This November 2011 edition of the IAS–USA drug resistance mutations list updates the figures last published in December 2010 (Johnson VA et al, Top HIV Med, 2010;18:156-163).
In this update, the format has changed to the use of bold type for most gene positions and corresponding amino acid substitutions. However, the substitutions for which data indicate that there is a lesser impact on susceptibility—certain mutations impacting drugs in the protease inhibitor class and those impacting the nonnucleoside analogue reverse transcriptase inhibitor (NNRTI) etravirine—are represented in plain (non-bold) type. For the protease inhibitors, the mutations are designated as “major” or “minor” (see user note q); for etravirine, see below.
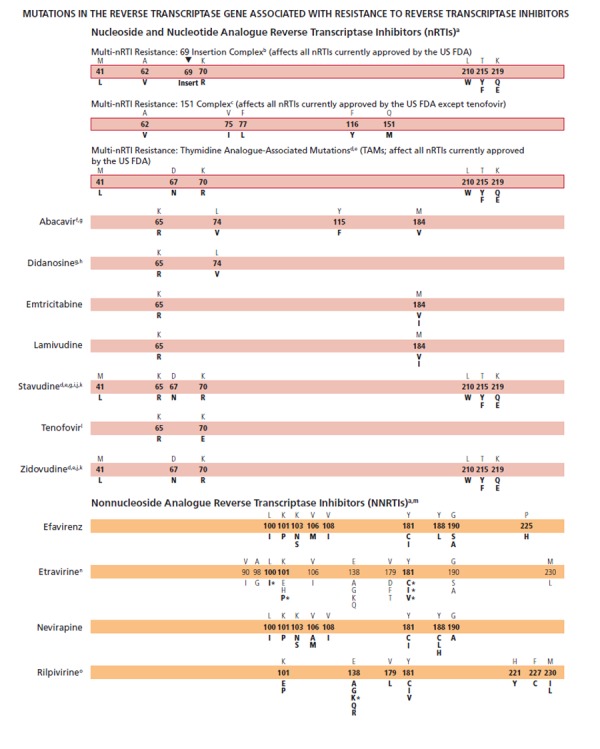
Rilpivirine (formerly TMC278), an NNRTI studied in antiretroviral treatment–naive patients and approved by the US Food and Drug Administration (FDA) this year, has been added. Fifteen mutations in HIV-1 reverse transcriptase have been observed to date from rilpivirine-treated patients with virologic failure: K101E/P; E138A/G/K/Q/R; V179L; Y181C/I/V; H221Y; F227C; and M230I/L. There are few data available on the clinical effectiveness of rilpivirine therapy for patients harboring NNRTI-resistant viruses. As a result, all of these mutations were bolded. The E138K mutation, especially with M184I or V, is found most frequently in patients in whom rilpivirine is failing, and is thus marked with an asterisk (*) because the combination of E138K and M184I showed a 6.7-fold reduced phenotypic susceptibility to rilpivirine compared with a 2.8-fold reduction for E138K alone (see user note o).
For etravirine, the Q substitution has been added to the E138 position on the reverse transcriptase gene, based on data from updated analyses of patients in the DUET trial (Tambuyzer L et al, JAIDS, 2011;58:18-22). Using the etravirine-weighted genotypic scoring system, reverse transcriptase mutations at positions L100I*, K101P*, and Y181C*/I*/V* are noted with an asterisk (*) to reflect that these mutations each have the greatest impact (ie, highest weighted scores) on reduced phenotypic susceptibility and impaired clinical response compared with other etravirine mutations (see user note n). For this reason, only those positions with asterisks are in bold type.
The S substitution has been added to the K103N mutation, which is associated with clinical resistance to efavirenz and nevirapine. This addition reflects the emerging understanding of substitutions other than N at the 103 position in the reverse transcriptase gene. (Harrigan PR et al, AIDS, 2005;19:549-554; Zhang Z et al, Antimicrob Agents Chemother, 2007;51:429-437; Tambuyzer L et al, Antivir Ther, 2009;14:103-109).
Methods
Mutations Panel
The IAS–USA Drug Resistance Mutations Group is an independent, volunteer panel of experts charged with delivering accurate, unbiased, and evidence-based information on these mutations to HIV clinical practitioners. As with all IAS–USA volunteer panels, members are rotated on a structured, planned basis. The group reviews new data on HIV drug resistance to maintain a current list of mutations associated with clinical resistance to HIV. This list includes mutations that may contribute to a reduced virologic response to a drug.
In addition, the group reviews only data that have been published or have been presented at a scientific conference. Drugs that have been approved by the US Food and Drug Administration (US FDA) as well as any drugs available in expanded access programs are included (listed in alphabetical order by drug class). User notes provide additional information as necessary. Although the Drug Resistance Mutations Group works to maintain a complete and current list of these mutations, it cannot be assumed that the list presented here is exhaustive.
Identification of Mutations
The mutations listed are those that have been identified by 1 or more of the following criteria: (1) in vitro passage experiments or validation of contribution to resistance by using site-directed mutagenesis; (2) susceptibility testing of laboratory or clinical isolates; (3) nucleotide sequencing of viruses from patients in whom the drug is failing; (4) correlation studies between genotype at baseline and virologic response in patients exposed to the drug.
The development of more recently approved drugs that cannot be tested as monotherapy precludes assessment of the impact of resistance on antiretroviral activity that is not seriously confounded by activity of other drug components in the background regimen. Readers are encouraged to consult the literature and experts in the field for clarification or more information about specific mutations and their clinical impact. Polymorphisms associated with impaired treatment responses that occur in wild-type viruses should not be used in epidemiologic analyses to identify transmitted HIV-1 drug resistance.
Clinical Context
The figures are designed for practitioners to use in identifying key mutations associated with viral resistance to antiretroviral drugs and in making therapeutic decisions. In the context of making clinical decisions regarding antiretroviral therapy, evaluating the results of HIV-1 genotypic testing includes: (1) assessing whether the pattern or absence of a pattern in the mutations is consistent with the patient’s antiretroviral therapy history; (2) recognizing that in the absence of drug (selection pressure), resistant strains may be present at levels below the limit of detection of the test (analyzing stored samples, collected under selection pressure, could be useful in this setting); and (3) recognizing that virologic failure of the first regimen typically involves HIV-1 isolates with resistance to only 1 or 2 of the drugs in the regimen (in this setting, resistance develops most commonly to lamivudine or emtricitabine or the nonnucleoside analogue reverse transcriptase inhibitors [NNRTIs]).
The absence of detectable viral resistance after treatment failure may result from any combination of the following factors: the presence of drug-resistant minority viral populations, nonadherence to medications, laboratory error, lack of current knowledge of the association of certain mutations with drug resistance, the occurrence of relevant mutations outside the regions targeted by routine resistance assays, drug-drug interactions leading to subtherapeutic drug levels, and possibly compartmental issues, indicating that drugs may not reach optimal levels in specific cellular or tissue reservoirs.
For more in-depth reading and an extensive reference list, see the 2008 IAS–USA panel recommendations for resistance testing (Hirsch MS et al, Clin Infect Dis, 2008;47:266-285) and 2010 IAS–USA panel recommendations for antiretroviral therapy (Thompson MA et al, JAMA, 2010;304[3]:321-333). Updates are posted periodically at www.iasusa.org.
Comments
Please send your evidence-based comments, including relevant reference citations, to the IAS–USA at resistance2011“at”iasusa.org or by fax at 415-544-9401. Please include your name and institution.
Reprint Requests
The Drug Resistance Mutations Group welcomes interest in the mutations figures as an educational resource for practitioners and encourages dissemination of the material to as broad an audience as possible. However, permission is required to reprint the figures and no alterations in format or the content can be made.
Requests to reprint the material should include the name of the publisher or sponsor, the name or a description of the publication in which you wish to reprint the material, the funding organization(s), if applicable, and the intended audience of the publication. Requests to make any minimal adaptations of the material should include the former, plus a detailed explanation of how the adapted version will be changed from the original version and, if possible, a copy of the proposed adaptation. To ensure the integrity of the mutations figures, IAS–USA policy is to grant permission for only minor, preapproved adaptations of the figures (eg, an adjustment in size). Minimal adaptations only will be considered; no alterations of the content of the figures or user notes will be permitted.
Please note that permission will be granted only for requests to reprint or adapt the most current version of the mutations figures as they are posted on the Web site (www.iasusa.org). Because scientific understanding of HIV drug resistance evolves rapidly and the goal of the Drug Resistance Mutations Group is to maintain the most up-to-date compilation of mutations for HIV clinicians and researchers, publication of out-of-date figures is counterproductive. If you have any questions about reprints or adaptations, please contact us.

User Notes
Some nucleoside (or nucleotide) analogue reverse transcriptase inhibitor (nRTI) mutations, like T215Y and H208Y,1 may lead to viral hypersusceptibility to the nonnucleoside analogue reverse transcriptase inhibitors (NNRTIs), including etravirine,2 in nRTI-treated individuals. The presence of these mutations may improve subsequent virologic response to NNRTI-containing regimens (nevirapine or efavirenz) in NNRTI-naive individuals,3-7 although no clinical data exist for improved response to etravirine in NNRTI-experienced individuals.
The 69 insertion complex consists of a substitution at codon 69 (typically T69S) and an insertion of 2 or more amino acids (S-S, S-A, S-G, or others). The 69 insertion complex is associated with resistance to all nRTIs currently approved by the US FDA when present with 1 or more thymidine analogue–associated mutations (TAMs) at codons 41, 210, or 215.8 Some other amino acid changes from the wild-type T at codon 69 without the insertion may be associated with broad nRTI resistance.
Tenofovir retains activity against the Q151M complex of mutations.8
Mutations known to be selected by thymidine analogues (M41L, D67N, K70R, L210W, T215Y/F, and K219Q/E, termed TAMs) also confer reduced susceptibility to all approved nRTIs.9 The degree to which cross-resistance is observed depends on the specific mutations and number of mutations involved.10-13 Mutations at the C-terminal reverse transcriptase domains (amino acids 293-560) outside of regions depicted on the figure bars may prove to be important for HIV-1 drug resistance. However, to date clinical relevance of these in vitro findings has not been established14 because the connection domain mutations arise mostly in conjunction with TAMs and M184V and do not seem to have major independent effects.15
Although reverse transcriptase changes associated with the E44D and V118I mutations may have an accessory role in increased resistance to nRTIs in the presence of TAMs, their clinical relevance is very limited.16-18
The M184V mutation alone does not appear to be associated with a reduced virologic response to abacavir in vivo.19,20 When associated with TAMs, M184V increases abacavir resistance.19,20
As with tenofovir, the K65R mutation may be selected by didanosine, abacavir, or stavudine (particularly in patients with nonsubtype-B clades) and is associated with decreased viral susceptibility to these drugs.19,21,22 Data are lacking on the potential negative impact of K65R on clinical response to didanosine.
The presence of 3 of the following mutations—M41L, D67N, L210W, T215Y/F, K219Q/E—is associated with resistance to didanosine.23 The presence of K70R or M184V alone does not decrease virologic response to didanosine.24
K65R is selected frequently (4%−11%) in patients with nonsubtype-B clades for whom stavudine-containing regimens are failing in the absence of tenofovir.25,26
The presence of M184V appears to delay or prevent emergence of TAMs.27 This effect may be overcome by an accumulation of TAMs or other mutations.
The T215A/C/D/E/G/H/I/L/N/S/V substitutions are revertant mutations at codon 215 that confer increased risk of virologic failure of zidovudine or stavudine in antiretroviral-naive patients.28-30 The T215Y mutant may emerge quickly from one of these mutations in the presence of zidovudine or stavudine.31,32
The presence of K65R is associated with a reduced virologic response to tenofovir.8 A reduced response also occurs in the presence of 3 or more TAMs inclusive of either M41L or L210W.8 The presence of TAMs or combined treatment with zidovudine prevents the emergence of K65R in the presence of tenofovir.33-35
The sequential use of nevirapine and efavirenz (in either order) is not recommended because of cross-resistance between these drugs.36
Resistance to etravirine has been extensively studied only in the context of coadministration with darunavir/ritonavir. In this context, mutations associated with virologic outcome have been assessed and their relative weights (or magnitudes of impact) assigned. In addition, phenotypic cutoff values have been calculated, and assessment of genotype-phenotype correlations from a large clinical database have determined relative importance of the various mutations. These 2 approaches are in agreement for many, but not all, mutations and weights.37-39 Asterisks (*) are used to emphasize higher relative weights with regard to reduced susceptibility and reduced clinical response compared with other etravirine mutations.40 The single mutations L100I*, K101P*, and Y181C*/I*/V* reduce clinical utility. The presence of K103N alone does not affect etravirine response.41 Accumulation of several mutations results in greater reductions in susceptibility and virologic response than do single mutations.42-44
A total of 15 mutations (K101E/P, E138A/G/K/Q/R, V179L, Y181C/I/V, H221Y, F227C, and M230I/L) associated with decreased susceptibility to rilpivirine have been described by in vitro studies and in patients in whom rilpivirine was failing.45-53 These mutations differ quantitatively in their impact on resistance. E138K, especially with M184I/V, is found most frequently in patients in whom rilpivirine is failing, and is thus marked with an asterisk (*) because the combination of E138K and M184I showed 6.7-fold reduced phenotypic susceptibility to rilpivirine compared with 2.8-fold reduction for E138K alone.45,53 The K103N substitution alone was not associated with reduced susceptibility to rilpivirine.52,53
Often, numerous mutations are necessary to substantially impact virologic response to a ritonavir-boosted protease inhibitor (PI).54 In some specific circumstances, atazanavir might be used unboosted. In such cases, the mutations that are selected are the same as with ritonavir-boosted atazanavir, but the relative frequency of mutations may differ.
Resistance mutations in the protease gene are classified as “major” or “minor.”
Major mutations in the protease gene (positions in bold type) are defined as those selected first in the presence of the drug or those substantially reducing drug susceptibility. These mutations tend to be the primary contact residues for drug binding.
Minor mutations generally emerge later than major mutations and by themselves do not have a substantial effect on phenotype. They may improve replication of viruses containing major mutations. Some minor mutations are present as common polymorphic changes in HIV-1 nonsubtype-B clades.
Ritonavir is not listed separately, as it is currently used only at low dose as a pharmacologic booster of other PIs.
Many mutations are associated with atazanavir resistance. Their impacts differ, with I50L, I84V, and N88S having the greatest effect. Higher atazanavir levels obtained with ritonavir boosting increase the number of mutations required for loss of activity. The presence of M46I plus L76V might increase susceptibility to atazanavir when no other related mutations are present.55
HIV-1 RNA response to ritonavir-boosted darunavir correlates with baseline susceptibility and the presence of several specific PI mutations. Reductions in response are associated with increasing numbers of the mutations indicated in the figure bar. The negative impact of the protease mutations I47V, I54M, T74P, and I84V and the positive impact of the protease mutation V82A on virologic response to darunavir/ritonavir were shown in 2 data sets independently.56,57 Some of these mutations appear to have a greater effect on susceptibility than others (eg, I50V vs V11I). A median darunavir phenotypic fold-change greater than 10 (low clinical cutoff) occurs with 3 or more of the 2007 IAS–USA mutations listed for darunavir58 and is associated with a diminished virologic response.59
The mutations depicted on the figure bar cannot be considered comprehensive because little relevant research has been reported in recent years to update the resistance and cross-resistance patterns for this drug.
In PI-experienced patients, the accumulation of 6 or more of the mutations indicated on the figure bar is associated with a reduced virologic response to lopinavir/ritonavir.60,61 The product information states that accumulation of 7 or 8 mutations confers resistance to the drug.62 However, there is emerging evidence that specific mutations, most notably I47A (and possibly I47V) and V32I, are associated with high-level resistance.63-65 The addition of L76V to 3 PI resistance–associated mutations substantially increases resistance to lopinavir/ritonavir.55
In some nonsubtype-B HIV-1, D30N is selected less frequently than are other PI mutations.66
Clinical correlates of resistance to tipranavir are limited by the paucity of clinical trials and observational studies of the drug. The available genotypic scores have not been validated on large, diverse patient populations. The presence of mutations L24I, I50L/V, F53Y/L/W, I54L, and L76V have been associated with improved virologic response to tipranavir in some studies.67-69
Resistance to enfuvirtide is associated primarily with mutations in the first heptad repeat (HR1) region of the gp41 envelope gene. However, mutations or polymorphisms in other regions of the envelope (eg, the HR2 region or those yet to be identified) as well as coreceptor usage and density may affect susceptibility to enfuvirtide.70-72
The activity of CC chemokine receptor 5 (CCR5) antagonists is limited to patients with virus that uses only CCR5 for entry (R5 virus). Viruses that use both CCR5 and CXC chemokine receptor 4 (CXCR4; termed dual/mixed [D/M]) or only CXCR4 (X4 virus) do not respond to treatment with CCR5 antagonists. Virologic failure of these drugs frequently is associated with outgrowth of D/M or X4 virus from a preexisting minority population present at levels below the limit of assay detection. Mutations in HIV-1 gp120 that allow the virus to bind to the drug-bound form of CCR5 have been described in viruses from some patients whose virus remained R5 after virologic failure of a CCR5 antagonist. Most of these mutations are found in the V3 loop, the major determinant of viral tropism. There is as yet no consensus on specific signature mutations for CCR5 antagonist resistance, so they are not depicted in the figure. Some CCR5 antagonist-resistant viruses selected in vitro have shown mutations in gp41 without mutations in V3; the clinical significance of such mutations is not yet known.
Raltegravir failure is associated with integrase mutations in at least 3 distinct genetic pathways defined by 2 or more mutations including (1) a signature (major) mutation at Q148H/K/R, N155H, or Y143R/H/C; and (2) 1 or more additional minor mutations. Minor mutations described in the Q148H/K/R pathway include L74M plus E138A, E138K, or G140S. The most common mutational pattern in this pathway is Q148H plus G140S, which also confers the greatest loss of drug susceptibility. Mutations described in the N155H pathway include this major mutation plus either L74M, E92Q, T97A, E92Q plus T97A, Y143H, G163K/R, V151I, or D232N.73 The Y143R/H/C mutation is uncommon.74-78 Another major mutation, E92Q, has also been described.79-81
References to the User Notes
- 1.Clark SA, Shulman NS, Bosch RJ, Mellors JW. Reverse transcriptase mutations 118I, 208Y, and 215Y cause HIV-1 hypersusceptibility to non-nucleoside reverse transcriptase inhibitors. AIDS. 2006;20:981-984. [DOI] [PubMed] [Google Scholar]
- 2.Picchio G, Vingerhoets J, Parkin N, Azijn H, de Bethune MP. Nucleoside-associated mutations cause hypersusceptibility to etravirine. [Abstract 23.] Antivir Ther. 2008;13(Suppl 3):A25. [Google Scholar]
- 3.Shulman NS, Bosch RJ, Mellors JW, Albrecht MA, Katzenstein DA. Genetic correlates of efavirenz hypersusceptibility. AIDS. 2004;18:1781-1785. [DOI] [PubMed] [Google Scholar]
- 4.Demeter LM, DeGruttola V, Lustgarten S, et al. Association of efavirenz hypersus-ceptibility with virologic response in ACTG 368, a randomized trial of abacavir (ABC) in combination with efavirenz (EFV) and indinavir (IDV) in HIV-infected subjects with prior nucleoside analog experience. HIV Clin TriSals. 2008;9:11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haubrich RH, Kemper CA, Hellmann NS, et al. The clinical relevance of nonnucleoside reverse transcriptase inhibitor hypersusceptibility: a prospective cohort analysis. AIDS. 2002;16:F33-F40. [DOI] [PubMed] [Google Scholar]
- 6.Tozzi V, Zaccarelli M, Narciso P, et al. Mutations in HIV-1 reverse transcriptase potentially associated with hypersusceptibility to nonnucleoside reverse-transcriptase inhibitors: effect on response to efavirenz-based therapy in an urban observational cohort. J Infect Dis. 2004;189:1688-1695. [DOI] [PubMed] [Google Scholar]
- 7.Katzenstein DA, Bosch RJ, Hellmann N, et al. Phenotypic susceptibility and virological outcome in nucleoside-experienced patients receiving three or four antiretroviral drugs. AIDS. 2003;17:821-830. [DOI] [PubMed] [Google Scholar]
- 8.Miller MD, Margot N, Lu B, et al. Genotypic and phenotypic predictors of the magnitude of response to tenofovir disoproxil fumarate treatment in antiretroviral-experienced patients. J Infect Dis. 2004;189:837-846. [DOI] [PubMed] [Google Scholar]
- 9.Whitcomb JM, Parkin NT, Chappey C, Hellman NS, Petropoulos CJ. Broad nucleoside reverse-transcriptase inhibitor cross-resistance in human immunodeficiency virus type 1 clinical isolates. J Infect Dis. 2003;188:992-1000. [DOI] [PubMed] [Google Scholar]
- 10.Larder BA, Kemp SD. Multiple mutations in HIV-1 reverse transcriptase confer high-level resistance to zidovudine (AZT). Science. 1989;246:1155-1158. [DOI] [PubMed] [Google Scholar]
- 11.Kellam P, Boucher CA, Larder BA. Fifth mutation in human immunodeficiency virus type 1 reverse transcriptase contributes to the development of high-level resistance to zidovudine. Proc Natl Acad Sci USA. 1992;89:1934-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calvez V, Costagliola D, Descamps D, et al. Impact of stavudine phenotype and thymidine analogues mutations on viral response to stavudine plus lamivudine in ALTIS 2 ANRS trial. Antivir Ther. 2002;7:211-218. [PubMed] [Google Scholar]
- 13.Kuritzkes DR, Bassett RL, Hazelwood JD, et al. Rate of thymidine analogue resistance mutation accumulation with zidovudine- or stavudine-based regimens. JAIDS. 2004;36:600-603. [DOI] [PubMed] [Google Scholar]
- 14.von Wyl V, Ehteshami M, Demeter LM, et al. HIV-1 reverse transcriptase connection domain mutations: dynamics of emergence and implications for success of combination antiretroviral therapy. Clin Infect Dis. 2010;51:620-628. [DOI] [PubMed] [Google Scholar]
- 15.von Wyl V, Ehteshami M, Symons J, et al. Epidemiological and biological evidence for a compensatory effect of connection domain mutation N348I on M184V in HIV-1 reverse transcriptase. J Infect Dis. 2010;201:1054-1062. [DOI] [PubMed] [Google Scholar]
- 16.Romano L, Venturi G, Bloor S, et al. Broad nucleoside-analogue resistance implications for human immunodeficiency virus type 1 reverse-transcriptase mutations at codons 44 and 118. J Infect Dis. 2002;185:898-904. [DOI] [PubMed] [Google Scholar]
- 17.Walter H, Schmidt B, Werwein M, Schwingel E, Korn K. Prediction of abacavir resistance from genotypic data: impact of zidovudine and lamivudine resistance in vitro and in vivo. Antimicrob Agents Chemother. 2002;46:89-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mihailidis C, Dunn D, Pillay D, Pozniak A. Effect of isolated V118I mutation in reverse transcriptase on response to first-line antiretroviral therapy. AIDS. 2008;22:427-430. [DOI] [PubMed] [Google Scholar]
- 19.Harrigan PR, Stone C, Griffin P, et al. Resistance profile of the human immunodeficiency virus type 1 reverse transcriptase inhibitor abacavir (1592U89) after monotherapy and combination therapy. CNA2001 Investigative Group. J Infect Dis. 2000;181:912-920. [DOI] [PubMed] [Google Scholar]
- 20.Lanier ER, Ait-Khaled M, Scott J, et al. Antiviral efficacy of abacavir in antiretroviral therapy-experienced adults harbouring HIV-1 with specific patterns of resistance to nucleoside reverse transcriptase inhibitors. Antivir Ther. 2004;9:37-45. [DOI] [PubMed] [Google Scholar]
- 21.Winters MA, Shafer RW, Jellinger RA, Mamtora G, Gingeras T, Merigan TC. Human immunodeficiency virus type 1 reverse transcriptase genotype and drug susceptibility changes in infected individuals receiving dideoxyinosine monotherapy for 1 to 2 years. Antimicrob Agents Chemother. 1997;41:757-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Svarovskaia ES, Margot NA, Bae AS, et al. Low-level K65R mutation in HIV-1 reverse transcriptase of treatment-experienced patients exposed to abacavir or didanosine. JAIDS. 2007;46:174-180. [DOI] [PubMed] [Google Scholar]
- 23.Marcelin AG, Flandre P, Pavie J, et al. Clinically relevant genotype interpretation of resistance to didanosine. Antimicrob Agents Chemother. 2005;49:1739-1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molina JM, Marcelin AG, Pavie J, et al. Didanosine in HIV-1-infected patients experiencing failure of antiretroviral therapy: a randomized placebo-controlled trial. J Infect Dis. 2005;191:840-847. [DOI] [PubMed] [Google Scholar]
- 25.Hawkins CA, Chaplin B, Idoko J, et al. Clinical and genotypic findings in HIV-infected patients with the K65R mutation failing first-line antiretroviral therapy in Nigeria. JAIDS. 2009;52:228-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallis CL, Mellors JW, Venter WD, Sanne I, Stevens W. Varied patterns of HIV-1 drug resistance on failing first-line antiretroviral therapy in South Africa. JAIDS. 2010;53:480-484. [DOI] [PubMed] [Google Scholar]
- 27.Kuritzkes DR, Quinn JB, Benoit SL, et al. Drug resistance and virologic response in NUCA 3001, a randomized trial of lamivudine versus zidovudine versus zidovudine plus lamivudine in previously untreated patients. AIDS. 1996;10:975-981. [DOI] [PubMed] [Google Scholar]
- 28.Riva C, Violin M, Cozzi-Lepri A, et al. Transmitted virus with substitutions at position 215 and risk of virological failure in antiretroviral-naive patients starting highly active antiretroviral therapy. [Abstract 124.] Antivir Ther. 2002;7:S103. [Google Scholar]
- 29.Chappey C, Wrin T, Deeks S, Petropoulos CJ. Evolution of amino acid 215 in HIV-1 reverse transcriptase in response to intermittent drug selection. [Abstract 32.] Antivir Ther. 2003;8:S37. [Google Scholar]
- 30.Violin M, Cozzi-Lepri A, Velleca R, et al. Risk of failure in patients with 215 HIV-1 revertants starting their first thymidine analog-containing highly active antiretroviral therapy. AIDS. 2004;18:227-235. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Lerma JG, MacInnes H, Bennett D, Weinstock H, Heneine W. Transmitted human immunodeficiency virus type 1 carrying the D67N or K219Q/E mutation evolves rapidly to zidovudine resistance in vitro and shows a high replicative fitness in the presence of zidovudine. J Virol. 2004;78:7545-7552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lanier ER, Ait-Khaled M, Craig C, Scott J, Vavro C. Effect of baseline 215D/C/S ‘revertant’ mutations on virological response to lamivudine/zidovudine-containing regimens and emergence of 215Y upon virological failure. [Abstract 146.] Antivir Ther. 2002;7:S120. [Google Scholar]
- 33.Parikh UM, Zelina S, Sluis-Cremer N, Mellors JW. Molecular mechanisms of bidirectional antagonism between K65R and thymidine analog mutations in HIV-1 reverse transcriptase. AIDS. 2007;21:1405-1414. [DOI] [PubMed] [Google Scholar]
- 34.Parikh UM, Barnas DC, Faruki H, Mellors JW. Antagonism between the HIV-1 reverse-transcriptase mutation K65R and thymidine-analogue mutations at the genomic level. J Infect Dis. 2006;194:651-660. [DOI] [PubMed] [Google Scholar]
- 35.von Wyl V, Yerly S, Boni J, et al. Factors associated with the emergence of K65R in patients with HIV-1 infection treated with combination antiretroviral therapy containing tenofovir. Clin Infect Dis. 2008;46:1299-1309. [DOI] [PubMed] [Google Scholar]
- 36.Antinori A, Zaccarelli M, Cingolani A, et al. Cross-resistance among nonnucleoside reverse transcriptase inhibitors limits recycling efavirenz after nevirapine failure. AIDS Res Hum Retroviruses. 2002;18:835-838. [DOI] [PubMed] [Google Scholar]
- 37.Benhamida J, Chappey C, Coakley E, Parkin NT. HIV-1 genotype algorithms for prediction of etravirine susceptibility: novel mutations and weighting factors identified through correlations to phenotype. [Abstract 130.] Antivir Ther. 2008;13(Suppl 3):A142. [Google Scholar]
- 38.Coakley E, Chappey C, Benhamida J, et al. Biological and clinical cut-off analyses for etravirine in the PhenoSense HIV assay. [Abstract 122.] Antivir Ther. 2008;13(Suppl 3):A134. [Google Scholar]
- 39.Peeters M, Nijs S, Vingerhoets J, et al. Determination of phenotypic clinical cut-offs for etravirine: pooled week 24 results of the DUET-1 and DUET-2 trials. [Abstract 121.] Antivir Ther. 2008;13(Suppl 3):A133. [Google Scholar]
- 40.Haddad M, Stawiski E, Benhamida J, Coakley E. Improved genotypic algorithm for predicting etravirine susceptibility: Comprehensive list of mutations identified through correlation with matched phenotype. [Abstract 574.] 17th Conference on Retroviruses and Opportunistic Infections (CROI). February 16-19, 2010; San Francisco, CA. [Google Scholar]
- 41.Etravirine [package insert]. Bridgewater, NJ: Tibotec Therapeutics; 2008. [Google Scholar]
- 42.Vingerhoets J, Peeters M, Azijn H, et al. An update of the list of NNRTI mutations associated with decreased virological response to etravirine: multivariate analyses on the pooled DUET-1 and DUET-2 clinical trial data. [Abstract 24.] Antivir Ther. 2008;13(Suppl 3):A26. [Google Scholar]
- 43.Scherrer AU, Hasse B, von Wyl V, et al. Prevalence of etravirine mutations and impact on response to treatment in routine clinical care: the Swiss HIV Cohort Study (SHCS). HIV Med. 2009;10:647-656. [DOI] [PubMed] [Google Scholar]
- 44.Tambuyzer L, Nijs S, Daems B, Picchio G, Vingerhoets J. Effect of mutations at position E138 in HIV-1 reverse transcriptase on phenotypic susceptibility and virologic response to etravirine. JAIDS. 2011;58:18-22. [DOI] [PubMed] [Google Scholar]
- 45.Rimsky L, Vingerhoets J, Van Eygen, V, et al. Genotypic and phenotypic characterization of HIV-1 isolates obtained from patients on rilpivirine therapy experiencing virologic failure in the phase 3 ECHO and THRIVE studies: 48-week analysis JAIDS. 2011;[published online ahead of print November 7, 2011]. [DOI] [PubMed] [Google Scholar]
- 46.Haddad M, Napolitano LA, Paquet AC, et al. Impact of HIV-1 reverse transcriptase E138 mutations on rilpivirine drug susceptibility. [Abstract 10.] Antivir Ther. 2011;16(Suppl 1):A18. [Google Scholar]
- 47.Xu H, Oliveira M, Asahchop E, Brenner BG, Wainberg MA. The fitness deficits of M184I/V in HIV reverse transcriptase are compensated by E138K that confers broad cross-resistance to second-generation NNRTIs. [Abstract 11.] Antivir Ther. 2011;16(Suppl 1):A19. [Google Scholar]
- 48.Hu ZX, Li J, Gallien S, Kuritzkes DR. Impact of the interactions of rilpivirine (E138K) and lamivudine/emtricitabine (M184V/I) resistance mutations on viral DNA synthesis and fitness of HIV-1. [Abstract 12.] Antivir Ther. 2011;16(Suppl 1):A20. [Google Scholar]
- 49.Kulkarni R, Babaoglu K, Lansdon EB, et al. Cross-talk between HIV reverse transcriptase NRTI and NNRTI binding pockets: interactions between E138K and M184I and drug resistance. [Abstract 13.] Antivir Ther. 2011;16(Suppl 1):A21. [Google Scholar]
- 50.Cohen CJ, Andrade-Villanueva J, Clotet B, et al. Rilpivirine versus efavirenz with two background nucleoside or nucleotide reverse transcriptase inhibitors in treatment-naive adults infected with HIV-1 (THRIVE): a phase 3, randomised, non-inferiority trial. Lancet. 2011;378:229-237. [DOI] [PubMed] [Google Scholar]
- 51.Molina JM, Cahn P, Grinsztejn B, et al. Rilpivirine versus efavirenz with tenofovir and emtricitabine in treatment-naive adults infected with HIV-1 (ECHO): a phase 3 randomised double-blind active-controlled trial. Lancet. 2011;378:238-246. [DOI] [PubMed] [Google Scholar]
- 52.Napolitano LA, Paquet AC, Petropoulos CJ, et al. Impact of genotypic mutations on phenotypic susceptibility to rilpivirine. [Abstract H1-371.] 51st Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC). September 17-20, 2011; Chicago, IL. [Google Scholar]
- 53.Rilpivirine [package insert]. Raritan, NJ: Tibotec Therapeutics; 2011. [Google Scholar]
- 54.Hirsch MS, Günthard HF, Schapiro JM, et al. Antiretroviral drug resistance testing in adult HIV-1 infection: 2008 recommendations of an International AIDS Society–USA panel. Clin Infect Dis. 2008;47:266-285. [DOI] [PubMed] [Google Scholar]
- 55.Young TP, Parkin NT, Stawiski E, et al. Prevalence, mutation patterns, and effects on protease inhibitor susceptibility of the L76V mutation in HIV-1 protease. Antimicrob Agents Chemother. 2010;54:4903-4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Meyer S, Descamps D, Van Baelen B, et al. Confirmation of the negative impact of protease mutations I47V, I54M, T74P and I84V and the positive impact of protease mutation V82A on virological response to darunavir/ritonavir. [Abstract 126.] Antivir Ther. 2009;14(Suppl 1):A147. [Google Scholar]
- 57.Descamps D, Lambert-Niclot S, Marcelin AG, et al. Mutations associated with virological response to darunavir/ritonavir in HIV-1-infected protease inhibitor-experienced patients. J Antimicrob Chemother. 2009;63:585-592. [DOI] [PubMed] [Google Scholar]
- 58.Johnson VA, Brun-Vézinet F, Clotet B, et al. Update of the drug resistance mutations in HIV-1: 2007. Top HIV Med. 2007;15:119-125. [PubMed] [Google Scholar]
- 59.De Meyer S, Dierynck I, Lathouwers E, et al. Phenotypic and genotypic determinants of resistance to darunavir: analysis of data from treatment-experienced patients in POWER 1, 2, 3 and DUET-1 and 2. [Abstract 31.] Antivir Ther. 2008;13(Suppl 3):A33. [Google Scholar]
- 60.Masquelier B, Breilh D, Neau D, et al. Human immunodeficiency virus type 1 genotypic and pharmacokinetic determinants of the virological response to lopinavir-ritonavir-containing therapy in protease inhibitor-experienced patients. Antimicrob Agents Chemother. 2002;46:2926-2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kempf DJ, Isaacson JD, King MS, et al. Identification of genotypic changes in human immunodeficiency virus protease that correlate with reduced susceptibility to the protease inhibitor lopinavir among viral isolates from protease inhibitor-experienced patients. J Virol. 2001;75:7462-7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lopinavir/ritonavir [package insert]. Abbott Park, IL: Abbott Laboratories; 2008. [Google Scholar]
- 63.Mo H, King MS, King K, Molla A, Brun S, Kempf DJ. Selection of resistance in protease inhibitor-experienced, human immunodeficiency virus type 1-infected subjects failing lopinavir- and ritonavir-based therapy: mutation patterns and baseline correlates. J Virol. 2005;79:3329-3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Friend J, Parkin N, Liegler T, Martin JN, Deeks SG. Isolated lopinavir resistance after virological rebound of a ritonavir/lopinavir-based regimen. AIDS. 2004;18:1965-1966. [DOI] [PubMed] [Google Scholar]
- 65.Kagan RM, Shenderovich M, Heseltine PN, Ramnarayan K. Structural analysis of an HIV-1 protease I47A mutant resistant to the protease inhibitor lopinavir. Protein Sci. 2005;14:1870-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gonzalez LM, Brindeiro RM, Aguiar RS, et al. Impact of nelfinavir resistance mutations on in vitro phenotype, fitness, and replication capacity of human immunodeficiency virus type 1 with subtype B and C proteases. Antivir Ther. 2004;9:3552-3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rhee S-Y, Taylor J, Fessel WJ, et al. HIV-1 protease mutations and protease inhibitor cross-resistance. Antimicrob Agents Chemother. 2010;54:4253-4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schapiro JM, Scherer J, Boucher CA, et al. Improving the prediction of virological response to tipranavir: the development and validation of a tipranavir-weighted mutation score. Antivir Ther. 2010;15:1011-1019. [DOI] [PubMed] [Google Scholar]
- 69.Marcelin AG, Masquelier B, Descamps D, et al. Tipranavir-ritonavir genotypic resistance score in protease inhibitor-experienced patients. Antimicrob Agents Chemother. 2008;52:3237-3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reeves JD, Gallo SA, Ahmad N, et al. Sensitivity of HIV-1 to entry inhibitors correlates with envelope/coreceptor affinity, receptor density, and fusion kinetics. Proc Natl Acad Sci USA. 2002;99:16249-16254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reeves JD, Miamidian JL, Biscone MJ, et al. Impact of mutations in the coreceptor binding site on human immunodeficiency virus type 1 fusion, infection, and entry inhibitor sensitivity. J Virol. 2004;78:5476-5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu L, Pozniak A, Wildfire A, et al. Emergence and evolution of enfuvirtide resistance following long-term therapy involves heptad repeat 2 mutations within gp41. Antimicrob Agents Chemother. 2005;49:1113-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hazuda DF, Miller MD, Nguyen BY, Zhao J, for the P005 Study Team. Resistance to the HIV-integrase inhibitor raltegravir: analysis of protocol 005, a phase II study in patients with triple-class resistant HIV-1 infection. Antivir Ther. 2007;12:S10. [Google Scholar]
- 74.Miller MD, Danovich RM, Ke Y, et al. Longitudinal analysis of resistance to the HIV-1 integrase inhibitor raltegravir: results from P005 a phase II study in treatment-experienced patients. [Abstract 6.] Antivir Ther. 2008;13:A8. [Google Scholar]
- 75.Fransen S, Gupta S, Danovich R, et al. Loss of raltegravir susceptibility by human immunodeficiency virus type 1 is conferred via multiple nonoverlapping genetic pathways. J Virol. 2009;83:11440-11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hatano H, Lampiris H, Fransen S, et al. Evolution of integrase resistance during failure of integrase inhibitor-based antiretroviral therapy. JAIDS. 2010;54:389-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wittkop L, Breilh D, Da Silva D, et al. Virological and immunological response in HIV-1-infected patients with multiple treatment failures receiving raltegravir and optimized background therapy, ANRS CO3 Aquitaine Cohort. J Antimicrob Chemother. 2009;63:1251-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ceccherini-Silberstein F, Armenia D, D’Arrigo R, et al. Virological response and resistance in multi-experienced patients treated with raltegravir. [Abstract 18.] Antivir Ther. 2008;13:A20. [Google Scholar]
- 79.Cooper DA, Steigbigel RT, Gatell JM, et al. Subgroup and resistance analyses of raltegravir for resistant HIV-1 infection. N Engl J Med. 2008;359:355-365. [DOI] [PubMed] [Google Scholar]
- 80.Malet I, Delelis O, Valantin MA, et al. Mutations associated with failure of raltegravir treatment affect integrase sensitivity to the inhibitor in vitro. Antimicrob Agents Chemother. 2008;52:1351-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Geretti AM, Fearnhill E, Ceccherini-Silber-stein F, et al. Prevalence and patterns of raltegravir resistance in treated patients in Europe. [Abstract 51.] Antivir Ther. 2010;159(Suppl 2):A62. [Google Scholar]


