Highlights
-
•
Although rare, paraganglioma should be included in the differential diagnosis of an intra-thoracic membranous tracheal mass.
-
•
Upfront non-circumferential surgical resection of paraganglioma may be indicated.
-
•
Airway reconstruction can be performed using a latissimus dorsi flap.
-
•
Extra-corporeal membrane oxygenation may be useful for tracheal repair.
Abbreviations: VA ECMO, veno-arterial extra-corporeal membrane oxygenation; FDG PET-CT, fluoro-deoxyglucose positron emission tomography – computer tomography; FEV1, forced expiratory volume in 1 s; DLCO, diffusing capacity of the lung for carbon monoxide CO
Keywords: Extra-corporeal membranous circulation, Tracheal tumor, Muscle flap
Abstract
Introduction
Paraganglioma is a rare neuroendocrine tumor and may sometimes be located in the membranous part of the trachea.
Presentation of case
We report the case of a 52-year-old man presenting a paraganglioma just above the carina with obstructive symptoms. The patient successfully underwent a non-circumferential tracheal membranous resection, followed by latissimus dorsi muscle flap repair, under peripheral extra-corporeal membrane oxygenation (ECMO).
Discussion
Complex carinal resection can be avoided for tracheal membranous tumors and replaced with non-circumferential resection and direct reconstruction with a muscle flap. In addition, ECMO support may be used for airway resection and reconstruction.
Conclusion
Tracheal membranous tumors can be managed without circumferential resection or direct anastomosis.
1. Introduction
Tracheo-carinal cancer is an infrequently occurring tumor. In a majority of cases, it is secondary to invasion from neoplasms located in adjacent organs. Primary tracheal tumors are even less frequent and often present histological features similar to adeno-squamous cell carcinomas or adenoid cystic carcinomas. Surgical management of the former type of tracheo-carinal cancer may require complex carinal resection with tracheo-bronchial anastomosis and may be associated with important post-operative morbidity rate [1].
Paraganglioma are rare neuroendocrine tumors arising from chromaffin tissue deriving from the neural crest. In exceptional cases, they may be located in the membranous part of the trachea [2]. They are often well vascularized and this may result in major bleeding during pre-operative biopsy by bronchoscopy [3]. Thus, special care must be exercised to perform a biopsy (rigid bronchoscopy with laser coagulation). This may complicate the exact diagnosis due to a lack of suitable cellular material and result, as in our case, in upfront surgery. Complete surgical resection remains the standard of care due to the risk of malignant degeneration and poor response to chemotherapy or radiotherapy [3,4].
We report the case of an occlusive paraganglioma located in the distal part of the membranous trachea and successfully managed by non-circumferential membranous airway resection followed by latissimus dorsi muscle flap repair. This work has been reported in line with the SCARE criteria [5].
2. Presentation of case
A 52-year-old man was referred with a 4-month history of progressive cough, dyspnea and stridor. Past medical history revealed an abdominal Heller myotomy for esophageal achalasia 15 years earlier and no tobacco abuse. Chest CT-scan and FDG-PET scan showed an intensely contrast-enhancing endoluminal mass of 2 × 2 cm with a high FDG uptake emerging from the membranous part of the lower trachea, along with sub-occlusion of the two main bronchi (Fig. 1, Fig. 2). Pulmonary functional spirometry revealed a FEV1 of 42% and DLCO of 71% of the predicted value. Echocardiography was considered normal. Upfront surgical resection was proposed due to the carinal sub-occlusion and high risk of bleeding with pre-operative biopsy by bronchoscopy from a radiologically highly vascularized tumor. This was further justified considering the absence of distant metastatic lesions and because the principles of management of all tracheal neoplasms are similar (resection with adequate margin and primary reconstruction). General anesthesia was performed using single lumen endotracheal intubation. Right-sided peripheral femoro-femoral veno-arterial (VA) ECMO was initially realized with the patient in supine position. Then, the patient was positioned in a left decubitus and a right postero-lateral thoracotomy was performed through the fourth intercostal space after harvesting a pedicled latissimus dorsi muscle flap. The trachea and carina were dissected away from mediastinal structures and showed no invasion. A posterior non-circumferential membranous tracheo-carinal resection was performed resulting in a membranous airway defect of 4 × 4 cm extending in the two main bronchi. Intraoperative frozen section examination confirmed negative surgical margins. The airway defect was closed by using the intrathoracically transposed pedicled latissimus dorsi muscle flap harvested earlier through the 2nd intercostal space. The muscle was placed over the membranous defect and sutured around it with interrupted resorbable stitches of PDS 3.0 (Ethicon, Inc, Somerville, NJ) (Fig. 3). Intraoperative bronchoscopy showed no dehiscence or endoluminal protrusion of the muscle patch allowing good ventilation. The VA ECMO was removed after closure of the thoracotomy. The final pathological examination confirmed the complete resection of paraganglioma with main cells positive for neuroendocrine markers (synaptophysine and chromogranine) and sustentacular cells positive to S-100 protein (Fig. 4).
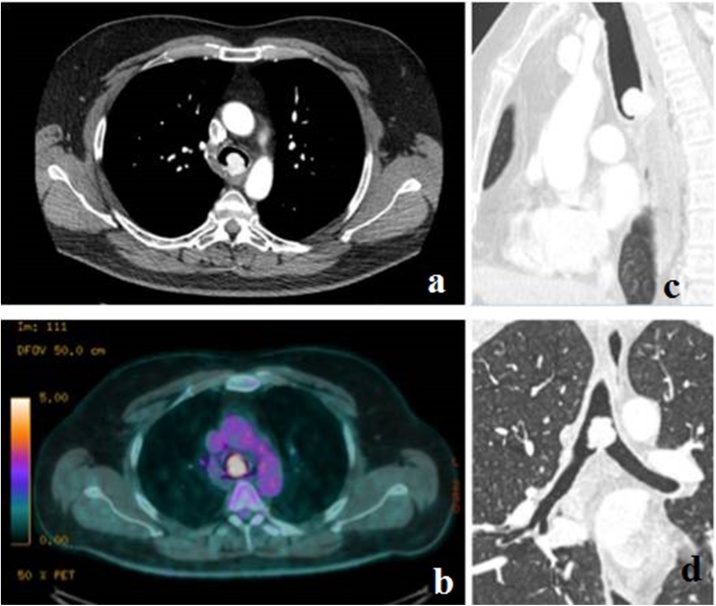
Fig. 1.
The computerized tomographic (a,c,d) showed an intensely contrast-enhancing 23 × 17 × 20 mm mass, arising from the membranous part of the posterior trachea, 1 cm above the carina. This mass showed high FDG uptake on PET-CT (b).
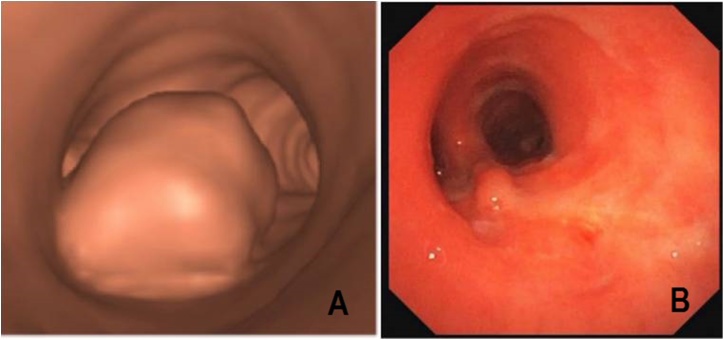
Fig. 2.
Pre-operative virtual bronchoscopy with the partially obstructive paraganglioma arising from the posterior membranous part of the distal trachea (a). Bronchoscopy at 6 weeks showing a complete healing of the airway without stenosis or muscle flap protrusion (b).
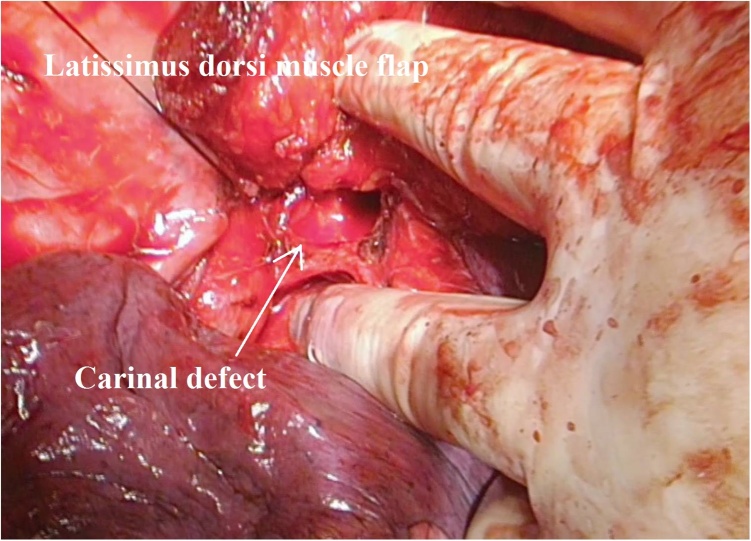
Fig. 3.
Intra-operative view of reconstruction of the carinal defect with the latissimus dorsi muscle flap.
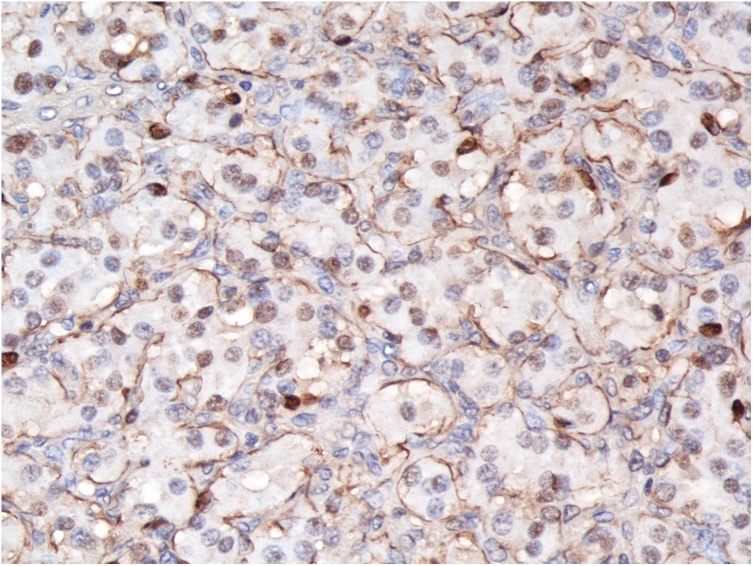
Fig. 4.
Photomicrographs of H&E and IHC (magnification: ×400) showing tumor cells with abundant eosinophilic cytoplasm arranged in clusters (zellballen pattern) surrounded by S-100–positive sustentacular cells.
The patient recovered uneventfully and was discharged on postoperative day 12. Control bronchoscopy at 6 weeks showed a complete healing of the airway without stenosis or muscle flap protrusion with macroscopic re-epithelization of the muscle flap (Fig. 2). In addition, the patient reported complete disappearance of respiratory symptoms. Additional investigations did not reveal hormonal hypersecretion. Plasmatic metanephrin and chromogranin-A dosages were normal. Hereditary paraganglioma syndrome was secondarily excluded by genetic consultation. After nine months of follow-up, the patient did not present local or distant recurrences.
3. Discussion
Paragangliomas are rare, normally benign, neuroendocrine tumors that arise from extra-adrenal chromaffin cells derived from the neural crest. These tumors can typically be located in the neck, mediastinum, abdomen or in association with cranial nerves where chromaffin cells can migrate during the embryological period [6]. Tracheal location is exceptional with fewer than 15 cases described in the literature [2]. Most of them were located in the membranous part of the trachea, as in our case. In spite of the fact that paraganglioma are related to pheochromocytoma, they are not known to secrete levels of catecholamine high enough to cause symptoms [2]. Typical paraganglioma symptoms may include hemoptysis, dysphagia, hoarseness, wheezing or dyspnea. These tumors have been reported to be highly vascularized with an increased risk of major bleeding during pre-operative bronchoscopy [[2], [3], [4]]. If pre-operative biopsy is mandatory, it should be performed carefully through rigid bronchoscopy. In the particular case that we report, since the tumor appeared to be well vascularized on chest CT-scan, bronchoscopy with biopsy was not performed to avoid risky bleeding and complex airway management. In addition, PET-CT showed high tumor FDG uptake without distant metastatic lesions, as is frequently reported for paraganglioma [7].
Airway management during the operation was performed with a single lumen tube. Then, peripheral femoral veno-arterial ECMO was introduced to ensure totally stable hemodynamic and respiratory states during airway resection and reconstruction. Jet ventilation or cross-field intubation were not necessary. This allowed us to perform the complex suture without disturbing lines crossing the operative field. Surgical resection is the standard of care, and several approaches have been described mainly by tracheal resection and direct anastomosis. Tracheostomy with endotracheal dissection through tracheofissure, followed by cauterization of the tumor bed has been proposed in the case of upper tracheal localizations [3]. Bronchoscopic laser ablation has been proposed as an alternative, but this technique is more delicate if local tracheomalacia is present [8]. In the case we report, the carinal location would have required a complex carinal resection with anastomosis of the two main bronchi with potentially hazardous results and high post-operative morbidity. We decided to perform a membranous resection only, with clear margins and subsequent closure of the defect by suturing a pedicled extrathoracic muscle flap into the debrided airway. Extrathoracic muscle flaps have been previously described for the management of complex tracheo-bronchial defects [9,10]. They are well described as a valid option to close airway defects and reduce anastomotic tension in complex tracheo-carinal surgeries whilst avoiding desmoplastic reactions of the resection margin or scarring. In this particular case, the size of the resection zone, its location necessitating several anastomosis sites, and the possibility to spare a fraction of the trachea-carinal circumference all favored the option we selected. This was compounded by the experience of our center with this technique and the generally low rate of long-term complications associated with it [11]. Moreover, the well-vascularized muscle flap can be wrapped around the repaired airway, leading to additional mediastinal reinforcement. The muscle should be sutured with interrupted stitches and gentle traction to avoid muscle protrusion or secondary stenosis. We recommend prompt extubation and routine bronchoscopy to avoid any pressure on suture line.
4. Conclusion
In conclusion, paraganglioma should be included in the differential diagnosis of an intrathoracic membranous tracheal mass. Upfront non-circumferential surgical resection may be indicated and airway reconstruction can be performed using a latissimus dorsi muscle flap.
Conflicts of interest
No conflict of interest.
Funding
No funding.
Ethical approval
The study is exempt from ethnical approval by our institution.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal upon request.
Author contribution
MG and LW collected the data and wrote the manuscript. EA, AR, IL, AL, CB, HBR and MB participated to the management of patients and corrected the manuscript. All authors read and approved the final manuscript.
Registration of research studies
Not applicable.
Guarantor
Michel Gonzalez.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Acknowledgement
We would like to acknowledge Matthieu Zellweger for his corrections and suggestions.
References
- 1.Shin S., Park J.S., Shim Y.M., Kim H.J., Kim J. Carinal resection and reconstruction in thoracic malignancies. J. Surg. Oncol. 2014;110:239–244. doi: 10.1002/jso.23643. [DOI] [PubMed] [Google Scholar]
- 2.Metzdorff M.T., Seaman J.C., Opperman D.A., Goates J.J., Musani A.I. Tracheal paraganglioma: an unusual neoplasm of the upper airway. Ann. Thorac. Surg. 2012;93:1717–1719. doi: 10.1016/j.athoracsur.2011.09.053. [DOI] [PubMed] [Google Scholar]
- 3.George M., Ayyappan A.P., Cherian R., Kurien M. Tracheal paraganglioma: a rare vascular neoplasm. AJR Am. J. Roentgenol. 2006;187:W231–2. doi: 10.2214/AJR.05.1116. [DOI] [PubMed] [Google Scholar]
- 4.Jones T.M., Alderson D., Sheard J.D., Swift A.C. Tracheal paraganglioma: a diagnostic dilemma culminating in a complex airway management problem. J. Laryngol. Otol. 2001;115:747–749. doi: 10.1258/0022215011908838. [DOI] [PubMed] [Google Scholar]
- 5.Agha R.A., Fowler A.J., Saeta A., Barai I., Rajmohan S., Orgill D.P. The SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;34:180–186. doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 6.Kantorovich V., King K.S., Pacak K. SDH-related pheochromocytoma and paraganglioma. Best Pract. Res. Clin. Endocrinol. Metab. 2010;24:415–424. doi: 10.1016/j.beem.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Berkel A., Rao J.U., Kusters B., Demir T., Visser E., Mensenkamp A.R. Correlation between in vivo 18F-FDG PET and immunohistochemical markers of glucose uptake and metabolism in pheochromocytoma and paraganglioma. J. Nucl. Med. 2014;55:1253–1259. doi: 10.2967/jnumed.114.137034. [DOI] [PubMed] [Google Scholar]
- 8.Sing T.M., Wong K.P., Young N., Despas P. Chemodectoma of the trachea. Thorax. 1996;51:341–342. doi: 10.1136/thx.51.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer A.J., Krueger T., Lepori D., Dusmet M., Aubert J.D., Pasche P. Closure of large intrathoracic airway defects using extrathoracic muscle flaps. Ann. Thorac. Surg. 2004;77:397–404. doi: 10.1016/S0003-4975(03)01462-0. discussion 05. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez M., Ma Tooh M., Krueger T., Ris H.B., Letovanec I., Wang Y. Repair of tracheal aspergillosis perforation causing tension pneumothorax. Ann. Thorac. Surg. 2013;96:2256–2258. doi: 10.1016/j.athoracsur.2013.06.064. [DOI] [PubMed] [Google Scholar]
- 11.Blatter J., Krueger T., Ris H.B., Baeriswyl M., Lovis A., Zellweger M. Complex tracheocarinal reconstructions using extrathoracic muscle flaps as airway substitutes. Ann. Thorac. Surg. 2018;105:1492–1498. doi: 10.1016/j.athoracsur.2018.01.021. [DOI] [PubMed] [Google Scholar]