Ergotism is a toxic condition resulting from overexposure to the ergot compounds produced by various fungi of the genus Claviceps. Traditionally, such exposure was due to ingestion of infected grains, but long-term or excessive use of medications containing ergot derivatives or drug-drug interactions between these medications can result in ergotism. Ergotamine, typically used to treat migraine, has less than 5% bioavailability due to extensive first-pass metabolism by cytochrome P450 3A4 (CYP3A4). Concurrent intake of ergotamine and strong CYP3A4 inhibitors, such as the HIV protease inhibitors (PIs), can lead to clinical ergotism.1 A total of 13 cases of clinical ergotism in HIV-infected patients has been published since 1997 (most recently reviewed by Frohlich et al2).
At a 2011 international HIV symposium3 in Bangkok, Thailand, a new case of ergotism in an HIV-infected patient was presented that led to subsequent notifications from symposium attendees about additional cases. A questionnaire was then sent to 5 large hospitals in Thailand requesting that they report any cases of ergotism in HIV-infected patients. The first submitted case and a summary of 22 additional cases are presented below.
Case Presentations
A 47-year-old HIV-infected woman was on an antiretroviral regimen consisting of lamivudine (300 mg once daily), tenofovir (300 mg once daily), and ritonavir-boosted (/r) saquinavir (1500 mg/100 mg once daily). She presented with necrosis of the left foot and generalized peripheral cyanosis (Figure 1) and reported having had severe headache the day before admission. The pharmacist at her local drugstore advised her to take ergotamine for the relief of her migraine. After taking ergotamine for 1 day, she developed severe pain in both legs and numbness in both feet. She was admitted to the intensive care unit and diagnosed with ergotism. Ergotamine was discontinued immediately and she was given sodium nitroprusside and heparin intravenously. The boosted PI was also discontinued and replaced with the HIV integrase inhibitor raltegravir (400 mg twice daily); the nucleos(t)ide analogue reverse transcriptase inhibitors (nRTIs) were continued. Although repeated surgical debridement of the wound was required, amputation was avoided. The patient was discharged after 2 weeks and had a full recovery. (21.7%) patients, amputation, gangrene, ulcer with persistent numbness, and 1 death occurred. There were no apparent risk factors, for example, length of ergotamine use, type of HIV PI, or administration of vasodilators, that could predict the outcome of the drug-drug interaction. The questionnaire asked only for the third agent in the antiretroviral drug regimen, assuming that the nRTIs were not responsible for the interaction.
Figure 1.
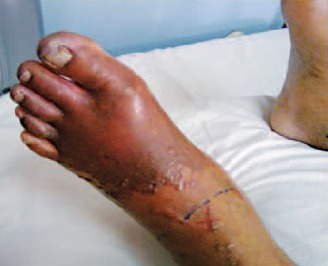
Peripheral ischemia of the left forefoot.
Other responses to the questionnaire yielded an additional 22 new cases of clinical ergotism in Thailand between 2004 and 2011 (Table 1). Patients had used ergotamine for a median of only 1 day (range, 1-14 days). Fortunately, 18 (78.3%) patients had full recovery, but in the remaining 5
Table 1.
Table 1. Cases of Clinical Ergotism Associated With Concurrent Use of HIV Protease Inhibitor– or Efavirenz-Containing Regimens and Ergotamine from a Survey of 5 Large Hospitals in Thailanda
| Patient Number | Sex | Age (years) | Days on Ergotamine | HIV Protease Inhibitor / Dose (mg) in the Regimen | Signs of Ergotism | Treatment for Ergotism | Treatment Outcome | Hospital Stay: Yes or No (days, if reported) | Prior to Ergotamine Use | |
|---|---|---|---|---|---|---|---|---|---|---|
| CD4+ Count (cells/µL) | HIV RNA Level (copies/mL) | |||||||||
| 1 | Female | 49 | 2 | ATV/r 300/100 qd | Severe headache, brain swelling, arterial spasm in all extremities with occlusion of bilateral radial and ulnar arteries on CTA | Heparin IV, nifedipine | Full recovery | Yes (11) | 360 | <50 |
| 2 | Male | 31 | 14 | SQV/r 1600/100 qd | Severe arterial spasm in all extremities with cyanosis and pain | Heparin IV, nifedipine | Full recovery | Yes (4) | 273 | <50 |
| 3 | Female | 44 | 1 | LPV/r 400/100 bid | Severe arterial spasm in all extremities with cyanosis and pain; nausea and vomiting | Nitroprusside, nifedipine | Full recovery | Yes (5) | 420 | <50 |
| 4 | Female | 44 | 1 | PEP: LPV/r 400/100 bid | Severe arterial spasm with cyanosis and pain | Nitroprusside, nifedipine, heparin | Full recovery | Yes (20) | Health care worker with history of a needle stick injury and PEP | |
| 5 | Female | 30 | 1 | IDV/r 400/100 bid | Severe arterial spasm with cyanosis of both legs | Nitroprusside, heparin, iloprost | Autoamputation of all toes on right foot | Yes (6) | 267 | <40 |
| 6 | Female | 47 | 1 | SQV/r 1500/100 qd | Severe arterial spasm with cyanosis and pain in all extremities | Nitroprusside, nifedipine, heparin | Dry gangrene in left big toe | Yes (16) | 300 | <40 |
| 7 | Female | 38 | 1 | LPV/r 400/100 bid | Severe arterial spasm with cyanosis and pain in all extremities | Nitroprusside, heparin, iloprost | Full recovery | Yes (5) | 556 | <40 |
| 8 | Female | 46 | 1 | SQV/r 1000/100 bid | Severe arterial spasm with cyanosis and pain in all extremities | Nitroprusside, nifedipine, heparin | Full recovery | Yes (4) | 800 | <40 |
| 9 | Male | 39 | 1 | DRV/r 600/100 bid | Severe nausea and vomiting; palpitation, fatigue, and numbness in right leg | None | Full recovery | No | 360 | <50 |
| 10 | Female | 36 | 1 | IDV/r 800/100 bid | Severe nausea and vomiting; palpitation, fatigue, and numbness in right leg; cyanosis of right leg; hypotension | Nitroprusside, nifedipine, heparin | Full recovery | Yes (5) | 460 | <50 |
| 11 | Female 38 1 IDV/r | 600/100 bid | Severe nausea and vomiting; | palpitation, fatigue, numbness, | and cyanosis in right leg | Nitroprusside, nifedipine, heparin | Full recovery | Yes (4) | 780 | <50 |
| 12 | Female | 4 | 3 | SQV/r 1500/100 qd | Nausea and vomiting; palpitation, fatigue, numbness, and cyanosis in right leg | Nitroprusside, nifedipine, heparin | Ulcer on right foot, persistent numbness in right leg (>3 mo) | Yes (8) | 630 | <50 |
| 13 | Female | 38 | 1 | SQV/r 1500/100 qd | Severe nausea and vomiting; palpitation and numbness in left leg | Observation only | Full recovery | No | 780 | <50 |
| 14 | Female | 38 | 1 | IDV/r 800/100 bid + EFV 600 qd | Fainting; nausea and vomiting; pulseless lower extremities; numbness and paresthesia in both legs | Nitroprusside, nifedipine | Full recovery | Yes | 757 (29%) | <50 |
| 15 | Female | NA | 7 | EFV 600 qd | Hypotension, limb ischemia | Nitroprusside, heparin, nifedipine | Full recovery | Yes | NA | <50 |
| 16 | Female | NA | 7 | IDV/r 800/100 bid | Hypoxemia, shock | Nitroprusside | Death | Yes | NA | NA |
| 17 | Female | 44 | 7 | LPV/r 400/100 bid | Peripheral cyanosis of both legs | Nitroprusside, amlodipine, heparin | Full recovery | Yes (11) | 799 | <40 |
| 18 | Female | 44 | 3 | LPV/r 400/100 bid | Numbness and pain in both legs, arterial occlusion | Warfarin, nitric oxide donor drug | Full recovery | Yes (10) | 200 | <40 |
| 19 | Female | 31 | 2 | LPV/r 400/100 bid | Diffuse ischemia and bluish discoloration of both lower extremities with nonpalpable pulse and undetectable BP | Off LPV/r; vasodilator | Full recovery | Yes | 320 | <50 |
| 20 | Female | 36 | NA LPV/r | 400/100 bid | Gangrene in feet and hands with an undetectable pulse and low BP | Off LPV/r | Amputation of both legs below the knee | Yes | 363 | <50 |
| 21 | Female | 33 | NA | LPV/r 400/100 bid | Dizziness, dropping BP, bluish extremities | Off LPV/r; vasodilator | Full recovery | Yes | 655 | <50 |
| 22 | Male | 38 | NA | IDV/r 400/100 bid | Bluish extremities | Off IDV/r | Full recovery | Yes | 266 | <50 |
| 23 | Female | 32 | 1 | LPV/r 400/100 bid | Severe nausea and vomiting; palpitation, fatigue, and numbness in left leg | Observation only | Full recovery | No | 459 | 50 |
ATV indicates atazanavir; bid, twice daily; BP, blood pressure; CTA, computed tomography angiography; DRV, darunavir; EFV, efavirenz; IDV, indinavir; LPV, lopinavir; NA, not available; PEP, postexposure prophylaxis; qd, once daily; /r, ritonavir boosted; SQV, saquinavir.
Cases occurred between 2004 and 2011.
This series of 23 cases of clinical ergotism in patients taking antiretroviral drugs is unique in many ways. It is the largest number of reported patients with clinical ergotism. It also includes the first cases of ergotism in patients on relatively new HIV PIs such as atazanavir and darunavir and in a health care worker who received post-exposure prophylaxis after a needle stick injury. One patient in the series (patient 15 in Table 1) was taking the nonnucleoside analogue reverse transcriptase inhibitor (NNRTI) efavirenz, which can act as a CYP3A inducer. Possibly, the drug-drug interaction in this patient occurred at the first dose of efavirenz, when CYP3A inhibition may be the dominant effect. In the combination of efavirenz and a boosted PI, the PI effect is stronger, thus the drug-drug interaction in patient 14 was likely caused by indinavir/r. It comes as no surprise that the majority of patients in this case series were women as migraine occurs more often in women than in men.
Conclusion
This report is a warning that increased global access to antiretroviral drugs, boosted PIs in particular, together with poor monitoring of potential drug-drug interactions can have severe clinical consequences. Also, the use of cobicistat, a CYP3A inhibitor prescribed as an alternative boosting agent to ritonavir, is contraindicated in patients taking ergot alkaloids. Patients and health care workers must be instructed to discuss the use of any coadministered medications, including over-the-counter products, with their HIV practitioner or supervising physician. The potential for drug-drug interactions can be further increased when individuals do not inform pharmacists of their positive HIV serostatus because of concerns about potential stigma. The importance of Internet access in resource-limited settings cannot be underestimated, and many excellent HIV-related online resources geared to the practicing clinician are available (reviewed in Krakower et al4). Short of dispensing ergotamine by prescription only, and in the absence of automated medication surveillance systems, education and effective communication about the potential for drug-drug interactions is necessary and important. Concurrent intake of ergotamine and strong CYP3A4 inhibitors, such as HIV PIs, can produce a potentially life-threatening drug-drug interaction that can be prevented.
References
- 1.Silberstein SD. The pharmacology of ergotamine and dihydroergotamine. Headache. 1997;37 Suppl 1:S15-S25. [PubMed] [Google Scholar]
- 2.Frohlich G, Kaplan V, Amann-Vesti B. Holy fire in an HIV-positive man: a case of 21st-century ergotism. CMAJ. 2010; 182(4):378-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. 14th Bangkok International Symposium on HIV Medicine, January 19-21, 2011, Bangkok, Thailand: http://www.hivnat.org/bangkoksymposium/14th. [Google Scholar]
- 4.Krakower D, Kwan CK, Yassa DS, Colvin RA. iAIDS: HIV-related internet resources for the practicing clinician. Clin Infect Dis. 2010;51(7):813-822. [DOI] [PubMed] [Google Scholar]


