Abstract
A new paradigm for atherogenesis in HIV infection is emerging, in which viral replication and microbial translocation result in ongoing T-cell and monocyte activation, with persistent inflammation leading to the development of atypical, high-risk morphology plaques. These plaques, characterized by low attenuation and positive remodeling, can be found even among HIV-infected patients who are at low risk for cardiovascular disease based on traditional risk factors. Prevention of cardiovascular events in HIV infection requires modulation of traditional risk factors and is also likely to require effective antiinflammatory treatment strategies. Statins, which are traditionally used to treat dyslipidemia, have also been shown to exert antiinflammatory effects associated with clinical benefit and maybe useful to treat and prevent cardiovascular disease in HIV-infected patients. However, large-scale studies of statins in the context of HIV infection must be conducted. This article summarizes a presentation by Steven K. Grinspoon, MD, at the IAS-USA continuing education program held in Chicago, Illinois, in May 2014.
Keywords: cardiovascular disease, CVD, HIV, inflammation, immune activation, plaque, antiinflammatory
The preponderance of evidence from many cohort studies indicates that HIV- infected patients are at a 1.5- to 2-fold greater risk for cardiovascular disease (CVD) than the general population.1,2 The traditional paradigm for explaining this increased risk includes the effects of HIV infection itself; the effects of antiretroviral therapy in causing dyslipidemia, ectopic fat accumulation, and diabetes or insulin resistance; and risk factors such as smoking, coinfections, or drug use. In this traditional paradigm, these factors contribute to the formation of calcified coronary plaques and resulting coronary events. However, accumulating evidence strongly indicates that persistent immune activation and inflammation play a major role in CVD risk in the setting of HIV infection, with atherosclerosis characterized by formation of atypical, noncalcified, high-risk plaques.
Traditional and Nontraditional CVD Risk Factors in HIV
HIV-infected patients have increased risk for diabetes, primarily associated with insulin resistance. Representative data from the MACS (Multicenter AIDS Cohort Study) study showed that the prevalence of diabetes was 5% in HIV-uninfected individuals, 7% in HIV- infected individuals who were not taking antiretroviral therapy, and 14% in HIV-infected individuals who were taking potent antiretroviral therapy.3 Dyslipidemia is common among HIV- infected patients. Representative data show considerably elevated triglyceride and considerably lower high-density lipoprotein (HDL) cholesterol levels in HIV-infected patients than in age-, sex-, and body mass index-matched controls.4 Low-density lipoprotein (LDL) levels may also increase, but increases are less consistent and LDL levels may often remain normal.4 Fat redistribution in HIV infection consists primarily of accumulation of abdominal (visceral) fat and loss of subcutaneous fat in the extremities and face. Fat redistribution has implications for survival in HIV infection. The FRAM (The Study of Fat Redistribution and Metabolic Change in HIV Infection) study, for example, showed an increased risk of death when visceral adipose tissue increased and fat in the extremities decreased, after adjustment for other risk factors.5
However, traditional risk factors account for only a portion of the excess CVD risk in HIV disease. In a large epidemiologic cohort study reported in 2007 that included 3851 HIV-infected patients and more than 1 million HIV-uninfected patients who received longitudinal care from 1996 to 2004, the relative risk for myocardial infarction (MI) in HIV-infected patients was 1.75 (P< .0001 ).1 Of this 75% increase in risk, approximately 25% was attributable to traditional risk factors (ie, diabetes, dyslipidemia, and hypertension), although smoking status was not included in the analysis. HIV infection itself was found to confer MI risk comparable to that conferred by traditional risk factors. In more recent years, persistent inflammation and immune activation have been found to be major contributors to CVD risk among HIV-infected individuals.
Initial studies of CVD risk in the context of HIV disease suggested that the excess risk was mainly attributable to the effects of antiretroviral drugs. However, the results of the SMART (Strategies for the Management of Antiretroviral Therapy) trial, reported in 2006, showed that an intermittent treatment strategy based on CD4+ cell count—hypothesized to reduce CVD risk by reducing drug exposure—was associated with greater MI risk than a continuous treatment strategy.6 The findings of the trial were pivotal in focusing attention on the role of viral infection and resultant inflammation in CVD risk. Among more recent studies of the relationship between immune markers and CVD is one recently reported by Silverberg and colleagues that showed a substantially increased MI risk based on lower recent CD4+ cell count and lower nadir CD4+ cell count among HIV-infected patients.7
Assessment of Nontraditional Risk Factors With Novel Techniques
Computed Tomography Angiography Studies of Plaques
In studies by Lo, Burdo, and colleagues, coronary computed tomography (CT) angiography was used to investigate the presence of plaques and plaque features in young HIV-infected men and matched controls with no dyslipidemia or hypertension, similar Framingham risk scores (FRSs), and similar smoking rates.8,9 The HIV-infected men did not have statistically significantly different Agatson calcium scores than controls or a greater number of calcified segments but did have a statistically significantly higher risk for plaques (59% vs 34%, respectively; P =.02), greater plaque volume (mean 173 μL vs 85 μL; P = .02), more segments with plaque (2.2 vs 1.2, respectively; P = .03), and more noncalcified segments (0.99 vs 0.46, respectively; P < .05), and a low and not statistically significantly greater prevalence of any stenosis greater than 70% (6.5% vs0%, respectively; P = .06).
Other studies have shown that whereas traditional CVD risk factors are associated with elevated calcium scores and calcium-enriched plaques, non calcified plaques are associated with monocyte or macrophage activation markers, such as soluble (s)CD163 and sCD14.9 Evidence is thus emerging that immune activation may lead to the development of atypical plaques in HIV disease.
Figure 1 shows the type of coronary lesions commonly found in HIV-infected patients.10,11 These lesions are not the calcified lesions typically associated with coronary disease and generally do not cause critical stenosis. They are atypical, noncalcified, high-risk morphology plaques characterized by low attenuation and positive remodeling. These plaques are characterized by a fatty core and are eccentric, building up under the luminal surface toward the outer wall of the vessel. If the lumen of the affected coronary artery is not compromised during the buildup of eccentric plaque, sufficient blood flow is preserved that collateral circulation may not develop. Further, these atypical lesions feature thin fibroatheroma caps, making rupture more likely. When rupture does occur, a greater portion of the myocar- dium may be at risk because of the absence of collateral circulation, es- pecially if the lesion is in a proximal segment. In a CT angiography study of 101 HIV-infected and 41 HIV-uninfected patients, Zanni and colleagues found that HIV-infected patients had a higher prevalence of these high-risk morphology plaques—at least 1 low attenuation plaque was present in 22.8% of HIV- infected patients compared with 7.3% of HIV-uninfected patients, and at least 1 positively remodeled plaque was present in 49.5% of HIV-infected patients compared with 31.7% of HIV-uninfected patients.12 Studies of HIV-uninfected patients have shown that event-free survival is statistically significantly lower (P < .01) in those with 2-feature plaques (low attenuation and positive remodeling) than in those with 1 or none of these features.13,14
Figure 1.
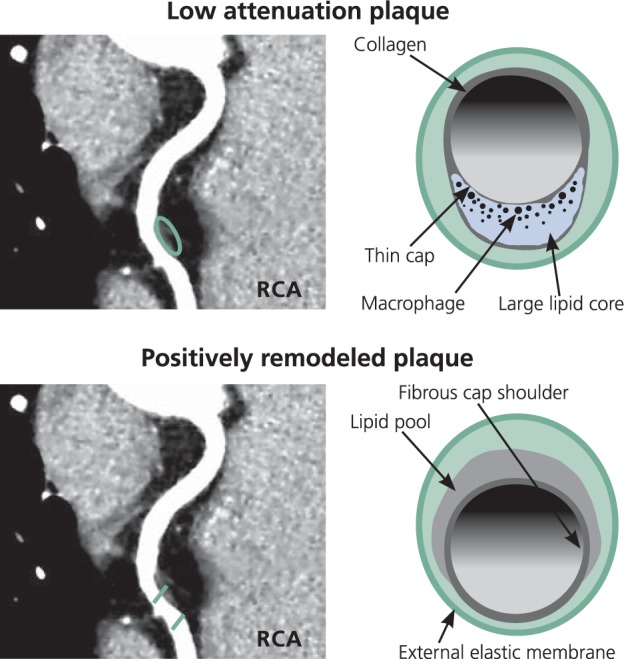
Low attenuation plaque and positively remodeled plaque seen on computed tomography angiography These types of high-risk morphology plaques are commonly observed in HIV-infected patients. RCA indicates right coronary artery. Adapted from Naghavi et al10 and Schoenhagen et al.11
Assessment of Vascular Inflammation in HIV
18Fluorine-2-deoxy-D-glucose positron emission tomography (18F-FDG-PET) provides a noninvasive method for arterial imaging and detection of arterial inflammation. 18F-FDG is taken up into metabolic pathways and allows for imaging of metabolically active cells. In atherosclerotic plaques, 18F-FDG is sequestered beneath the fibrous cap in the lipid core area and within activated macrophages.15 Increased vascular 18F-FDG signaling has been shown to be associated with statistically significant reduction in event-free survival (Pc.OOl).16
In a study by Subramanian and colleagues, cardiac 18F-FDG-PET was used to assess 27 HIV-infected patients with no known CVD; 27 age-, sex-, and FRS-matched, HIV-uninfected patients that did not have atherosclerosis; and 27 HIV-uninfected, sex-matched patients with known atherosclerotic disease.17 HIV-infected patients and HIV-uninfected, FRS-matched patients without atherosclerosis were balanced in demographic and clinical characteristics: mean ages were 52 years and 54 years, respectively; mean FRSs were 6.4 and 6.6, respectively, indicative of very low risk; mean LDL cholesterol levels were 113 mg/L and 118 mg/L, respectively; and median calcium scores on CT angiography were 24 and 0, respectively. HIV-infected patients had a mean duration of HIV infection of 15.5 years, all were on antiretroviral therapy (mean duration of 12.3 years), and 81% had undetectable HIV RNA levels; mean current CD4+ cell count was 641/μL and mean nadir CD4+ cell count was 99/μL.
This study provided some of the first direct evidence of increased arterial inflammation among HIV-infected patients. Inflammation—measured as aortic target-to-background ratio (TBR) of 18F-FDG-PET uptake—among HIV- infected patients was statistically significantly greater than among HIV- uninfected controls who did not have atherosclerosis (P < 0.001) and equal to that among HIV-uninfected controls that did have atherosclerosis. Subgroup analysis showed that TBR was statistically significantly higher among HIV-infected patients than HIV- uninfected patients in analyses restricted to those with no measured calcium (P = .009), FRSs of less than 10 (P = .002), LDL cholesterol levels of less than 100 mg/dL (P = .01), those who did not use statins (P=.001) or were nonsmokers (P=.001), and among HIV-infected patients with undetectable HIV RNA levels (Pc.001). Increased aortic TBR was associated with increased levels of the monocyte or macrophage activation marker SCD163 (P= .04) among HIV-infected patients.
These investigators subsequently showed that increased arterial inflammation, indicated by higher TBR on 18F-FDG-PET imaging, was associated with increased prevalence of high-risk morphology lesions.18 When patients with higher and lower arterial inflammation were compared, 40% and 10%, respectively, (P = .02) had at least 1 low attenuation plaque; 85% and 67%, respectively, (P =.17) had at least 1 positively remodeled plaque; 90% and 67%, respectively, (P = .06) had at least 1 plaque with 1 feature; and 35% and 10%, respectively, (P = .04) had at least 1 plaque with 2 features.
A New Paradigm and New Treatment Strategies
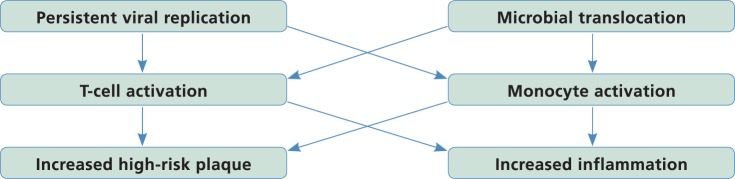
Taken together, these data suggest a new mechanistic paradigm for atherogenesis in HIV disease, and potential strategies for reducing inflammation and atherosclerosis (Figure 2). In this paradigm, viral replication and microbial translocation result in ongoing T-cell and monocyte activation, with persistent inflammation leading to the development of atypical, high-risk plaques.
Figure 2.
Recent data suggest a potential new paradigm for cardiovascular disease risk in HIV disease. Persistent low-grade undetectable viral replication and ongoing microbial translocation, along with hepatitis C virus or other viral coinfection, may lead to increased T-cell activation and ongoing monocyte activation, which may contribute to the development of high-risk plaque morphology and increased arterial inflammation.
Given the evolving understanding of atherogenesis in HIV infection, strategies to reduce CVD risk include minimizing traditional risk factors (ie, smoking cessation, blood pressure control, correction of insulin resistance and dyslipidemia, reduction of excess visceral adipose tissue, and use of less-toxic antiretroviral therapy). Viral burden and latency can be reduced through earlier initiation of antiretroviral therapy and use of intensification strategies. Potential strategies to counter inflammation include modulation of T-cell and monocyte activation, chemokine receptor antagonism, use of proinflammatory cytokine antagonists (eg, anti-inter- leukin-6 agents), and use of low-dose methotrexate. These strategies remain under investigation.
Statin therapy warrants particular attention, given its pleiotropic effects. Statins have the potential to markedly reduce cardiovascular events among HIV-infected patients. In addition to their traditional ability to lower LDL cholesterol level (generally comparable between HIV-infected and HIV-uninfected patients19), statins reduce monocyte activation, chemoattraction, and vascular inflammation in HIV-infected patients20, and reduce noncalcified plaque volume in HIV-uninfected patients 21 Initial data from nonrandomized studies indicate that statin use is associated with reduced mortality among HIV-infected patients (hazard ratio [HR], 0.33; P = .009).22 The JUPITER (Justification for the Use of Statins in Primary Prevention: an Intervention Trial Evaluating Rosuvastatin) trial of rosuvastatin in HIV-uninfected individuals showed that primary prevention treatment with statins reduced cardiovascular events in those with low LDL cholesterol levels and elevated inflammation, indicated by raised C-reactive protein levels (events per 100 person-years were 0.77 with rosuvastatin and 1.36 with placebo; HR, 0.56).23 In this trial, rosuvastatin treatment was associated with an increased risk of diabetes that, although not large, was statistically significant. There has been some study of pitavastatin, a potent statin that lowers LDL cholesterol level, in HIV-infected patients; it has not been associated with diabetes in small studies to date 24
Statins are associated with a somewhat elevated risk for adverse events among HIV-infected patients (eg, myositis was reported in 1.9% of HIV- infected patients vs 0.5% of HIV- uninfected patients).19 However, the overall risk of adverse events appears to remain generally low. Despite the potential benefits of statin therapy, statin use is uncommon among the HIV-infected population. The AIDS Clinical Trials Group reported that only 19.6% of HIV-infected patients use statins. Large clinical trials are needed to better identify the potential benefits and risks of statin therapy for the HIV- infected population. Indeed, a large randomized study, REPRIEVE (A Randomised Study to Prevent Vascular Events in HIV), was recently funded by the National Institutes of Health to address this question.
Summary
CVD risk is higher in the setting of HIV infection. As of 2014, preventing CVD is an important but unmet goal for HIV-infected patients. Traditional risk factors (eg, ectopic fat, insulin resistance, dyslipidemia) and nontraditional risk factors (eg, immune activation, inflammation) contribute to this increased risk, which manifests in the development of noncalcified, high-risk coronary plaques. Modulation of traditional and nontraditional risk factors is necessary to prevent CVD in HIV- infected patients. Antiinflammatory strategies, including use of statins, may prove effective in reducing CVD risk. El
References
- 1.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92(7):2506-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013; 173(8):614-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown TT, Cole SR, Li X, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the Multicenter AIDS Cohort Study. Arch Intern Med 2005;165:1179-1184. [DOI] [PubMed] [Google Scholar]
- 4.Hadigan C, Meigs JB, Corcoran C, et al. Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy. Clin Infect Dis. 2001;32(1):130-139. [DOI] [PubMed] [Google Scholar]
- 5.Scherzer R, Heymsfield SB, Lee D, et al. Decreased limb muscle and increased central adiposity are associated with 5-year all-cause mortality in HIV infection. AIDS. 2011;25(11): 1405-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strategies for Management of Antiretroviral Therapy Study Group (SMART), El-Sadr WM, Lundgren JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283-2296. [DOI] [PubMed] [Google Scholar]
- 7.Silverberg MJ, Leyden WA, Xu L, et al. Immunodeficiency and risk of myocardial infarction among HIV-positive individuals with access to care. J Acquir Immune Defic Syndr. 2014;65(2): 160-166. [DOI] [PubMed] [Google Scholar]
- 8.Lo J, Abbara S, Shturman L, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS. 2010;24(2):243-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burdo TH, Lo J, Abbara S, et al. Soluble CD 163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011; 204(8): 1227-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation. 2003; 108(14): 1664-1672. [DOI] [PubMed] [Google Scholar]
- 11.Schoenhagen P, Ziada KM, Vince DG, Nissen SE, Ttizcu EM. Arterial remodeling and coronary artery disease: the concept of ”dilated” versus ”obstructive” coronary atherosclerosis. J Am Coll.Cardiol. 2001; 38(2):297-306. [DOI] [PubMed] [Google Scholar]
- 12.Zanni MV, Abbara S, Lo J, et al. Increased coronary atherosclerotic plaque vulnerability by coronary computed tomography angiography in HIV-infected men. AIDS. 2013;27(8): 1263-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Motoyama S, Kondo T, Sarai M, et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll.Cardiol. 2007;50(4):319-326. [DOI] [PubMed] [Google Scholar]
- 14.Kitagawa T, Yamamoto H, Ohhashi N, et al. Comprehensive evaluation of noncalcified coronary plaque characteristics detected using 64-slice computed tomography in patients with proven or suspected coronary artery disease. Am Heart J. 2007; 154(6): 1191-1198. [DOI] [PubMed] [Google Scholar]
- 15.Rudd JH, Warburton EA, Fryer TD, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxygIucose positron emission tomography. Circulation. 2002;105(23):2708-2711. [DOI] [PubMed] [Google Scholar]
- 16.Rominger A, Saam T, Wolpers S, et al. 18F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J Nucl.Med. 2009;50(10): 1611-1620. [DOI] [PubMed] [Google Scholar]
- 17.Subramanian S, Tàwakol A, Burdo TH, et al. Arterial inflammation in patients with HIV. JAMA. 2012;308(4):379-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tawakol A, Lo J, Zanni MV, et al. Increased arterial inflammation relates to high-risk coronary plaque morphology in HIV- infected patients. J Acquir Immune Defic Syndr. 2014;66(2):164-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silverberg MJ, Leyden W, Hurley L, et al. Response to newly prescribed lipid- lowering therapy in patients with and without HIV infection. Ann Intern Med. 2009; 150(5) :301-313. [DOI] [PubMed] [Google Scholar]
- 20.McComsey G, Jiang Y, Debanne S, et al. Effect of statins on immune activation and inflammation in HIV+ subjects on ART: a randomized placebo controlled trial [Abstract 186LB], 20th Conference on Retroviruses and Opportunistic Infections (CROI). March 3-6, 2013; Atlanta, Georgia. [Google Scholar]
- 21.Burgstahler C, Reimann A, Beck T, et al. Influence of a lipid-lowering therapy on calcified and noncalcified coronary plaques monitored by multislice detector computed tomography: results of the New Age II Pilot Study. Invest Radiol. 2007; 42(3):189-195. [DOI] [PubMed] [Google Scholar]
- 22.Moore RD, Bartlett JG, Gallant JE. Association between use of HMG CoA reductase inhibitors and mortality in HIV-infected patients. PLoS One. 2011;6(7):e21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. NEnglJMed. 2008;359(21):2195-2207. [DOI] [PubMed] [Google Scholar]
- 24.Sponseller CA, Aberg J, INTREPID Team. After 52 weeks, pitavastation is superior to pravastatin for LDL-C lowering in patients with HIV [Abstract 751 LB], In Special Issue: Abstracts from the 2014 Conference on Retroviruses and Opportunistic Infections. Top Antivir Med. 2014;22(e-l):385. [Google Scholar]