Abstract
The 20th Conference on Retroviruses and Opportunistic Infections (CROI) presented important highlights of advances in antiretroviral therapy. Investigators emphasized new approaches to finding a cure for HIV infection, with a special focus on an infant who received combination antiretroviral therapy at 30 hours of age and may have achieved a functional cure in the absence of continued antiretroviral therapy. Challenges and opportunities for sustainable antiretroviral therapy under the Patient Protection and Affordable Care Act (PPACA) were discussed, and investigators around the globe examined attrition through the cascade of care for HIV disease and its implications. Knowledge of barriers to antiretroviral therapy in resource-limited settings (RLSs) continues to expand, as do innovative strategies for improving antiretroviral therapy access and uptake in these settings. Encouraging results from expanded prevention of mother-to-child transmission programs, including option B+, were presented. Prevalence of transmitted (primary) drug resistance appears to be increasing in the United States, and new detection techniques may increase access to resistance testing in RLSs.
Keywords: HIV, antiretroviral, therapy, treatment, CROI, cure, resource-limited, cascade of care
Clinical Studies Investigating HIV-1 Cure Strategies
Persaud and colleagues presented data describing a possible functional cure of an HIV-1–infected infant (Abstract 48LB). The infant was born to an HIV-infected mother who had not received prenatal care and was diagnosed with HIV-1 infection during delivery. The delivery was precipitous and no antiretroviral prophylaxis was given to the mother. The child was transferred to a tertiary medical center at 30 hours of age and was started on full treatment doses of zidovudine, lamivudine, and nevirapine. Initial testing showed that the mother had a preserved CD4+ cell count of 644/μL and a plasma HIV-1 RNA level of 2423 copies/mL. The infant had a plasma HIV-1 RNA level of 19,812 copies/mL and detectable HIV-1 DNA in her peripheral blood cells, suggesting in utero transmission of HIV-1 infection. The infant’s HIV-1 RNA levels were detectable in 3 sequential plasma specimens before declining to undetectable levels over the next 28 days and remained undetectable though 15 months of age. The infant was not brought in for medical care and antiretroviral therapy was discontinued. At 23 months of age, the infant was brought in for medical care and HIV-1 RNA was still undetectable in the absence of antiretroviral therapy.
Extensive testing was undertaken to assess a possible cure. The child had no HIV-specific antibody or cellular immune responses and levels of immune activation were within the normal range. No infectious virus was recovered, although the sensitivity of testing was limited by blood volume. Tests for proviral DNA were undetectable in various subsets except for 2 evaluations with very low levels, and HIV-1 RNA has remained undetectable since these evaluations. The investigators suggested that this is a functional cure of HIV-1 infection in this infant. Future investigations will attempt to replicate this functional cure in infants perinatally infected with HIV-1.
In a related abstract (Abstract 171LB), Luzuriaga and colleagues evaluated 5 teenagers infected with HIV as infants who received early antiretroviral therapy by 2 months of age, and 4 teenagers, also infected with HIV as infants, who started antiretroviral therapy later in childhood. Replication-competent virus was not found in the early antiretroviral therapy group and was found in all 4 subjects in the delayed treatment group. The early treatment group had lower proviral DNA levels and lower residual plasma viremia. HIV-specific antibody and CD8+ T-cell responses were identified in 1 of 5 subjects in the early treatment group and 4 of 4 in the delayed treatment group. These results suggest that early antiretroviral treatment of perinatally infected infants with HIV-1 leads to a marked decrease in HIV viral reservoir.
Very Early Antiretroviral Therapy and HIV-1 Reservoir Size
In a clinical study conducted by Ananaworanich and colleagues, pooled HIV-1 RNA testing was used to identify acute HIV-1 infection in nearly 53,000 people presenting for HIV testing in Thailand (Abstract 47). Of the 89 participants with acute HIV-1 infection identified, 75 were enrolled in a prospective trial in which they were given antiretroviral therapy within 5 days of presenting for HIV testing. Those who presented at the earliest phase of HIV-1 infection (Fiebig stage I: negative p24 antigen and positive HIV-1 RNA) had lower levels of HIV-1 DNA prior to initiation of antiretroviral therapy than those in Fiebig stage II or III. Only 2 of 24 (8%) participants in Fiebig stage I had detectable integrated HIV-1 DNA. After initiation of antiretroviral therapy, nearly all participants (93%) in Fiebig stages I to III developed undetectable integrated HIV-1 DNA levels.
Raltegravir Intensification
A debate continues as to whether ongoing cycles of HIV-1 replication typically occur in patients with effective virologic suppression on antiretroviral therapy. If ongoing replication exists, it may continually replenish the latent reservoir of HIV-1 infection, a major obstacle to curing HIV-1 infection; 2-long terminal repeat (2-LTR) circles are markers of ongoing replication. Hatano and colleagues randomly assigned participants taking effective antiretroviral therapy to receive either raltegravir or a placebo for 24 weeks (Abstract 42). The researchers measured 2-LTR circles using droplet digital polymerase chain reaction (ddPCR) technology, which is more sensitive at low levels than traditional assays. Raltegravir blocks the integration of HIV-1 complementary DNA (cDNA) into host DNA and promotes conversion of HIV-1 cDNA to 2-LTR circles. Raltegravir use resulted in higher 2-LTR circles after 2 weeks, 4 weeks, and 8 weeks, with no difference thereafter in 9 of 15 subjects. The researchers concluded that ongoing HIV-1 replication was blocked by raltegravir. This study confirms prior observations by Buzon and colleagues.1
Histone Deacetylase Inhibition
In an emerging strategy for HIV-1 infection cure known as “kick and kill,” interventions “kick” the latent reservoir of HIV-1 infection into activation and replication allowing the immune system to “kill” the infected cell. A major focus of the kick portion of this strategy is histone deacetylase inhibitors (HDACis). In a prior study, vorinostat, an HDACi approved by the US Food and Drug Administration (FDA) for the treatment of cutaneous T-cell lymphoma, was shown to activate HIV-1, leading to a transient increase in intracellular unspliced HIV-1 RNA.2 Lewin and colleagues presented data from a single-arm, open-label, 14-day trial of vorinostat given to 20 HIV-infected participants with virologic suppression who were taking effective antiretroviral therapy (Abstract 50LB). Vorinostat was generally well tolerated and no grade 3 or 4 adverse events were observed. Of the 20 participants, 18 experienced a substantial increase in intracellular HIV-1 RNA at least 1 time after vorinostat dosing. In the study group as a whole, HIV-1 RNA levels increased substantially as soon as 8 hours after the first dose of vorinostat and remained elevated up to 10 weeks after completion of dosing. A nonstatistically significant increase in HIV-1 RNA in rectal tissues was seen after 14 days of dosing, but levels of HIV-1 DNA in the blood or rectal tissue did not change during the trial. Data from this trial support the use of HDACis as activators of latent HIV-1 infection.
Wei and colleagues investigated the in vitro efficacy of romidepsin, another HDACi (Abstract 376). They found that romidepsin was 500 times more potent than vorinostat in inducing HIV-1 expression in vitro, supporting clinical trials of this drug as part of a cure strategy.
Interleukin-7 and Antiretroviral Therapy Intensification
Katlama and colleagues presented results from the ERAMUNE-01 (Therapeutic Intensification Plus Immunomodulation in HIV-Infected Patients) trial (Abstract 170aLB). Participants with well-controlled HIV-1 infection (n = 29) were randomly assigned to receive interleukin-7 (IL-7) in combination with antiretroviral therapy intensified by maraviroc and raltegravir, or intensified antiretroviral therapy alone. The goal was to evaluate which option would be most successful at reducing the HIV-1 viral reservoir, as measured by proviral DNA. HIV-1 DNA levels did not change in the group that received antiretroviral therapy intensification alone. CD4+ cell counts increased more dramatically in the arm that received IL-7 (median increase, 1400 cells/μL) than in the arm receiving intensification alone. The difference in CD4+ cell counts declined over time but was still statistically significantly higher 1 year later. In the IL-7 group, total HIV-1 DNA levels increased but log10 copies of HIV-1 DNA per million peripheral blood mononuclear cells (PBMC) did not change appreciably over time. Neither strategy examined in the study successfully reduced the HIV-1 viral reservoir, as measured by HIV-1 DNA.
Investigational Antiretroviral Drugs
CC Chemokine Receptor 5 Antagonists
Cenicriviroc is an investigational CC chemokine receptor 5 (CCR5) antagonist that also antagonizes CC chemokine receptor 2 (CCR2) and may lead to antiinflammatory effects in addition to antiretroviral activity. Gathe and colleagues presented data from a phase II study of cenicriviroc versus efavirenz. One hundred forty-three, HIV-infected, treatment-naive patients were randomly assigned to 1 of 3 study arms (2:2:1) in which they received cenicriviroc (100 mg or 200 mg daily) or efavirenz, each given with tenofovir and emtricitabine (Abstract 106LB). Eligible participants had a CD4+ cell count greater than 200/μL, plasma HIV-1 RNA levels greater than 1000 copies/mL, and documented CCR5-using HIV. In the cenicriviroc arms, 76% (100 mg group) and 73% (200 mg group) of patients achieved plasma HIV-1 RNA levels below 50 copies/mL compared with 71% of those in the efavirenz arm, using the FDA snapshot algorithm, a composite end point of virologic failure, treatment discontinuation, and missing data. The proportion of patients who experienced virologic failure was higher in the cenicriviroc arms (12% and 14%, respectively) than in the efavirenz arm (4%). Of the 3 groups, more participants in the efavirenz arm discontinued treatment due to adverse events. These data suggest that the antiretroviral potency of cenicriviroc combined with 2 nucleos(t)ide analogue reverse transcriptase inhibitors (nRTIs) is not sufficient for use as an initial antiretroviral therapy regimen. Levels of soluble CD14 (sCD14), a marker of monocyte activation, were lower in the cenicriviroc arms, suggesting a possible antiinflammatory effect.
nRTIs
Tenofovir disproxil fumarate (tenofovir) is part of IAS–USA and US Department of Health and Human Services (DHHS; https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescent-gl.pdf) recommended initial antiretroviral therapy regimens.3 This drug is well tolerated but leads to a loss in bone density and may cause renal dysfunction in some patients. Tenofovir alefenamide fumarate (tenofovir AF), an investigational prodrug of tenofovir, contains a lipid side chain that enhances intracellular concentrations of tenofovir and minimizes plasma concentrations. Its developers suggest that this will reduce the adverse bone and renal effects associated with tenofovir.
Zolopa and colleagues presented data from a phase II clinical trial comparing tenofovir AF and tenofovir, each given with elvitegravir, cobicistat, and emtricitabine (Abstract 99LB). One hundred seventy HIV-infected, treatment-naive participants were randomly assigned (2:1) to receive tenofovir AF or tenofovir. Similar proportions in each group (tenofovir AF, 88%; tenofovir, 90%) achieved HIV-1 RNA levels below 50 copies/mL at 24 weeks, and similar gains in CD4+ cell numbers were seen. Increases in serum creatinine levels were smaller in the tenofovir AF group than in the tenofovir group (0.07 mg/dL vs 0.12 mg/dL, respectively; P = .02), and the tenofovir group experienced larger decreases in bone density in the spine and hip. This study confirms the purported advantages of tenofovir AF over tenofovir. However, the clinical significance of the difference in serum creatinine levels is not clear. Phase III clinical trials of tenofovir AF are under way and should help clarify this finding.
Nonnucleoside Analogue Reverse Transcriptase Inhibitors
Data were presented by Anderson and colleagues on an investigational nonnucleoside analogue reverse transcriptase inhibitor (NNRTI), MK-1439 (Abstract 100). This compound retains activity against isolates with common mutations associated with efavirenz or nevirapine resistance, but not those associated with rilpivirine or etravirine resistance. The pharmacokinetic properties of MK-1439 support once-daily dosing. In a phase Ib study, treatment-naive subjects were randomly assigned to receive MK-1439 (25 mg or 200 mg) or a placebo once daily, without additional antiretroviral therapy drugs, for 7 days. The researchers observed a 1.5 log10 copy/mL decline in plasma HIV-1 RNA in the arms receiving MK-1439 and no change in the arm receiving placebos. The authors conclude that these results support further development of this compound.
Maturation Inhibitors
Maturation inhibitors affect gag processing; it has been shown that the maturation inhibitor bevirimat may also inhibit HIV-1 replication in vivo, although the compound did not complete clinical development, because of limited activity against a substantial proportion of circulating strains. Urano and colleagues presented data on numerous investigational maturation inhibitors that have in vitro activity against a substantially broader range of strains of HIV than bevirimat by retaining activity against HIV-1 isolates with naturally occurring polymorphisms that render bevirimat ineffective (Abstract 105). These data suggest that 1 or more of these compounds may be a viable candidate to enter clinical development.
Clinical Trials of Antiretroviral Drugs
Antiretroviral Therapy for Patients With Controlled HIV-1
Hatano and colleagues investigated the effects of antiretroviral therapy on treatment-naive patients with very low levels of HIV-1 RNA (Abstract 75LB). The researchers enrolled 16 participants, with a median HIV-1 RNA level of 77 copies/mL and a median CD4+ cell count of 615/μL. The participants started a regimen of raltegravir plus tenofovir and emtricitabine. After 24 weeks, CD4+ cell counts did not increase substantially, although HIV-1 RNA levels did decline substantially. Levels of immune activation decreased after initiation of antiretroviral therapy. These data suggest that HIV-infected participants with very low HIV-1 RNA levels in the absence of antiretroviral therapy are in a chronic state of immune activation, and that antiretroviral therapy initiation should be considered for these patients.
Maraviroc and Immune Reconstitution Inflammatory Syndrome
Immune reconstitution inflammatory syndrome (IRIS) is a relatively common condition occurring in patients starting a new effective antiretroviral therapy regimen, especially those with low CD4+ cell counts. Maraviroc, a CCR5 antagonist, may have antiinflammatory effects.4 Sierra-Madero and colleagues conducted a randomized, double-blind, placebo-controlled trial of maraviroc versus a placebo, each given with efavirenz, emtricitabine, and tenofovir (Abstract 182LB). The 276 study participants had CD4+ counts below 100 cells/μL (median, 30 cells/μL), a median HIV-1 RNA level of 5.3 log10 copies/mL, and were not required to have CCR5-using HIV-1. The primary end point of the study was the occurrence of IRIS, which was similar in the maraviroc-and placebo-receiving groups (24% and 23%, respectively; P = not significant). There was no difference in the severity of these events between the groups. There was a slight increase in CD4+ cell counts in the maraviroc group at week 12 that was not sustained to week 24. This study does not support the immunomodulatory use of maraviroc to reduce IRIS events.
Maraviroc for Suboptimal CD4+ Cell Gains
Van Lelyveld and colleagues conducted a randomized placebo-controlled trial, adding maraviroc to an existing antiretroviral therapy regimen in order to increase CD4+ cell counts in participants with suboptimal CD4+ cell gain despite virologic suppression (Abstract 555). The median baseline CD4+ cell count was 237/μL. After 24 weeks, CD4+ cell gain did not differ between maraviroc and placebo recipients (+23 vs +15, respectively; P = .50). Changes in immune activation and sCD4 levels were not different between the 2 groups. These results do not support the use of maraviroc to increase CD4+ cell counts or as an immune modulator.
Dolutegravir for Treatment-Experienced Patients
Dolutegravir, an investigational integrase strand transfer inhibitor (InSTI), is in phase III clinical development. Previous trials have established its efficacy as part of combination antiretroviral therapy in treatment-naive and InSTI-experienced populations. Pozniak and colleagues reported interim data from SAILING, a double-blind placebo-controlled trial of dolutegravir (50 mg once daily) versus raltegravir (400 mg twice daily) with an optimized background regimen in treatment-experienced, InSTI-naive populations (Abstract 179LB). The 715 trial participants, 32% of whom were women, with a median age of 42 years, median baseline CD4+ counts of 200 cells/μL, and median HIV-1 RNA levels of 4.2 log10 copies/mL, were randomly assigned to the dolutegravir or the raltegravir group. At week 24, using the FDA snapshot algorithm, 79% of participants receiving dolutegravir achieved plasma HIV-1 RNA levels below 50 copies/mL compared with 70% of those receiving raltegravir (difference 9.7%; 95% confidence interval [CI], 3.1%-16.9%). CD4+ cell gains were similar between the 2 groups. The difference in efficacy was because of differences in virologic efficacy, although the drugs were well tolerated by subjects in both arms. One important difference was the emergence of InSTI-associated mutations. Nine subjects in the raltegravir group developed mutations in integrase, conferring resistance to raltegravir. Two subjects in the dolutegravir group developed the integrase R263K mutation, which resulted in minimal changes in phenotypic susceptibility to either InSTI. These data in combination with data from SPRING-2, a study comparing dolutegravir and raltegravir in treatment-naive patients, suggest that emergence of InSTI resistance is rare with dolutegravir use in InSTI-treatment-naive patients.
nRTIs for Highly Treatment-Experienced Patients
Newer antiretroviral drugs that have become available in the last 5 years have resulted in the ability to construct effective combination regimens for highly treatment-experienced patients. Nearly all clinical trials of antiretroviral therapy have included nRTIs as part of the studied regimen. Tashima and colleagues presented data from the AIDS Clinical Trials Group (ACTG) A5241 study, which investigated whether nRTIs were a necessary component of antiretroviral treatment regimens containing at least 2 fully active agents (Abstract 153LB). Treatment-experienced patients whose current antiretroviral regimen was failing had a new regimen chosen based on antiretroviral therapy history, resistance testing, and coreceptor tropism testing. An expert panel provided recommendations for the antiretroviral treatment regimens and nRTIs to be used. Participants with a continuous phenotypic score greater than 2 were randomly assigned to receive nRTIs or to omit nRTIs from their antiretroviral regimen.
Three hundred sixty racially and ethnically diverse participants, 25% of whom were women, with a median age of 46 years, a median CD4+ cell count of 200/μL, and a mean HIV-1 RNA level of 4.2 log10 copies/mL, were enrolled in the trial. Investigators hypothesized that omitting nRTIs was noninferior to including them, using a noninferiority margin of 15% and a primary end point of regimen failure, a composite of virologic failure or change in assigned nRTI strategy. Regimen failure occurred in 30% of participants in the no-NRTI arm and 26% in the nRTI arm (difference − 3.2%; 95% CI, − 2.5% to +6%). The no-NRTI arm was judged to be noninferior. Of note, virologic failure was similar in both arms, at 25%. There were 6 deaths in the nRTI arm and no deaths in the no-NRTI arm. These results were unexpected and require further investigation. The study suggests that nRTIs may be safely omitted in this patient population, which may reduce treatment cost and pill burden.
Comparison of Second-Line Therapies
Boyd and colleagues presented data from a randomized, open-label, clinical trial comparing raltegravir plus ritonavir-boosted (/r) lopinavir with 2 to 3 nRTIs plus lopinavir/r in patients in whom their initial regimens of NNRTIs plus 2 nRTIs had failed (Abstract 180LB). The 558 subjects had a mean HIV-1 RNA level of 4.3 log10 and a median CD4+ cell count of 190/μL. Genotypic testing was used to select the nRTIs for the majority of subjects (n = 492; 88%). At 48 weeks, virologic suppression (plasma HIV-1 RNA level < 200 copies/mL) in the raltegravir arm was noninferior to that in the nRTI arm, 83% and 81%, respectively (difference 1.8%; 95% CI, − 4.7% to 8.3%). The researchers concluded that both types of regimen were reasonable choices for second-line therapy.
Pharmacokinetic Considerations
Antiretroviral Therapy in End-Stage Renal Disease
Teicher and colleagues presented data on the safety and pharmacokinetics of raltegravir in 10 HIV-infected subjects with end-stage liver disease (Abstract 528). Patients’ antiretroviral regimens were changed to raltegravir plus 2 nRTIs. Concentrations of raltegravir, protein-unbound raltegravir, and raltegravir glucuronide (the raltegravir metabolite) after 1 and 3 months were similar to those observed in patients without liver disease. Raltegravir was found to be safe and well tolerated.
Ramanathan and colleagues investigated the pharmacokinetics of tenofovir AF in 14 HIV-1–uninfected participants with severe renal impairment (creatinine clearance, 15 mL/min-29 mL/min) and in 13 HIV-1–uninfected participants with normal renal function (Abstract 529). Tenofovir AF concentrations were increased in participants with impaired renal function, but these differences were not thought to be clinically significant. The tenofovir concentrations were approximately 6 times higher in those with impaired renal function as compared to those without renal impairment but were still lower than those typically seen in patients with normal renal function receiving tenofovir disproxil fumarate. The investigators concluded that tenofovir AF should be safe for patients with severe renal impairment. A clinical trial evaluating the safety and efficacy of tenofovir AF in patients with severe renal impairment is currently under way.
Dolutegravir Interactions
Dolutegravir does not inhibit or induce cytochrome P450 (CYP450) or uridine diphosphate glucuronyl-transferase (UDPGT) and has a low likelihood of drug-drug interactions. Piscitelli and colleagues investigated possible drug-drug interactions between dolutegravir and methadone or oral contraceptives (Abstract 535). HIV-1–uninfected participants receiving methadone chronically were enrolled in the study. They found that dolutegravir did not lead to appreciable changes in concentrations of methadone or its metabolites. Similarly, a second study enrolling HIV-uninfected women receiving oral contraception (ethinyl estradiol and norgestimate) found that levels of these hormones and their metabolites were not affected by dolutegravir.
Higher Dose Lopinavir/r in Pregnant Women
Drug concentrations of lopinavir are known to decrease in the second and third trimesters of pregnancy, and higher doses of lopinavir/r are recommended for pregnant women. Bonafe and colleagues conducted a randomized, open-label clinical trial to examine the safety, tolerability, and efficacy of higher dose lopinavir/r (600 mg/150 mg twice daily) compared with standard dose lopinavir/r (400 mg/100 mg twice daily) in 63 HIV-1–infected pregnant women between 14 weeks and 33 weeks gestation (Abstract 935). Of women randomized to the high- and standard-dose groups, 17% and 9%, respectively, discontinued treatment due to adverse effects (P = .29). Women who entered the study with HIV-1 RNA levels of 50 copies/mL or higher were more likely to achieve an HIV-1 RNA level below 50 copies/mL at the time of delivery in the high-dose group (89% vs 55%; P = .01). There was no difference among women who entered the trial with HIV-1 RNA levels below 50 copies/mL. The investigators concluded that higher dose lopinavir/r was needed for HIV-infected pregnant women with HIV-1 RNA levels of 50 copies/mL or higher but not for those with HIV-1 RNA levels below 50 copies/mL.
Abacavir and Ribavirin
Andrade and colleagues evaluated whether there are intracellular interactions between abacavir and ribavirin that explain poorer sustained virologic responses to hepatitis C virus (HCV) therapy in patients receiving abacavir (Abstract 538). They randomly assigned 28 HIV-uninfected participants to receive ribavirin alone or ribavirin with abacavir. When they compared intracellular ribavirin and ribavirin triphosphate concentrations, no appreciable difference was found between the 2 groups. The investigators did not find any evidence of intracellular interactions between these drugs.
Raltegravir and Rifampin
Sauvageon and colleagues investigated whether higher doses of raltegravir could be used to overcome the interactions between raltegravir and rifampin in a pharmacokinetic substudy of a larger trial of tuberculosis (TB) treatment in HIV-infected patients (Abstract 539). Participants taking a rifampin-containing TB regimen were randomly assigned to receive standard- (400 mg twice daily) or high-dose (800 mg twice daily) raltegravir. The investigators compared drug concentrations with those obtained after completion of TB treatment while on standard-dose raltegravir. Similar to results from other studies, there was a large variability in raltegravir concentrations, and high-dose raltegravir partially compensated for the lower concentrations observed when coadministered with rifampin. However, a separate presentation (Abstract 853) suggested that higher doses of raltegravir did not result in improved virologic control.
Political and Economic Context for Sustainable Antiretroviral Therapy
Kates, of the Kaiser Family Foundation in Washington, DC, provided an overview of the global and US political and economic challenges to antiretroviral therapy implementation (Abstract 119). Kates asserted that sustainable antiretroviral therapy is a misnomer, because only 24% of those living with HIV worldwide and 33% of those living with HIV in the United States are currently receiving antiretroviral therapy. The US government provided 68.2% of the international AIDS assistance from donor governments in 2011, but disbursements from the United States have been declining since 2009. Federal funding for the Ryan White HIV/AIDS Program has been stable, after adjustment for inflation, since 1999, despite an increase of more than 40% in HIV/AIDS prevalence in the United States during that time. The gap remains between those in need of and those receiving antiretroviral therapy. Maintenance of antiretroviral therapy and expansion of existing programs are in jeopardy in an era of US budget sequestration and global reductions in funding of antiretroviral therapy in resource-limited settings (RLSs).
Kates discussed how the Patient Protection and Affordable Care Act (PPACA) will impact US domestic antiretroviral therapy programs and access to those programs. The PPACA will likely increase access to care because it raises the age limit for dependent coverage, eliminates lifetime and annual coverage limits, and removes limitations on preexisting conditions, a particularly relevant limitation for those living with HIV. Prescription drugs, including antiretroviral drugs, will no longer be subject to the coverage gap “donut hole” within Medicare Part D, and states can choose to define antiretroviral therapy as an essential health benefit required by insurers. However, the ability to opt out of Medicaid expansion in the PPACA, guaranteed by a US Supreme Court decision in 2012, could substantially impact this opportunity for expanded antiretroviral therapy access. Currently, 68% of people living with HIV in the United States reside in states that are likely to support Medicaid expansion. However, the majority of states opposed to Medicaid expansion are in the South, which means that current regional disparities in access to antiretroviral therapy could be exacerbated by the implementation of the PPACA. Kates emphasized that health insurance coverage does not ensure access to antiretroviral therapy and the important role that the Ryan White HIV/AIDS Program should play during this transition, by supplementing coverage for poorer patients and providing care for those who fall out of Medicaid expansion or who are not US citizens.
Cascade of HIV Care
Since the results of the HPTN (HIV Prevention Trials Network) 052 Study demonstrated that patients receiving potent antiretroviral therapy were less likely to transmit HIV to their partners, antiretroviral therapy has been seen as serving the dual purpose of saving the life of the infected partner and preventing transmission to uninfected individuals.5 Treatment as prevention is limited, however, to those achieving virologic suppression on antiretroviral therapy. In 2010, it was estimated that only 28% of HIV-infected individuals in the United States had suppressed HIV-1 RNA levels.6 The process of achieving virologic suppression proceeds through 5 stages: HIV diagnosis, linkage to care, retention in care, receipt of antiretroviral therapy, and virologic suppression. This progression is often called the cascade of care. The proportion of people who make it through the cascade and achieve virologic suppression is a metric of the efficacy of HIV testing and treatment programs. Several presentations highlighted successes and gaps in the cascade in different settings.
Greenberg reviewed national US data and used Washington DC’s efforts to expand HIV testing and treatment as a case study, as discussed in the article “New Tools to Understand Transmission Dynamics and Prevent HIV Infections” by Buchbinder and Liu (Abstract 58). Session 31 included data from the United States, Canada, and France, highlighting the need for consistent definitions in discussions of the cascade of care and for examination of the cascade of care nationally and locally to address gaps. Althoff and colleagues applied relevant DHHS indicators of the cascade—percentage of patients retained in care, percentage of patients prescribed antiretroviral therapy, and percentage of patients with suppressed HIV-1 RNA levels—to data from 10 clinics participating in the NA-ACCORD (North American AIDS Cohort Collaboration on Research and Design) (Abstract 1026).
The investigators noted that those participating in NA-ACCORD were all engaged in care at some point. To be included in the cohort, participants must have 2 HIV clinic visits within a 12-month period. Thus, these data do not reflect early steps in the cascade, such as testing and linkage to care. Of 25,235 patients, 87% met DHHS definitions for retained in care, 85% were prescribed antiretroviral therapy, and 76% achieved virologic suppression. Statistically significant disparities in age, race and ethnicity, and HIV transmission risk category existed for all indicators after adjustment. Among patients with access to HIV care, only one-quarter did not achieve virologic suppression, but this success was attenuated for younger people, African Americans, and heterosexual individuals.
Data on the cascade of care in Kings County, Washington, were presented by Dombrowski and colleagues (Abstract 1027). Their unique strategy used laboratory data on CD4+ cell counts and HIV-1 RNA levels in addition to case investigations to define indicators at various steps in the cascade and accounted for patient migration in and out of care. They confirmed a linkage-to-care rate of 78% of people living with HIV, of which 54% were retained and engaged in continuous care and 52% were virologically suppressed. These data are higher than the national estimate of 28% virologic suppression.6 The investigators speculated that their ability to account for in-and-out migration and use of laboratory data as a surrogate for retention in care may have led to a more accurate depiction of the care cascade. The reliability of laboratory data as a surrogate for engagement in care is currently under investigation, and Kings County is now using a lack of laboratory data after 6 months as a trigger for outreach efforts to prevent patients from being lost to follow-up.
The US Medical Monitoring Project (MMP) is a national supplemental surveillance system that monitors the clinical outcomes of HIV-infected adults engaged in care across 23 health department jurisdictions in the United States. Quinn and colleagues used MMP data from January through April of 2009 to examine the association between insurance type and continuous virologic suppression, defined as all HIV-1 RNA levels of 200 copies/mL or lower within the past 12 months (Abstract 1028). Sixty percent of patients in the cohort met criteria for durable virologic suppression, and after adjustment for sociodemographic and clinical factors, this was associated with insurance coverage through Medicare (odds ratio [OR], 1.13; 95% CI, 1.05-1.21) and the military (OR, 1.26; 95% CI, 1.09-1.45). Virologic suppression was also inversely associated with black race, younger or older age (compared with those 40 years to 49 years of age), homelessness, and more advanced disease stage (AIDS or nadir CD4+ cell count < 500/μL). The investigators concluded that these data support the expansion of quality HIV care delivery models, though confounding by the sociodemographic factors associated with various types of insurance is also probable.
Montaner and colleagues used the robust longitudinal dataset available in British Columbia to examine the evolution of the cascade of care during the period from 1996 to 2010 (Abstract 1029). They found that the estimated percentage of those with undiagnosed HIV infection fell from 53% to 14% during the study period. In 2009, linkage to care was 79%, retention in care was 56%, and an estimated 38% of HIV-infected individuals were virologically suppressed, defined as having no detectable (using assay threshold at the time) HIV-1 RNA over 3 or more months within the calendar year. Sensitivity analyses revealed that estimates of virologic suppression varied widely, from 38% to 55%, by the definition of suppression applied to the data. It is remarkable that despite universal access to care in Canada, there is still substantial attrition through the care cascade. The investigators are examining the sociodemographic and geographic predictors of achievement of virologic suppression.
Supervie and Constagliola used several French national datasets, including HIV surveillance data, French national health insurance data, and the French hospital database on HIV (Agence Nationale de Recherche sur le Sida [ANRS]-CO4 study), to estimate engagement throughout the HIV care cascade in France (Abstract 1030). Of all HIV-infected adults older than 15 years in France in 2010, it is estimated that 81% were diagnosed, 74% were in care, 60% were taking antiretroviral therapy, and 52% were virologically suppressed. Disparities occur early in HIV care by HIV transmission risk group, with consistently higher engagement at all levels for injection drug users and lowest engagement for non–French national heterosexual men, although the investigators did not report statistical tests for differences between these groups.
Data from the Ryan White HIV/AIDS Program were used by Doshi and colleagues to examine the cascade of care for 546,156 Ryan White HIV/AIDS Program clients in 2010 (Abstract 1031a). Of 291,449 persons with documented HIV seropositive status receiving Ryan White HIV/AIDS Program–funded medical care, 80% were prescribed antiretroviral therapy, 76% were retained in care, and 60% had a most-recent viral load below 200 copies/mL. Retention in care was statistically significantly associated with female sex, Hispanic or multiracial race and ethnicity compared with white race, and all age groups between 13 years and 54 years compared with those older than 65 years. Blacks and American Indian or Alaska natives were statistically significantly less likely to be retained in care.
Horberg and colleagues used data from the Kaiser Permanente Medical Care Program to illustrate a flaw in the cascade of care: progression to subsequent steps in the cascade is not necessarily dependent on achievement of success at earlier stages (Abstract 1033). They argue that, depending on how the variables are defined, one can fail to be engaged in care but still receive antiretroviral therapy or achieve virologic control. They analyzed achievement of cascade stages by forcing each stage to be a subset of the previous or by allowing stages to be independent. In the Kaiser Permanente Medical Care Program in 2010, 97% of 16,816 known HIV seropositive patients were linked to care and 78% were retained in care. Of those patients retained in care in 2010, defined as at least 2 medical visits in 2010 with at least 60 days between visits, 66% filled 3 months worth of antiretroviral therapy prescriptions. However, if the calculation is not restricted to those retained in care, 83% received antire-troviral therapy. Similarly, for virologic suppression, 61% of HIV-infected patients had last-measured HIV-1 RNA levels below 200 copies/mL, if this stage is taken as a subset of all prior stages. If the cascade steps are independent, 80% of HIV-infected patients in the Kaiser program in 2010 had a last-measured HIV-1 RNA level below 200 copies/mL. There were also statistically significant differences in achievement of various stages in the cascade of care by sex and age.
Data from a single site in Johannesburg, South Africa, was used to examine attrition in 3 cohorts of individuals: those testing seropositive for HIV, those linking to HIV care, and those starting antiretroviral therapy between March 2010 and March 2012 (Abstract 1103). The investigators defined lost to follow-up (LTFU) as at least 1 month late for linkage to care for those who tested HIV seropositive and at least 3 months late for the pre–antiretroviral therapy and on antiretroviral therapy cohorts. Of 229 patients testing HIV seropositive, 54.6% were not linked to care. Of 134 pre–antiretroviral therapy patients, 17.2% were LTFU prior to antiretroviral therapy initiation and 13 of 152 (8.6%) were LTFU while on antiretroviral therapy. Predictors of LTFU included transportation-related barriers for the HIV-seropositive testing cohort, and young age and South African birth for the pre–antiretroviral therapy cohort. These findings are concerning but, because they come from a single site, do not account for the substantial in-and-out migration that may be impacting the LTFU measures.
Taken collectively, findings on the cascade of care presented at CROI illustrate several key points. First, uniformity of definitions and methodology for assessment of the numbers of patients reaching each stage is crucial. As demonstrated, percent attainment can be affected by the definitions of engagement in care and virologic suppression (Abstract 1033) and the type of dataset used, as illustrated by the use of laboratory data to determine care engagement (Abstract 1027). Even in settings with integrated medical systems and success in diagnosis and linkage to care, such as Seattle, Washington (Abstract 1027), British Columbia (Abstract 1029), Canada, and France (Abstract 1030), substantial health disparities by race and ethnicity, sex, and HIV transmission category exist. Finally, as the moderators emphasized, these data must be used to address gaps throughout the care cascade and to translate lessons from programs with good care cascades to other settings (Figure).
Figure.
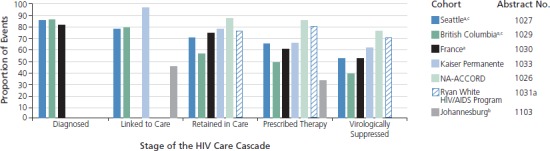
Attrition through the cascade of care reported at the 20th Conference on Retroviruses and Opportunitic Infections (CROI 2013) varies widely by cohort and metrics utilized. aThe Seattle, British Columbia, and French cohorts began calculations with the number of HIV seropositive individuals diagnosed. The remaining cohorts began with the number of known diagnoses linked to care, thus the denominators are not comparable between the first 3 and latter 4 studies. bThe Johannesburg cohort percentage of prescribed antiretroviral therapy is defined as the percentage of individuals retained from seropositive HIV test through 1 year on antiretroviral therapy. cIn the Seattle and British Columbia cohorts, “virologically suppressed” was defined as a plasma HIV-1 RNA level below 200 copies/mL for the most recent value reported for the observation period in 2010.
Antiretroviral Therapy in RLSs
Engagement in Care in RLSs
Teasdale and colleagues from the International Center for AIDS Care and Treatment Programs (ICAP) presented data on the cascade of care and mortality from 34,892 patients in care at 41 ICAP-affiliated health care facilities in Rwanda between 2005 and 2010 (Abstract 92). They examined LTFU and mortality in patients pre–antiretroviral therapy and on antiretroviral therapy and found that LFTU was 11.2% and 4.4% and mortality was 4.4% and 6.3% at 2 years for pre– and on antiretroviral therapy, respectively. Both adverse outcomes were associated with male sex, and receiving care at a rural facility was associated with mortality pre– and on antiretroviral therapy. There is a strong likelihood that deaths were underreported in this clinical cohort without active case finding. Regardless, retention was higher in this cohort than in others reporting pre–antiretroviral therapy LTFU but remains a challenge for individuals pre–antiretroviral therapy who had higher CD4+ cell counts and World Health Organization (WHO) clinical disease stages at enrollment.
Guffey and colleagues asked a similar question regarding LTFU for individuals with immediate (within 180 days of enrollment) antiretroviral therapy initiation versus delayed initiation for 118,935 adults enrolled in a public sector cohort in Lusaka, Zambia (Abstract 93). To mitigate confounding by disease severity, the investigators examined LTFU, defined as 60 days or more late for a clinical or pharmacy visit, in 6419 individuals falling within a narrow disease status band: a CD4+ cell count of 175/μL to 225/μL and WHO disease stage I or II. This allowed for inclusion of individuals falling on either side of the antiretroviral therapy initiation threshold of 200 cells/μL in Zambia. Despite similar baseline characteristics, they found LTFU rates of 10.2 per 100 person-years (95% CI, 14.1-15.7) in the immediate antiretroviral therapy start group (n = 4810) and 33.9 per 100 person-years (95% CI, 31.8-36.1) in the delayed antiretroviral therapy start group (n = 1609). In a Kaplan-Meier analysis of the proportion of LTFU by antiretroviral therapy initiation status, it was evident that the discrepancy between the 2 groups occurred within the first year of enrollment, with rates of LTFU leveling out after that. The investigators acknowledged that unreported deaths or transfers may be misclassified as LTFU. Questions from the audience also raised the concern that people may not have initiated antiretroviral therapy because they were LTFU, particularly considering the 60-day follow-up definition.
Hoffman and colleagues from ICAP examined another aspect of engagement in care in RLSs, late enrollment into care (Abstract 94). They determined the proportion of patients enrolling into care late, defined as entering into care after being eligible for antiretroviral therapy (CD4+ cell count =≤ 350/μL or WHO stage III or IV by 2010 WHO guidelines), in 302,777 patients drawn from 193 ICAP-affiliated HIV care clinics in 4 countries in sub-Saharan Africa. The percentage of individuals enrolling late decreased substantially from 68% in 2006 to 55% in 2011, and the median CD4+ cell count at enrollment rose from 238/μL to 286/μL during the same period; both tests for trends were statistically significant (P<0001). This decrease in risk for late enrollment was observed across all patient demographic groups (sex, age, marital status, enrollment point of entry, and country). A second analysis of factors associated with late enrollment was conducted for 45,113 patients in 193 clinics providing services in 2011. The investigators included program-level and regional data in this analysis and found that increased population knowledge of HIV and testing uptake were protective against late enrollment. The investigators concluded that, though late enrollment is declining, it was still substantial (55%) in 2011, and that efforts to increase HIV knowledge and testing at the population level could have an impact.
MacPherson and colleagues presented data from a possible intervention to address the above issues in engagement in HIV care, particularly regarding delays in antiretroviral therapy initiation (Abstract 95LB). Using a cluster-randomized trial design of 14 neighborhoods in Blantyre, Malawi, they randomized 7 regions to home-based HIV testing with referral to facility-based HIV care (control arm) and 7 regions to home-based testing with the option of home assessment of HIV serostatus and initiation of antiretroviral therapy if HIV infected and eligible for antiretroviral therapy initiation by national guidelines (intervention arm). Community-based counselors were selected and trained within each cluster to perform testing, assessment, and initiation of 2 weeks of antiretroviral therapy for those in the intervention arm. After 2 weeks of therapy, those patients in the intervention arm opting for home initiation were required to engage in care at a local clinic. Patients in the intervention arm were more likely to disclose their HIV seropositive status to the counselors (risk ratio [RR], 1.86; 95% CI, 1.16-2.97) and more likely to initiate antiretroviral therapy (RR, 2.94; 95% CI, 2.10-4.12). The investigators are conducting a more in-depth analysis of these late-breaking data, including important results on the number of individuals who initiated antiretroviral therapy at home but did not subsequently link to care and other adverse outcomes. Regardless, home-based antiretroviral therapy initiation appeared acceptable to many participants and increased treatment initiations in this context.
Retention and Adherence in RLSs
Session 48 covered similar themes of retention and adherence in RLSs. Bangsberg began the session with a review of the current literature. He highlighted that any analysis generated from programmatic data without sampling those LTFU may generate biased information, overestimating survival and underestimating engagement in care. Studies in RLSs demonstrate that many people who are disengaged from care have died, and many others have transferred to other clinics that are more convenient.7 Adherence in RLSs remains good and may be enhanced by some technology interventions. However, initiating antiretroviral therapy at higher CD4+ cell counts and duration on antiretroviral therapy are both risk factors for poor adherence and suggest that programs may have more trouble as they mature and CD4+ cell count thresholds for antiretroviral therapy initiation rise.
Transportation cost and distance to clinic are frequently noted barriers to maintenance in care in RLSs. Seidner and colleagues sought to measure and validate transportation time and distance using 4 different measures of transportation for 188 patients in the UARTO (Ugandan AIDS Rural Treatment Outcomes) study (Abstract 1101). They found a high correlation between global positioning system (GPS) measures of distance to clinic: GPS straight-line distance and GPS tracked distance using the distance measured by a clinic staffer driving the patient home by his or her regular route (R2 = 0.92). There was poor correlation between GPS straight-line distance and either self-reported travel time or self-reported travel cost. They validated these measures using linear regression models for the association between transportation measure and days missing from clinic per year. Both GPS measures (straight-line and tracked distance) had a statistically significant correlation (P < .001) with days missing, but the self-reported measures did not. The investigators suggest that objective measures of distance to clinic should be used to stratify patients in rural RLSs by level of risk for loss to follow-up.
Ugoji and colleagues examined predictors of patient retention in care, defined as 1 or more clinic visits during the 1-year review period, for 5176, randomly selected, HIV seropositive patients from a retrospective review of quality of care in 2010 at 37 Nigerian treatment facilities (Abstract 1102). They found that 1 of 4 pre–antiretroviral therapy patients and 3 of 4 patients on antiretroviral therapy were retained in care. After controlling for patient-level characteristics known to be associated with LTFU (age, sex, antiretroviral therapy status, baseline CD4+ cell count, and WHO stage), several treatment-site characteristics were found to be statistically significantly associated with retention in care, including rural site, younger age of treatment site, stability of site clinicians, and support group linkages. Interestingly, the presence of leadership-supported quality improvement teams and a high nurse-to-patient ratio were inversely associated with retention in care. These findings are encouraging for the ability of programs to enhance retention with support groups, even in decentralized and newly established rural clinics.
Retention in care prior to antiretroviral therapy initiation was examined using routinely collected data from 17 primary health care clinics in a decentralized HIV program in KwaZulu-Natal, South Africa (Abstract 1104). Overall, 31,767 patients 15 years of age or older met inclusion criteria of a seropositive rapid HIV test and a CD4+ cell count at a participating site between January 2007 and March 2011, with follow-up through March 2012. Based on CD4+ cell count criteria for initiation (≤ 200/μL prior to April 2010, or ≤ 350/μL thereafter), 13,761 (43%) were eligible for antiretroviral therapy immediately, 5404 (17%) became eligible during follow-up, and 7630 (24%) were LTFU without subsequent CD4+ cell count monitoring to determine eligibility. Of the 19,165 patients eligible for antiretroviral therapy at any point, only 66% initiated antiretroviral therapy during the observation period. Patients who were eligible for antiretroviral therapy after 2 or more CD4+ cell count measurements were more likely to initiate antiretroviral therapy than those eligible at the first CD4+ cell count, after adjustment for sex, age, CD4+ cell count, and year of eligibility (adjusted odds ratio [aOR], 1.39; 95% CI, 1.33-1.45). The investigators felt that LTFU and failure to initiate antiretroviral therapy after the first CD4+ cell count could be mitigated by interventions at the first clinic visit.
Myer and colleagues asked whether HIV-infected women have better outcomes on antiretroviral therapy when other HIV-infected family members are enrolled at the same site (Abstract 1105). They included women initiating antiretroviral therapy with up to 5 years of follow-up from 12 ICAP-affiliated sites in 8 African countries. Of these 2877 women, 10% had an HIV-infected child enrolled in care at the same site, and 24% had an HIV-infected partner enrolled. Comparing women with co-enrollment of either a child or partner with those without co-enrollment, they found no differences in mortality. However, LTFU was statistically significantly lower among women with either a child or a partner co-enrolled in the same program (adjusted hazard ratio [aHR], 0.26; 95% CI, 0.15-0.46 for children co-enrolled; aHR, 0.41; 95% CI, 0.30-0.56 for partner co-enrolled). The investigators acknowledge that without active patient tracking, it is likely that some of those LTFU actually died and mortality is underestimated for the cohort.
Geng and colleagues investigated reasons for disengagement from care for patients on antiretroviral therapy at 14 clinics in Kenya, Uganda, and Tanzania (Abstract 1106). Of 6687 patients on antiretroviral therapy who were more than 3 months late for their last appointment (ie, LTFU), they generated a random sample of 1024 (15%) patients and attempted to ascertain their current status. Of 907 patients whose outcome was ascertained, 27% had died, 65% were in care, and 35% had disengaged from care. Those who were disengaged were asked open-ended questions regarding reasons for disengagement categorized as structural: transportation difficulty or expense (29% reported), work interference (23%), or lack of money to access care (12%); clinic-based: fear of scolding for missed appointment (13%), spending too much time at clinic (4%), and staff not being nice (3%); and patient-based: felt well and did not need care (23%), seeing a traditional healer instead (19%), and family obligations (16%). There were substantial between-country differences in the number of participants reporting structural and patient-based barriers, but all participants reported similar prevalence (13%-21%) of clinic-based barriers. These data shed light yet again on the high percentage of those LTFU who are deceased, and barriers to care for those disengaged, particularly transportation-based issues and feeling well, that could be mitigated.
Standard adherence measures can predict virologic rebound on antiretroviral therapy but usually do so after the fact. A wireless adherence monitor attached to a pillbox sends a signal via a cellular phone network each time it is opened, allowing real-time tracking of potential lapses in antiretroviral therapy. Haberer and colleagues used this technology in 447 individuals initiating antiretroviral therapy within the UARTO study and detected 134 virologically suppressed individuals with a signal lapse longer than 48 hours (Abstract 1107). Of these, virologic rebound (HIV-1 RNA levels, 430-24,278 copies/mL) was seen in 9 lapses, and this was associated with lapse duration (OR, 4.4 for lapses > 6 days; P = .03). Eight of nine patients with lapses resuppressed HIV-1 RNA levels on subsequent measurements after intervention by the study team. The investigators believe that this technology is feasible and could potentially replace some routine HIV-1 RNA monitoring.
Overall, the data presented on engagement in care and adherence in RLSs highlight several challenges: (a) the lack of uniformity of definitions for engagement in care and outcomes to allow comparison across studies and cohorts, (b) the likely underestimation of mortality that occurs whenever outcome ascertainment in LTFU is not completed, and (c) the need for innovative technology-based and other strategies to improve the HIV care continuum, from testing to virologic suppression, in RLSs.
Prevention of Mother-to-Child Transmission of HIV
This year’s N’Galy-Mann lecture was presented by Mofenson of the National Institute of Child Health and Human Development (Abstract 15). Her address provided an overview of the global scale-up of prevention of mother-to-child transmission (PMTCT) programs since the 1990s. The historical trajectory of PMTCT—from monotherapeutic options to lifelong antiretroviral therapy for pregnant women—provided a hopeful yet sobering view of ongoing PMTCT, primarily in RLSs. Mofenson argued that maternal antiretroviral therapy, even at coverage rates of 90%, would not be sufficient to achieve the WHO goal of less than 5% mother-to-child transmission (MTCT) globally by 2015. She delineated the crucial programmatic interventions needed to eliminate pediatric HIV infection, including overall reductions in incident HIV infections among women, universal access to family planning services, early antenatal services, and availability of safe alternatives to breast feeding when appropriate. The following sections summarize the most salient PMTCT data to be presented at this year’s CROI.
Uptake and Retention in PMTCT Programs
Data from the implementation of WHO options A, B, and B+ for PMTCT were presented in a number of oral and poster abstracts. Options A and B are CD4+ cell count–driven protocols for PMTCT. In both options, all pregnant women with CD4+ cell counts below 350/μL receive combination antiretroviral therapy during pregnancy and continue for their lifetimes. In Option A, women presenting with a CD4+ cell count above 350/μL receive prophylaxis with zidovudine monotherapy in the antepartum period; single-dose (sd) nevirapine, zidovudine, and lamivudine in the intrapartum period; and zidovudine with lamivudine for 7 days postpartum. In Option B, women with CD4+ cell counts above 350/μL receive antiretroviral therapy from week 14 of pregnancy until 1 week following cessation of breast feeding. A third option, Option B+, provides lifetime antiretroviral therapy to all pregnant women irrespective of CD4+ cell count results.
In an oral abstract session dedicated to PMTCT, Barr and colleagues described the implementation of an Option B+ PMTCT program in Malawi (Abstract 82). Designed by the Malawian Ministry of Health, the program modified WHO Option B+ by offering antiretroviral therapy to all pregnant or breast-feeding women. Program implementation began in the third quarter of 2011, and data are presented through year-end 2012. From a programmatic perspective, the implementation of Option B+ resulted in the successful integration of antenatal and antiretroviral therapy programs, the decentralization of antiretroviral therapy programs and expansion into rural settings, and the complete replacement of sd nevirapine protocols with antiretroviral therapy. Quantitatively, a 49% increase in the total antiretroviral therapy coverage in known HIV seropositive pregnant women was demonstrated, from 13,910 women in the 2 quarters preceding program implementation to 20,687 women in the most recent 2 quarters of program implementation, which represents an almost 800% increase in the use of antiretroviral therapy in pregnant women. The authors also noted that 41% of those starting antiretroviral therapy were breast-feeding mothers and described this as an unexpected and “client-driven phenomenon.”
In the same session, a South African study compared 3 programmatic strategies for uptake of PMTCT: standard of care (in which antenatal care and antiretroviral therapy are provided in separate settings); enhanced linkage (in which a lay counselor acts as a patient navigator to link patients to the antiretroviral therapy program); and an integrated approach (in which antiretroviral therapy and antenatal services are collocated at the same site). Not surprisingly, the integrated approach resulted in far higher rates of linkage to care and antiretroviral therapy initiation. Antiretroviral therapy initiation rates were 21% in the standard of care group, 49% in the enhanced linkage group, and 86% in the integrated approach group. Integration of care also resulted in a statistically significant reduction in time from antiretroviral therapy eligibility to time of antiretroviral therapy initiation: 29 days in the standard of care, 15 days in enhanced linkage, and 7 days in the integrated model (Abstract 83).
An analysis from the United Kingdom and Ireland compared rates of MTCT for 2 periods, 2000 to 2006 and 2007 to 2011 (Abstract 906). Analyzing data from more than 6000 women, the authors note a substantial decrease in MTCT across the 2 periods, from 1.28% in the early period to 0.68% in the later period. This decline in MTCT was accounted for by near universal use of antiretroviral therapy in pregnant women.
Timely HIV testing during pregnancy is an essential inflection point in the HIV care cascade. Using insurance claims, investigators calculated the percent coverage of HIV testing among commercially insured women who gave birth in the United States between 2009 and 2010 (Abstract 904). Extracting from a database of more than 20,000,000 women, 177,930 deliveries were identified. An insurance claim for HIV testing within 293 days before delivery was identified in 75.7% of women. No data on prevalence of HIV infection in this population was presented, and the authors noted that the study group may not be representative of the US female population. A study from rural Uganda estimated the incidence of HIV infection among pregnant women: 863 women who presented for nonemergent delivery with a prior seronegative HIV test at least 3 months prior to delivery were enrolled. Six new HIV diagnoses were made, yielding an incidence rate of 1.6 per 100 person-years, comparable to background rates in the population (Abstract 903).
Efficacy of PMTCT
Prevention of HIV transmission during breast-feeding is a crucial component of PMTCT efforts and overall child wellness goals. Fowler and colleagues presented 18-month follow-up results from HPTN 046 (Abstract 84LB). HPTN 046 is a large, randomized study comparing 6 months versus 6 weeks of infant nevirapine for prevention of postnatal HIV transmission. The study is being conducted in Zimbabwe, South Africa, Tanzania, and Kenya. All infants received 6 weeks of nevirapine after birth. At 6 weeks, 1505 HIV seronegative infants were randomized to continue nevirapine or receive placebo for a total of 6 months.
Women were instructed to exclusively breast-feed up to the 6-month point. In both arms, 29% of mothers were receiving antiretroviral therapy at randomization, and the median maternal CD4+ cell count was more than 500/μL. Self-reported adherence to study medication was 88% to 96%, and 95% of mothers reported cessation of breast-feeding at 12 months. At 18 months, data for the primary safety and efficacy end points were available for 679 infants randomized to extended nevirapine and 683 to placebo. No differences were seen in overall mortality or HIV infection–free survival between the arms. Postnatal transmission was observed in 2.2% of infants in the extended nevirapine arm and 3.1% in the placebo arm (P = .28). Statistically significantly higher rates of HIV transmission were observed in those women not receiving antiretroviral therapy. In women with a CD4+ cell count of above 350/μL who were not receiving antiretroviral therapy, transmission rates at 6 months were 0.7% in the extended nevirapine arm and 2.8% in the placebo arm (P=.014). Thirty-six percent of transmissions occurred before 6 months, and 18% occurred after reported cessation of breast-feeding, underscoring the risk conferred by early and abrupt cessation of breast-feeding in this study setting. No differences in rates of adverse events were observed between the arms.
The authors conclude that extended nevirapine is a safe and effective strategy for postnatal prevention of HIV transmission in women who are not on antiretroviral therapy. The strategy may be a bridge to universal implementation of maternal antiretroviral therapy.
Preliminary data from the French ANRS 12174 study were presented (Abstract 912). ANRS 12174 is a randomized, controlled study comparing the safety and efficacy of lamivudine-based with lopinavir/r–based prophylaxis in breast-fed infants of mothers deemed ineligible for antiretroviral therapy. The study is ongoing in Burkina Faso, Uganda, Zambia, and South Africa. Infant prophylaxis is administered for 12 months, during which mothers continue breast-feeding. Preliminary blinded data from 763 of the 1273 children enrolled show a total of 9 transmission events and an overall transmission rate of 1.3 per 100 child-years. The mortality rate thus far is reported to be 2.6 per 100 child-years. None of the deaths are attributable to HIV seroconversion or HIV-related disease. Six of the transmission events occurred after 6 months of breastfeeding. Although the study is ongoing, the authors note that the low rate of postnatal transmission observed is within the WHO target for reduction of breast-feeding transmission.
Data from the Kisumu Breastfeeding Study compared infant death rates in Kenyan women receiving PMTCT Option B with those receiving PMTCT Option B+ (Abstract 921). Data were collected prospectively between 2003 and 2009 in this open-label study. Maternal antiretroviral therapy discontinuation occurred in 82% of women in the Option B arm. The investigators found a statistically significant increase in risk of HIV transmission and infant death in the Option B arm compared with the Option B+ arm, 10.1% and 2.4%, respectively (P = .04).
A study of temporal trends in virologic failure among pregnant women receiving antiretroviral therapy in a European cohort showed reductions in virologic failure over time (Abstract 909). The authors analyzed data from 396 women who received a minimum of 28 days of antiretroviral therapy during pregnancy. Virologic failure was defined as any HIV-1 RNA level above 200 copies/mL at any point from conception to delivery. Between 2000 and 2001, virologic failure was observed in 34% of women, compared with 3% between 2010 and 2011. In a partially adjusted analysis, virologic failure was associated with use of an unboosted protease inhibitor (PI), younger age, previous deliveries, duration of HIV infection, as well as calendar year of delivery. Despite the high rates of viral resistance in the early analysis period, 63% of women with virologic failure subsequently achieved suppression.
A related analysis from Canada estimated the proportion of pregnant women who maintained virologic suppression to delivery (Abstract 908). Of 178 women with virologic suppression during pregnancy, 9.5% had a detectable HIV-1 RNA level (> 250 copies/mL) at delivery. In a multivariate analysis, only poor adherence was associated with loss of virologic control. Despite the surprisingly high rates of virologic failure reported in this study, no MTCT events occurred.
Safety and Complications of PMTCT
Sibiude and colleagues presented long-term teratogenicity data from more than 13,000 children enrolled in the French Perinatal Cohort study (Abstract 81). The analysis included women and their children enrolled since 1985 and represents approximately 70% of HIV-infected women in metropolitan France. Children who were uninfected at birth were followed up for 2 years, whereas infected children were followed up for 18 years. Of the total cohort, 370 women were exposed to efavirenz in the first trimester. Efavirenz exposure in the first trimester was not associated with an overall increase in birth defects. However, efavirenz exposure in the first trimester was associated with an increase in neurologic defects, with an aOR of 2.3 (95% CI, 1.1-9.1; P = .03). Four neurologic birth defects were observed: pachygyria, cerebral cyst, agenesis of the corpus callosum, and hydrocephaly. No neural tube defects were identified. All neurologic defects occurred in children exposed to efavirenz since conception. Zidovudine exposure in the first trimester was associated with an overall increased risk of a birth defect, with an aOR of 1.4 (95% CI, 1.1-1.8; P = .002). There was a statistically significant association between zidovudine exposure in the first trimester and congenital heart defects, with an aOR of 2.5 (95% CI, 1.6-4.2; P = .001); the majority of these were ventricular septal defects. Didanosine was associated with head and neck defects (aOR, 1.93; 95% CI, 1.1-3.3; P < .05). The investigators noted that the analysis did not control for maternal drug use. Referencing the recent change to WHO guidelines, the authors urged caution in the use of efavirenz in the first trimester and the ongoing need for surveillance of the benefits and risks of PMTCT.
A study conducted in Kisumu, Kenya, compared risk of birth defects and infant mortality among infants exposed to nevirapine versus nelfinavir (Abstract 924). Only women with a CD4+ cell count above 250/μL were included in the analysis. A total of 392 women and 401 births were included: 215 women received nevirapine and 177 women received nelfinavir. All women were treated with a zidovudine plus lamivudine nRTI backbone. Overall, there were 394 live births and 7 stillbirths (1.8%). Rates of preterm delivery and low birth weight were 12.2% and 9.6% of live births, respectively. No statistically significant differences were observed between nevirapine-and nelfinavir-exposed infants.
Expansion of PMTCT programs has been associated with the emergence of mutations associated with drug resistance. A study from Brazil showed high rates of these mutations in both antiretroviral therapy–naive and –experienced pregnant women (Abstract 905). Two hundred thirteen pregnant women presenting for care with a HIV-1 RNA level above 2000 copies/mL were included in the analysis; 77% were antiretroviral therapy–naive and 24% were primigravida. Drug resistance mutations were found in 10% of antiretroviral therapy–naive women and in 37% of antiretroviral therapy–experienced women. The K103N accounted for 79% of the mutations detected. Overall, 80% of antiretroviral therapy–naive women achieved an HIV-1 RNA level below 400 copies/mL at 34 weeks gestation, compared with 57% of antiretroviral therapy–experienced women. Suboptimal antiretroviral therapy in pregnancy is known to lead to the emergence of resistance. In a study from Thailand, zidovudine monotherapy was associated with a 16% rate of zidovudine-associated resistance mutations (Abstract 937).
Antiretroviral Therapy Resistance
In a symposium presentation entitled ARV Drug Resistance: Global Challenges, Hamers discussed the implications of antiretroviral resistance to global antiretroviral therapy success (Abstract 121). The presentation addressed a number of crucial issues in the emergence of drug resistance globally, including the implications of antiretroviral therapy scale-up on acquired and transmitted drug resistant virus; the impact of novel treatment strategies, including preexposure prophylaxis (PrEP), treatment as prevention, and early antiretroviral therapy initiation on the emergence of drug resistance mutations; and the implications of limited access to HIV-1 RNA level testing and sequencing technologies on resistance trends in low- and middle-income countries. Noting that antiretroviral therapy resistance has stabilized or decreased in resource-rich settings, Hamers attributed this trend to the availability of potent antiretroviral therapy, effective HIV-1 RNA level monitoring, widespread availability of resistance testing, and access to individualized second-line and salvage regimens.
Transmitted drug resistance (TDR) is an epidemiologic warning sign of future compromise to antiretroviral therapy efficacy. Since the rollout of antiretroviral therapy, aggregated data from East and southern Africa show an average annual rate of increase in TDR of 29% and 14%, respectively. This increase in resistance is driven mainly by NNRTI resistance. Data also suggest that TDR is associated with a doubled risk of future virologic failure.
Mathematical modeling has been used to predict the impact of antiretroviral therapy scale-up on TDR prevalence in Africa. Expansion of antiretroviral therapy treatment criteria to all patients with a CD4+ cell count below 500/μL is predicted to increase TDR prevalence to 19%. Nevertheless, a benefit-to-harm analysis favors antiretroviral therapy expansion. Treatment initiation based on a CD4+ cell count threshold of below 500/μL was modeled for 2 urban settings in Uganda and Kenya and found to be associated with 1 case of TDR for every 22 and 32 new cases of HIV infection averted, respectively (Abstract 1116). Acquired drug resistance mutations are also on the rise. Lack of timely identification of virologic failure, coupled with a paucity of second-line options, leads to accumulation of resistance in patients maintained on failing regimens. Hamers argued that large-scale expansion of antiretroviral therapy programs will result in progressively increasing rates of resistance, unless virologic monitoring, resistance sequencing, and second-line options become available concomitantly.
Acquired Drug Resistance
In a large study from Senegal, investigators calculated rates of resistance in the ANRS 1215 cohort (Abstract 592). Subjects included in the analysis initiated therapy with either an NNRTI- or an unboosted indinavir–based regimen, had a minimum of 6 months follow-up, and had at least 1 HIV-1 RNA measurement. Virologic failure was defined as 2 consecutive HIV-1 RNA level measurements above 1000 copies/mL; 366 subjects were included, of which 89% achieved virologic suppression. Cumulative risk of failure at 6 months, 12 months, and 24 months was 5%, 16%, and 25%, respectively. Resistance testing was available in 64% of subjects with virologic failure. NNRTI-associated mutations were noted in 77% of patients failing NNRTI-based therapy. PI-associated mutations were noted in 21% of those on a failing PI-based therapy, despite the observation that PI-based therapy was associated with a greater risk of virologic failure. The authors also provide data on the success of second-line regimens in those for whom the first regimen had failed: 81% achieved virologic suppression. However, at 6 months, 12 months, and 24 months, virologic failure was again noted in 18%, 20%, and 27%, respectively, of subjects on second-line therapy.
A similar study conducted in Namibia calculated the prevalence of resistance after 12 months of follow-up on an initial antiretroviral therapy (Abstract 593). Patients enrolled in treatment programs since 2009 and who had undergone pretreatment genotyping were included in the analysis. NNRTI-based therapy was prescribed to almost all participants. Of the 394 subjects enrolled, 80% remained on their initial antiretroviral therapy at 12 months; of these, 94% achieved virologic suppression. Of the total study population, 7% had resistance mutations at baseline and 5% of those with virologic failure at 12 months had detected resistance mutations. No PI resistance was noted, but high-level NNRTI resistance was prevalent. Virologic failure with resistance was associated with the presence of resistance mutations at baseline and strongly associated with markers of poor adherence.
Canadian researchers analyzed the prevalence of resistance mutations in previously suppressed patients who rebounded to low-level viremia, defined as an HIV-1 RNA level between 50 copies/mL and 1000 copies/mL. Two hundred patients with no baseline resistance mutations were included in the analysis. Approximately 5000 sequencing attempts were made. The success of sequencing increased with HIV-1 RNA strata from 75% in the 51 copies/mL to 249 copies/mL strata to 90% in the 749 copies/mL to 1000 copies/mL strata. Mutations were found in 20% of patients. The most common mutation detected was M184V (16%), followed by K103N (10.5%). HIV-1 RNA strata did not predict the presence of mutations.
TDR
In an oral abstract session, investigators from the Centers for Disease Control and Prevention (CDC) presented data on TDR rates from 10 US surveillance sites (Abstract 149). Persons with newly diagnosed HIV infection between 2007 and 2010, with no prior history of antiretroviral treatment, and with viral sequencing evaluations available were included in the analysis. The surveillance sites included 6 states, mostly in the southern United States, and 4 municipalities. Of the 77,000 newly diagnosed cases reported to the CDC, sequencing data was available for 18,144 (23%). Overall, 16.2% of sequences harbored mutations. Single, dual, and triple class mutations were identified in 13.6%, 2.1%, and 0.5% of sequences, respectively. NNRTI mutations were most frequent, detected in 8.1% of specimens, followed by nRTI-associated mutations in 6.7% and PI-associated mutations in 4.5%. Seventy-two percent of NNRTI mutations were K103N. The most common nRTI mutations identified were M41L (24.8%), T69N (19.5%), and M184V (8.2%); transmitted resistance to tenofovir was minimal (0.9%). The investigators calculated an annual percentage change in rates of TDR. Statistically significant increases in single class resistance and NNRTI resistance were found, with an estimated annual percentage increase of 4.3% (P = .01) and 5.2% (P = .03), respectively.
Investigators from the HOPS (HIV Outpatient Study) analyzed trends in resistance between 1999 and 2011 among antiretroviral therapy–naive individuals undergoing commercial genotypic testing prior to antiretroviral therapy initiation (Abstract 615). Of 711 samples analyzed, 10.4% harbored major drug resistance mutations. Mutations associated with nRTIs were observed in 8.9%, with NNRTIs in 7.8%, and with PIs in 3.2% of samples. Of note, no temporal trends in prevalence of TDR were observed in the reported time period. A similar analysis was conducted in Spain (Abstract 619). Rates reported were lower overall than in the US data cited above, but similarly, the researchers did not detect any temporal trends in TDR prevalence. An analysis of TDR in Guatemala, Panama, and Nicaragua showed prevalence rates of 7.5%, 9.0%, and 10.1%, respectively (Abstract 617).
Increased rates of TDR over time were reported in a study of clinical samples obtained at a single center in New York City (Abstract 616). The patients were primarily immigrants with subtype B strains. A statistically significant increase in TDR from 9% (2000-2005) to 18% (2006-2011) was observed. Overall TDR rates were 15%. High TDR rates were also reported in an adolescent HIV clinic in New York City (Abstract 952b). There were 331 behaviorally infected adolescents, with duration of infection of less than 1 year, enrolled in the clinical program between 2007 and 2011. Genotypic analysis data were available for 64% of these patients prior to initiation of antiretroviral therapy. Prevalence of resistance mutations was 19%; NNRTI-associated, nRTI-associated, and PI-associated resistance mutations accounted for 64%, 21%, and 15%, respectively.
Transmission of multiple drug–resistant HIV strains has been reported previously. Authors from Spain report on a multi-nRTI– and NNRTI-resistant strain causing a cluster of infections in men who have sex with men (Abstract 618).
Drug Resistance Mutation Detection Techniques
A novel technique using a single-tube, allele-specific assay for detection of tenofovir and emtricitabine resistance mutations was presented in Abstract 597. The clinical application of this assay would be useful in determining eligibility for tenofovir and emtricitabine–based PrEP. The authors compared the results of the single tube, allele-specific assay to a PCR sequence–based assay. Of the 75 specimens analyzed, the single-tube assay showed a high sensitivity, detecting 100% of all relevant tenofovir and emtricitabine resistance mutations at codon 65, 98.7% at co-don 184, and 92% at codon 70.
ANRS researchers reported on the operative characteristics of sequencing assays in dried blood spot (DBS) samples compared with paired plasma specimens (Abstract 606). Subjects included were those for whom their initial antiretroviral regimen was failing as indicated by an HIV-1 RNA level of above 1000 copies/mL. Sequencing was successful in 89 of 93 DBS samples. Sensitivity of the various assays used to sequence from DBS ranged from 82% to 93%. These findings suggest that resistance testing may become feasible within the constraints of existing laboratory infrastructures in RLSs.
Subtype- and Class-Specific Resistance Mutations
Investigators showed a decrease in darunavir-associated resistance mutations between 2006 and 2012 (Abstract 590). More than 78,000 specimens submitted to a commercial laboratory in the United States were analyzed. In 2006, 77.7% of specimens harbored no darunavir resistance mutations compared with 92.8% in 2012. The presence of more than 3 darunavir–associated resistance mutations were found in 7.5% of sequences in 2006 and in 2.6% in 2012. Among specimens with any resistance to PIs, presence of darunavir resistance mutations also decreased, as did phenotypic evidence of resistance to darunavir.
Data on integrase resistance from nearly 2000 sequences submitted to a commercial US laboratory were presented in Abstract 591. These clinical specimens were submitted specifically for InSTI resistance testing, suggesting clinical concern for InSTI failure. InSTI-associated resistance was observed in 25.6% of samples. InSTI-associated resistance was more frequent in older subjects and those with a higher resistance score to other classes of antiretrovirals (P < .001).
Investigators evaluated the ability of proviral env sequencing in virologically suppressed subjects to predict phenotypic tropism during viral rebound at treatment interruption (Abstract 586). Seventy-two subjects enrolled in 2 ACTG treatment interruption trials (ACTG 5102 and 5170) under went sequencing of the V1 through V5 regions while virologically suppressed. At time of treatment interruption, a commercial tropism assay was performed. Concordance between the proviral genotype assay results and phenotypic tropism assay results was 91.7%; sensitivity of genotype for predicting R5 tropism was 96.6% and specificity was 69.2%. In another study, mutations conferring resistance to maraviroc were assessed in 951 maraviroc treatment–naive individuals with R5 tropic virus by phenotype (Abstract 587). The investigators found genotypic evidence of resistance to maraviroc in 9.7% of subjects and suggested that genotypic analysis of tropism may overestimate phenotypic evidence of resistance to maraviroc.
Abstract 598 reports on the development of a standardized rule set for interpretation of resistance mutations in HIV-2. The tool is available at http://www.HIV-grade.de.
A list of all cited abstracts appears on pages 90-95 and is available online at www.iasusa.com.
References
Additional References
- 1.Buzon MJ, Massanella M, Llibre JM, et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med. 2010;16(4):460-465. [DOI] [PubMed] [Google Scholar]
- 2.Archin NM, Liberty AL, Kashuba AD, et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487(7408):482-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson MA, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA. 2012;308(4):387-402. [DOI] [PubMed] [Google Scholar]
- 4.Wilkin TJ, Lalama CM, McKinnon J, et al. A pilot trial of adding maraviroc to suppressive antiretroviral therapy for suboptimal CD4(+) T-cell recovery despite sustained virologic suppression: ACTG A5256. J Infect Dis. 2012;206(4):534-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention (CDC). Vital signs: HIV prevention through care and treatment--United States. MMWR Morb Mortal Wkly Rep. 2011;60(47):1618-1623. [PubMed] [Google Scholar]
- 7.Geng EH, Bangsberg DR, Musinguzi N, et al. Understanding reasons for and outcomes of patients lost to follow-up in antiretroviral therapy programs in Africa through a sampling-based approach. JAIDS. 2010;53(3):405-411. [DOI] [PMC free article] [PubMed] [Google Scholar]


