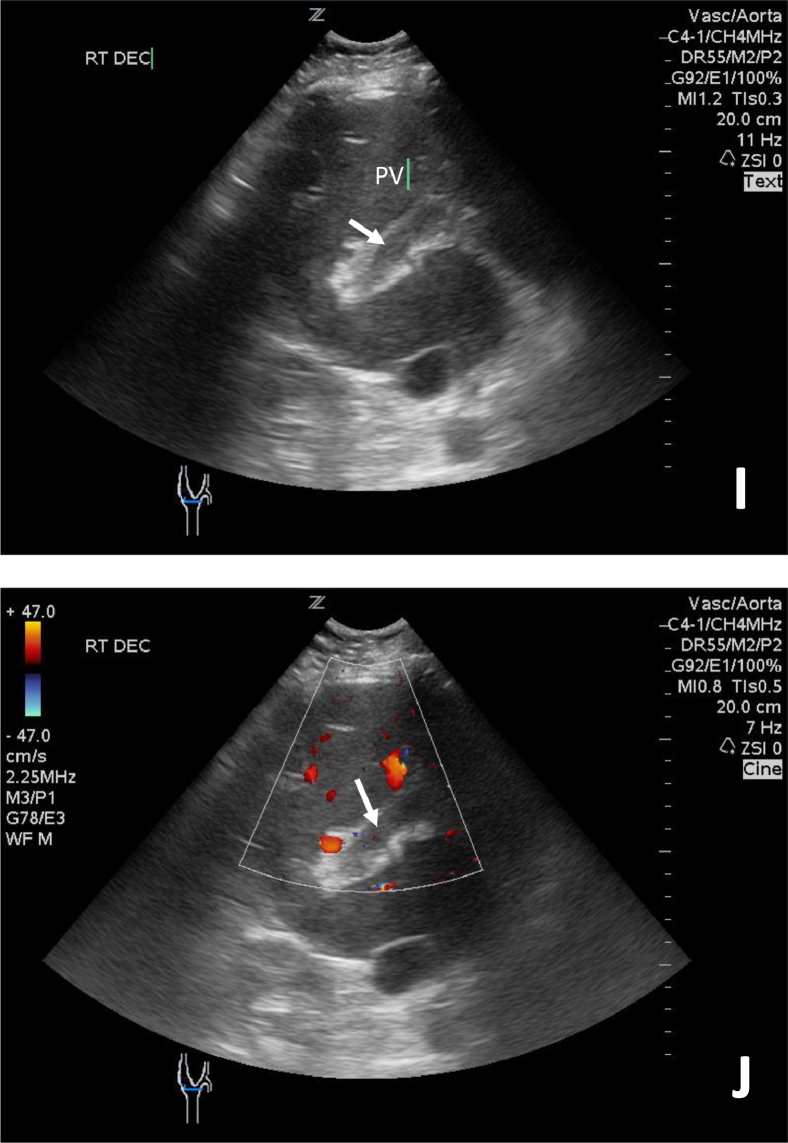
Fig. 3.
Portal vein ultrasound images (I, J) without and with color Doppler demonstrate increased echogenicity and no flow within the portal vein consistent with portal vein thrombosis.
The patient was made nil per os and received intravenous fluid hydration with supportive care for pain and nausea. Anticoagulation therapy was initiated with intravenous heparin and then transitioned to therapeutic enoxaparin. Hematology-oncology was consulted for further evaluation of acute unprovoked PVT without evidence of local disease. Peripheral blood testing detected the presence of JAK2 V617F mutation. Though family history was positive for factor V Leiden, testing for inherited or acquired thrombophilia including factor V Leiden, protein C, S, antithrombin III (ATIII), and prothrombin (G20210A) mutations yielded negative results. There was no evidence of paroxysmal nocturnal hemoglobinuria with flow cytometry. Serum CA19-9 levels were also tested and found to be normal.
The patient remained inpatient for 7 days and was discharged on enoxaparin to be continued for 6 months. Seven months later, the patient followed up as an outpatient with hematology-oncology and reported their symptoms resolved. Approximately 1-month prior to this follow-up visit, the patient presented to the emergency department and received contrast enhanced CT imaging due to concerns of urinary tract infection vs pyelonephritis with history of recurrent nephrolithiasis. Incidentally noted on this imaging was the presence of cavernous transformation of the portal vein surrounding the thrombosed portal vein and gallbladder (Fig. 4).
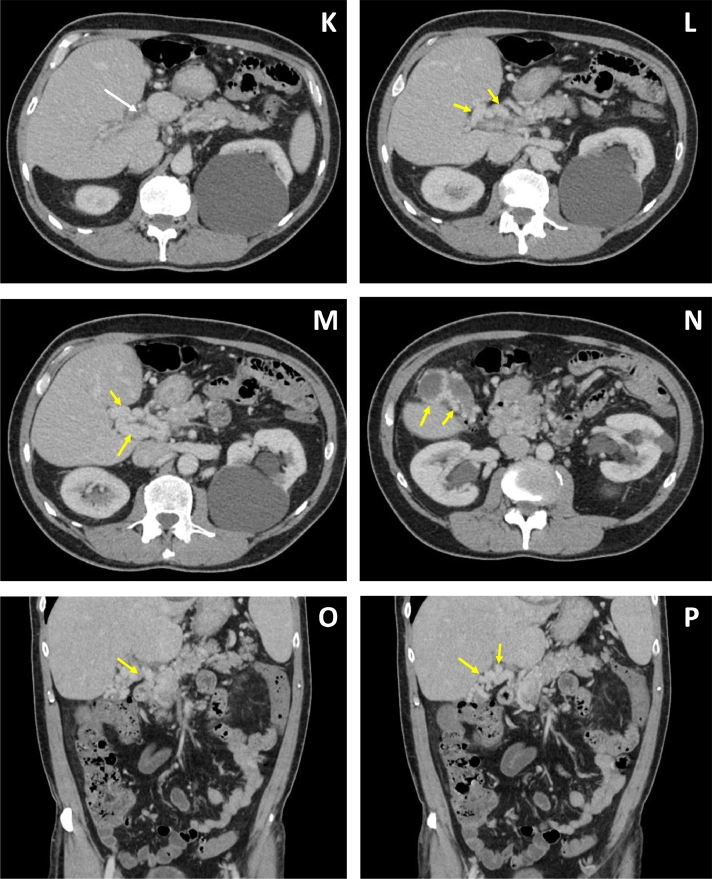
Fig. 4.
Six months after the initial imaging, IV contrast enhanced CT images in axial (K-N) and coronal (O, P) plane depict decreased attenuation in the portal vein consistent with thrombosis (white arrows). Tubular enhancing structures around the thrombosed portal vein are consistent with cavernous transformation of the portal vein (yellow arrows). Collateral vessels are noted to surround the gall bladder demonstrated in image N. (Color version of this figure is available online.)
Discussion
PVT can present acutely or exist in chronic states with a broad array of underlying causes [3]. Acute PVT typically presents with symptoms of acute abdominal pain, fever, ascites, or splenomegaly [1], [2], [3]. Useful laboratory studies for evaluating PVT include liver function testing, CBC, and testing for prothrombotic risk factors. Liver chemistry may be normal in the absence of liver disease or biliary obstruction. Abnormalities on CBC suggestive of PVT include leukocytosis in the setting of intraabdominal infection, erythrocytosis in PCV, or thrombocytosis in ET [3]. Chronic PVT is generally asymptomatic when detected and is more commonly an incidental finding on radiological imaging. The presence of collateral veins surrounding the region of occlusion, thrombus calcifications, or signs of portal hypertension are often visualized in the setting of chronic PVT [1], [3]. Signs of malignancy, cirrhosis, ascites, hepatic congestion, or ischemia should be noted on imaging as well as identifying the age of thrombus and extent of splenomesenteric vessel involvement [9]. Due to the broad range of conditions that predispose PVT formation, management involves both diagnostic imaging and testing for prothrombotic risk factors in all patients who present with local or systemic disease [3], [9] (Table 2).
Diagnostic imaging is warranted in patients with sudden epigastric or diffuse abdominal pain with criteria for systemic inflammatory response syndrome and/or has a suspected prothrombotic state [9]. When PVT is suspected, ultrasound is recommended as first-line imaging with an accuracy of 88%-98% and a specificity of 60%-100% [1], [2], [3]. Color Doppler ultrasonography and pulsed wave ultrasonography can be used to determine the degree of flow within the portal vein and whether there is partial or complete occlusion [1], [2]. Presence of PVT is suspected on ultrasound, when thrombosis is directly visualized or when there is echogenicity within the portal vein with loss of venous flow, inability to identify the portal vein, and/or dilation of the thrombosed portion [3]. In cirrhotic patients, the risk of PVT is greater with decreased flow velocity of <15 cm/s [3], [10]. CT with IV contrast is preferred in patients with acute abdominal symptoms for better visualization of anatomy and extension of occlusion [2]. CT additionally provides advantages of visualizing bowel ischemia, presence of malignancy, or cirrhosis [1], [2], [3], [9]. Decreased enhancement inside the portal vein lumen, increased hepatic enhancement during the arterial phase, and/or decreased venous enhancement in the liver during the portal venous phase raises clinical suspicion [3]. Magnetic resonance imaging (MRI) has comparable sensitivity to CT for detection of thrombus. Both modalities can be used to visualize bowel ischemia, septic foci, intraabdominal malignancies, and cavernous transformation [1]. MRI additionally can be used to evaluate abnormalities of the biliary system and gallbladder and is useful in the setting of extrahepatic portal vein obstruction [1]. MRI, however, is limited by its reduced availability in practice and its inability to demonstrate calcification within the thrombi [1], [3].
In almost half of PVT cases, more than 1 risk factor is identified. Investigation into inherited and acquired prothrombotic risk factors including MPN is recommended [10]. PCV increases prevalence of thrombus formation by 34%-39% and poses additional risks of hemorrhage, thrombocytosis, and neutrophilia [7], [11]. The World Health Organization 2016 criteria states 3 major criteria and 1 minor criterion for diagnosis of PCV (Table 3). Diagnosis of PCV is made, when either all 3 major criteria are met, or if the first 2 major criteria and the minor criterion are positive [12]. Secondary causes of erythrocytosis including respiratory disease, renal disease, decreased plasma volume, or congenital etiologies should be excluded before a diagnosis of PCV is made [11]. Symptoms of PCV, when present, are related to increased blood viscosity and release of cytokines. Patients may complain of visual disturbances, fatigue, pruritus, sweats, and myalgias [11].
Table 3.
2016 WHO major and minor criteria for PCV diagnosis [12].
| Major criteria | 1 | Hemoglobin > 16.5 g/dL in males and > 16.0 g/dL in females or Hematocrit > 49% in males and > 48% in females or Increased red cell mass |
| 2 | Bone marrow showing hypercellularity for age with panmyelosis and presence of proliferation of mature megakaryocytes | |
| 3 | Positive testing for JAK2 V617F or JAK2 exon 12 mutation | |
| Minor criterion | Subnormal serum erythropoietin level |
Treatment of PVT is directed towards reducing the advancement of thrombosis and preventing development of portal hypertension or intestinal infarction [13]. Due to lack of randomized controlled trials, treatment decisions are made based on individual expertise [2]. Restoring portal venous flow in both partial and complete occlusion is achieved with anticoagulation in patients with prothrombotic states without contraindications to therapy [3]. The mainstay therapy immediately following diagnosis of acute noncirrhotic nonmalignant PVT is low molecular weight heparin followed by long-term use of vitamin K antagonists with targeted INR of 2-3 [3,6,9]. Anticoagulation is effective in achieving 42%-100% recanalization of the portal vein and is associated with a low rate of thrombus extension following treatment (0%-15%) [4]. Because recanalization occurs between 4 to 6 months, it is recommended that anticoagulation be continued for at least 6 months [1], [2], [3]. Treatment beyond 6 months is dependent on patients with high recurrence risk, underlying risk factors, or genetic predispositions [6]. Permanent anticoagulation is favored for reducing recurrence of PVT secondary to genetic predispositions including MPN [9]. Endovascular interventions to restore portal flow, including placement of a transjugular intrahepatic portosystemic shunt or local thrombolysis have been utilized in patients that fail conservative management or demonstrate signs of bowel ischemia [14]. Five- and 10-year survival in patients with noncirrhotic nonmalignant PVT is 90% and 80%, respectively. Patients with cirrhosis or hepatobiliary malignancy tend to have a poorer prognosis [6]. The safety and efficacy of treating PVT with anticoagulation in patients with cirrhosis remains unclear [3], [4]. Monitoring and follow-up without anticoagulation is advised in cirrhotic patients with asymptomatic PVT and <50% occlusion [4]. Once diagnosis of PVT is made, follow-up imaging is recommended every 6 months with ultrasound or contrast enhanced CT or MRI when ultrasound demonstrates suboptimal visualization. Magnetic resonance cholangiopancreatography is utilized for grading portal vein obstruction and monitoring for biliary complications if the portal vein fails to recanalize after 12 months or in states of cavernous transformation [1].
Conclusion
In conclusion, this case provides a unique example of PVT that arose in the context of JAK2 positive genetic predisposition without discernable liver disease. The presented patient did not meet criteria for PCV based on World Health Organization classification at the time PVT was diagnosed. However, due to the presence of the JAK2 V617F mutation, it was assumed that the patient was predisposed to a systemic hypercoagulable state, like those formally diagnosed with PCV and as such, anticoagulation was initiated. CBC would be monitored for manifestations of PCV at subsequent patient follow-up and consideration will be given for further imaging if symptoms recur. Recommendations suggest close examination of PVT and documentation of radiological findings visualized in the hepatobiliary system, portal, and splenomesenteric vessels, and pancreatic anatomy to better guide the multidisciplinary team in determining local or systemic causes. Communication between radiologists, clinicians, and surgeons can provide a more comprehensive approach to identifying underlying causes for PVT and initiating appropriate treatment.
Footnotes
Declarations of interest: None.
Informed consent was obtained from all individual participants involved in this study.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.radcr.2018.08.023.
Appendix. Supplementary materials
References
- 1.Margini C, Berzigotti A. Portal vein thrombosis: the role of imaging in the clinical setting. Dig Liver Dis. 2017;49(2):113–120. doi: 10.1016/j.dld.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Chawla YK, Bodh V. Portal vein thrombosis. J Clin Exp Hepatol. 2015;5(1):22–40. doi: 10.1016/j.jceh.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basit SA, Stone CD, Gish R. Portal vein thrombosis. Clin Liver Dis. 2015;19(1):199–221. doi: 10.1016/j.cld.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Qi X, Han G, Fan D. Management of portal vein thrombosis in liver cirrhosis. Nat Rev Gastroenterol Hepatol. 2014;11(7):435–446. doi: 10.1038/nrgastro.2014.36. [DOI] [PubMed] [Google Scholar]
- 5.Yerdel MA, Gunson B, Mirza D, Karayalçin K, Olliff S, Buckels J. Portal vein thrombosis in adults undergoing liver transplantation—risk factors, screening, management, and outcome. Transplantation. 2000;69(9):1873–1881. doi: 10.1097/00007890-200005150-00023. [DOI] [PubMed] [Google Scholar]
- 6.Leebeek FWG, Smalberg JH, Janssen HLA. Prothrombotic disorders in abdominal vein thrombosis. Neth J Med. 2012;70(9):400–405. [PubMed] [Google Scholar]
- 7.Tevet M, Ionescu R, Dragan C, Lupu AR. Influence of the JAK2 V617F mutation and inherited thrombophilia on the thrombotic risk among patients with myeloproliferative disorders. Mædica. 2015;10(1):27–32. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4496761&tool=pmcentrez&rendertype=abstract [PMC free article] [PubMed] [Google Scholar]
- 8.Nielsen C, Birgens HS, Nordestgaard BG, Kjaer L, Bojesen SE. The JAK2 V617F somatic mutation, mortality and cancer risk in the general population. Haematologica. 2011;96(3):450–453. doi: 10.3324/haematol.2010.033191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plessier A, Rautou PE, Valla DC. Management of hepatic vascular diseases. J Hepatol. 2012;56(Suppl. 1) doi: 10.1016/S0168-8278(12)60004-X. [DOI] [PubMed] [Google Scholar]
- 10.Trebicka J, Strassburg CP. Etiology and complications of portal vein thrombosis. Visz Gastrointest Med Surg. 2014;30(6):375–380. doi: 10.1159/000369987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grinfeld J, Godfrey AL. After 10 years of JAK2V617F: disease biology and current management strategies in polycythaemia vera. Blood Rev. 2017;31(3):101–118. doi: 10.1016/j.blre.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2406. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 13.Lisman T. Low molecular weight heparin in management and prevention of portal vein thrombosis. Thromb Res. 2014;134(4):761–762. doi: 10.1016/j.thromres.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Rosenqvist K, Eriksson L-G, Rorsman F, Sangfelt P, Nyman R. Endovascular treatment of acute and chronic portal vein thrombosis in patients with cirrhotic and non-cirrhotic liver. Acta Radiol. 2016;57(5):572–579. doi: 10.1177/0284185115595060. [DOI] [PubMed] [Google Scholar]
- 15.Thoeni RF. Imaging of acute pancreatitis. Radiol Clin North Am. 2015;53(6):1189–1208. doi: 10.1016/j.rcl.2015.06.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.