Abstract
Hyperreactio luteinalis (HL) is a rare pregnancy-related condition in which the ovaries become massively enlarged bilaterally, occupied by multiple benign theca lutein cysts, secondary to increased ovarian stimulation by beta-human chorionic gonadotropin (B-hCG). HL should resolve spontaneously postpartum, however, their occurrence has led some physicians unfamiliar with the natural history of the condition to perform unnecessary ovarian cystectomies or oophorectomies. A healthy 32-year-old woman was incidentally found to have new onset multicystic ovaries on ultrasound at 31 + 3 weeks gestational age, which continued to enlarge, with a maximum volume of ∼448.0 cm3 and ∼323.5 cm3 in right and left ovaries, respectively. She also developed signs and symptoms of hyperandrogenism, and later abdominal pain which ultimately expedited delivery. This paper demonstrates that familiarity with HL as a clinical entity, its typical presentation and natural history, and targeting conservative management is paramount in minimizing iatrogenic harm by obstetricians given the increased use of ultrasound in pregnancy. Patients presenting after the first trimester with bilateral multicystic ovaries with a "spoke wheel" appearance on ultrasound, hyperandrogenism, abnormally elevated B-hCG, or symptoms consistent with elevated B-hCG should prompt a possible diagnosis.
Keywords: Hyperreactio luteinalis, Theca lutein cysts, Hyperandrogenism in pregnancy, Multicystic ovaries in pregnancy, Adnexal mass in pregnancy, Elevated B-hCG
Introduction
Hyperreactio luteinalis (HL) is a rare pregnancy-related condition in which maternal ovaries become massively enlarged bilaterally, occupied by multiple benign theca lutein cysts (TLCs). TLCs develop secondary to increased ovarian stimulation by beta-human chorionic gonadotropin (B-hCG) [1]. Their occurrence has been more commonly documented in cases of multiple pregnancies, gestational trophoblastic disease, or choriocarcinoma, where B-hCG is excessively elevated [2], [3].
In singleton pregnancies, the literature consists mainly of case reports, with the natural history thus far demonstrating spontaneous postpartum resolution. Given the rarity of HL, patients have unfortunately undergone cystectomies or oophorectomies by surgeons unfamiliar with the condition [4], [5].
With the increasing use of ultrasound during pregnancy, more TLCs will likely be found incidentally. It is therefore essential that physicians practicing obstetrics be familiar with HL to direct management and avoid unnecessary iatrogenic morbidity. Here, we present the case of a conservatively managed singleton pregnancy with an incidental finding of TLCs on ultrasound.
Case
A healthy 32-year-old Rh+ primigravida woman of Chinese descent presented for prenatal care. She conceived spontaneously with her husband, also of Chinese descent. Family history was significant only for paternal hypertension. The pregnancy was complicated by nausea and vomiting in the first trimester, new onset suppressed TSH at 0.07µU/L (with normal free T4 at 11pmol/L), 2 enterococcus urinary tract infections (each treated with amoxicillin), carpal tunnel syndrome, pruritic urticarial papules, and plaques of pregnancy and diet controlled gestational diabetes.
At the patient's 19 + 4 weeks anatomy sonogram the maternal ovaries were normal, measuring 4.7 × 1.7 cm on the right and 4.0 × 2.0 cm on the left.
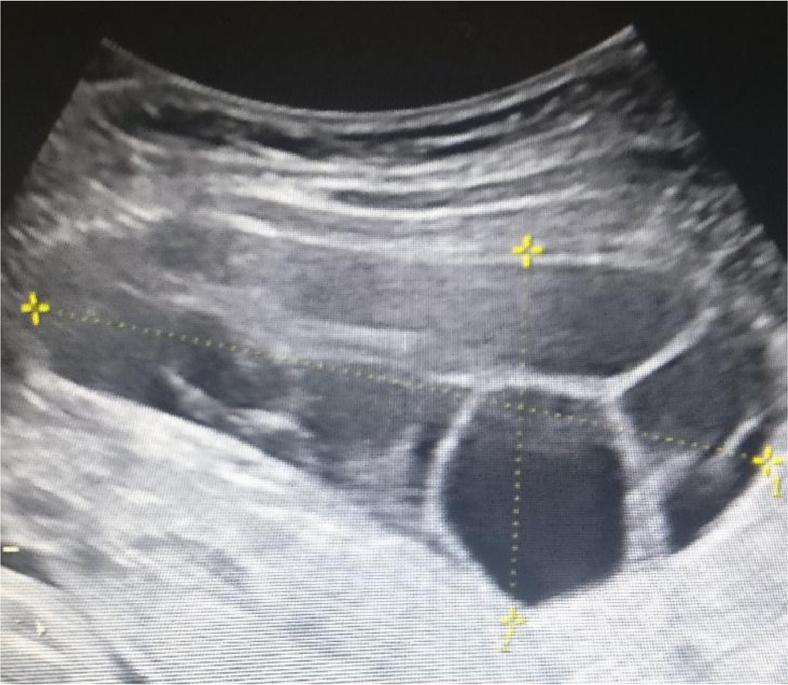
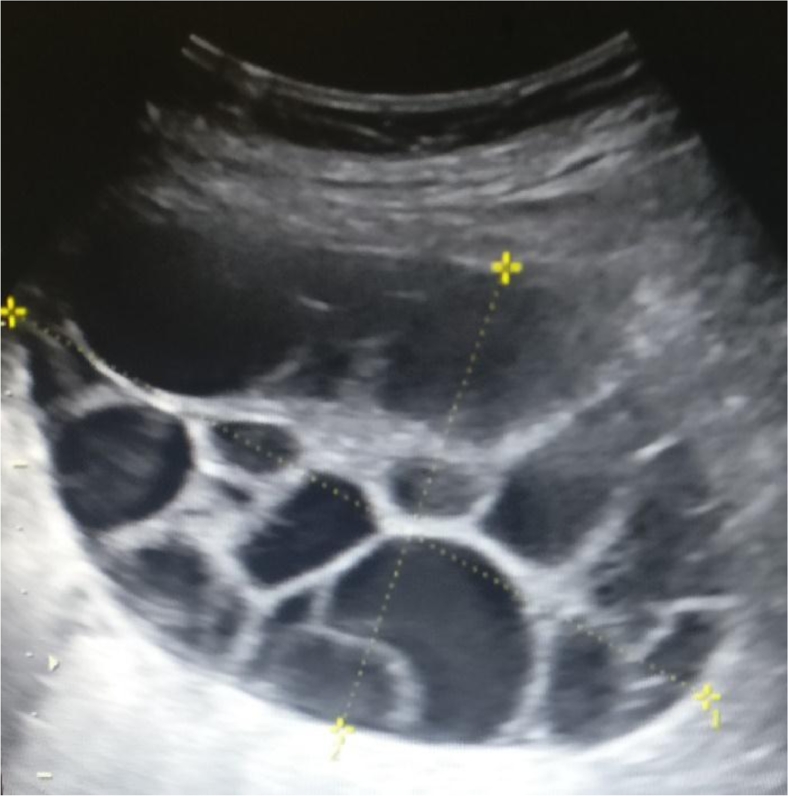
At 31 + 3 weeks, a fetal growth ultrasound was performed. Estimated weight was normal (1869 g, 70th percentile), however, multicystic enlarged maternal ovaries with thin septations were noted bilaterally, with the right measuring 11.9 × 7.3 × 7.1 cm (∼308.4 cm3), and the left measuring 9.3 × 6.7 × 4.4 cm (∼137.1 cm3). There were no solid components or abnormal vascularity (Figs. 1 and 2).
Fig. 1.
Ultrasound of right ovary at 31 + 3 weeks. Simple appearing cysts, thin septations, and no solid components
Fig. 2.
Ultrasound of right ovary at 31 + 3 weeks. Simple appearing cysts, thin septations, and no solid components
New facial acne and hirsutism were present at 37 weeks. Laboratory investigations demonstrated hyperandrogenism (testosterone 146 nmol/L, normal <1.9 nmol/L; bioavailable testosterone 2.4 nmol/L, normal <0.3 nmol/L; sex hormone-binding globin 595 nmol/L, normal 20-122 nmol/L; albumin 38 g/L, normal 35-50 g/L).
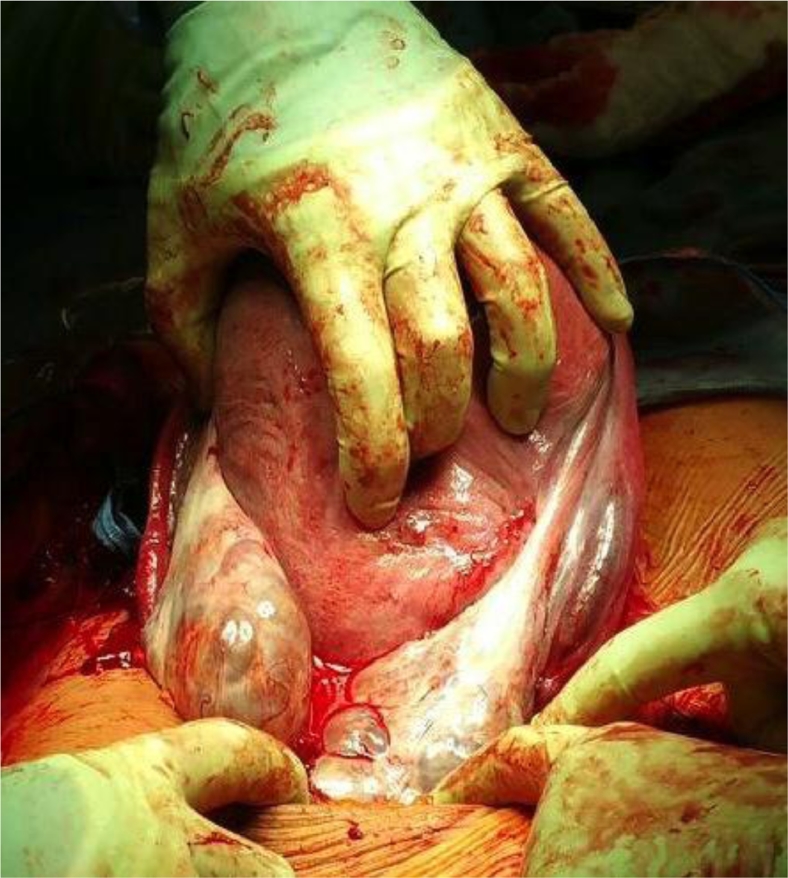
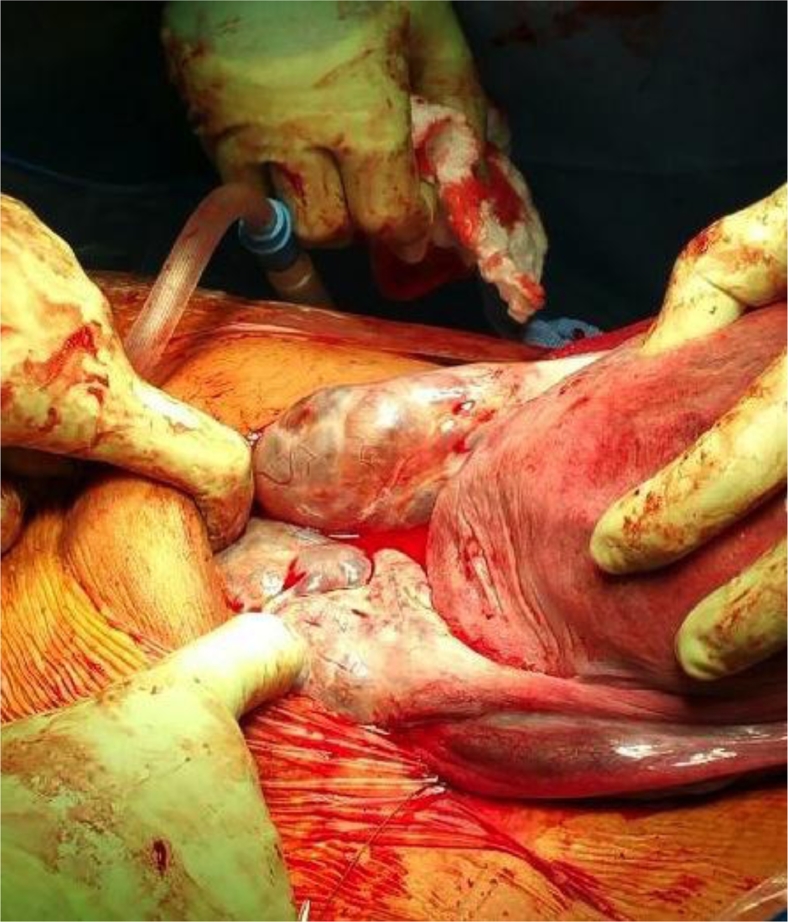
At 38 + 2 weeks, a follow-up ultrasound was performed in response to right lower quadrant pain. The ovaries had further increased in size (right ovary 14.4 × 6.1 × 10.2 cm, ∼448.0 cm3; and left 13 × 7.9 × 6.3 cm, ∼323.5 cm3). Estimated fetal weight had dropped to 37% percentile (3144 g). The patient underwent an elective lower segment cesarean section 3 days later for persistent abdominal discomfort. A live female infant was born weighing 3048 g with no evidence of virilization. The placenta appeared normal. At the time of surgery, the ovaries were confirmed to be occupied by multiple simple cysts (Figs. 3 and 4) and they were left in situ. Her postoperative course was unremarkable and she was discharged home on postoperative day 2.
Fig. 3.
Multicystic ovaries at time of cesarean section
Fig. 4.
Multicystic ovaries at time of cesarean section
By the ninth week postpartum, the patient's free testosterone and TSH normalized to 3 nmol/L and 1.21 µU/L, respectively. Her follow-up ultrasound revealed complete resolution of her ovarian cysts (right ovary 4.4 × 3.2 × 3.9 cm (∼27.5 cm3) with a 3.1 cm dominant simple follicle and left ovary 4.0 × 2.0 × 4.5 cm (∼18.0 cm3). Her acne was the only abnormality that persisted.
Discussion
This case serves as evidence of the self-resolving nature of HL. Clinicians must recognize the typical presentation and acute issues that may arise in these patients. HL may manifest with symptoms typical of large ovarian cysts including torsion, peritonitis from hemorrhage or rupture, and mass effects [5]. Ultrasound is the imaging modality of choice. Sonographic findings of a classic “spoke wheel” appearance, bilateral cysts, normal doppler flow, and a lack of solid components differentiate TLCs from ovarian malignancies [6].
It is believed that TLCs develop in response to elevated B-hCG or increased ovarian stromal sensitivity to B-hCG although the underlying cause of this elevation is not understood [1] B-hCG has an α-subunit identical to TSH. Elevated levels can therefore have a thyrotrophic effect causing suppressed TSH, and in some cases, thyrotoxicosis [7]. High levels of B-hCG have also been positively correlated with severe nausea and vomiting of pregnancy [8]. Our patient presented with both these symptoms as well as suppressed TSH.
Maternal hyperandrogenism during pregnancy is rare; however, TLCs are among the most common causes .[9]. Signs include temporal balding, clitoromegaly, deepening of the voice, and as in our patient, hirsutism, and acne. In pregnancy, increased levels of sex hormone-binding globin reduce the clinical manifestations of hyperandrogenism as the molecule has a high-affinity for both dihydrotestosterone and testosterone, reducing their effects once bound [9]. In the majority of HL patients (estimated at 70%) there is no clinical evidence of hyperandrogenism [5]. Fetal virilization is even rarer as HL typically presents in the second or third trimester, avoiding interference with fetal labioscrotal fusion, which typically happens prior to 12 weeks [5].
In a 2015 publication, Malinowski et al [5] conducted the largest review to date of HL in the English literature drawing on cases from PubMed between 1993 and 2014. This yielded 58 pregnancies. Of these, similar to our patient, 81% were singleton (93.1% spontaneously conceived) with 66.7% occurring in primiparous women. Comorbidities relevant to our patient, hyperthyroidism and gestational diabetes, occurred in 8.6% and 5.2%, respectively. The study also reported preterm delivery in 28.9% of singleton pregnancies, preeclampsia in 19%, intrauterine growth restriction in 31.7%, and suggested a possible relationship between those complications and increased B-hCG level in pregnant patients with HL. None of these complications were present in our patient, albeit the fetus demonstrated a falloff in growth at 38 weeks.
During labor, dependent on the position of the enlarged ovaries within the abdomen and pelvis, there is a theoretical potential for obstruction of fetal descent, fetal malpresentation, and cyst rupture. However, HL is not considered a contraindication to vaginal delivery and successful cases are documented in the literature [5]. Despite an initial plan for spontaneous vaginal delivery, our patient elected for a cesarean section due to persistent abdominal pain.
Postpartum, high maternal levels of both B-hCG and testosterone, as in HL, have been associated with delayed lactation [10]. Early involvement of a lactation consultant and nipple stimulation strategies are essential components of the postpartum management of these patients [5]. Our patient had a transient delay in lactation and low milk supply, which resolved with professional guidance.
Conclusion
Patients presenting after the first trimester of pregnancy with bilateral mulitcystic ovaries with a “spoke wheel” appearance on sonogram, hyperandrogenism, abnormally elevated B-hCG or symptoms consistent with elevated B-hCG should prompt a possible diagnosis of HL. An understanding of the natural history of this condition and targeting conservative management is paramount in minimizing unnecessary iatrogenic harm to patients. Close monitoring with ultrasound examinations antepartum and postpartum to ensure return to normal ovarian architecture is recommended.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.radcr.2018.08.022.
Contributor Information
Humara Edell, Email: humara.edell@mail.utoronto.ca.
Omid Shearkhani, Email: omid.shearkhani@mail.utoronto.ca.
M. Rose Rahmani, Email: roserahmani@gmail.com.
Rose C. Kung, Email: rose.kung@sunnybrook.ca.
Appendix. Supplementary materials
References
- 1.Telischak NA, Yeh BM, Joe BN, Westphalen AC, Poder L, Coakley F V. MRI of adnexal masses in pregnancy. AJR Am J Roentgenol. 2008;191:364–370. doi: 10.2214/AJR.07.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wajda KJ, Lucas JG, Marsh WL. Hyperreactio luteinalis. Benign disorder masquerading as an ovarian neoplasm. Arch Pathol Lab Med. 1989;113:921–925. [PubMed] [Google Scholar]
- 3.Montz FJ, Schlaerth JB, Morrow CP. The natural history of theca lutein cysts. Obstet Gynecol. 1988;72:247–251. [PubMed] [Google Scholar]
- 4.Skandhan AK, Ravi V. Hyperreactio luteinalis: an often mistaken diagnosis. Indian J Radiol Imaging. 2014;24:84–86. doi: 10.4103/0971-3026.130711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malinowski AK, Sen J, Sermer M. Hyperreactio luteinalis: maternal and fetal effects. J Obstet Gynaecol Canada. 2015;37:715–723. doi: 10.1016/S1701-2163(15)30176-6. [DOI] [PubMed] [Google Scholar]
- 6.Van Holsbeke C, Amant F, Veldman J, De Boodt A, Moerman P, Timmerman D. Hyperreactio luteinalis in a spontaneously conceived singleton pregnancy. Ultrasound Obstet Gynecol. 2009;33:371–373. doi: 10.1002/uog.6325. [DOI] [PubMed] [Google Scholar]
- 7.Glinoer D, De Nayer P, Robyn C, Lejeune B, Kinthaert J, Meuris S. Serum levels of intact human chorionic gonadotropin (HCG) and its free alpha and beta subunits, in relation to maternal thyroid stimulation during normal pregnancy. J Endocrinol Invest. 1993;16:881–888. doi: 10.1007/BF03348950. [DOI] [PubMed] [Google Scholar]
- 8.Lee NM, Saha S. Nausea and vomiting of pregnancy. Gastroenterol Clin North Am. 2011;40:309–334. doi: 10.1016/j.gtc.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaňová N, Bičíková M. Hyperandrogenic states in pregnancy. Physiol Res. 2011;60:243–252. doi: 10.33549/physiolres.932078. [DOI] [PubMed] [Google Scholar]
- 10.Betzold CM, Hoover KL, Snyder CL. Delayed lactogenesis II: a comparison of four cases. J Midwifery Womens Health. 2004;49:132–137. doi: 10.1016/j.jmwh.2003.12.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.