Abstract
Numerous investigational antiretroviral agents are in clinical development. Among them are festinavir (BMS986001), a thymidine analogue similar to stavudine with reduced potential for toxicity; GS-7340, a prodrug of tenofovir that achieves greater intracellular concentrations; MK-1439, a nonnucleoside analogue reverse transcriptase inhibitor (NNRTI) that retains activity against common NNRTI-associated resistance mutations; and albuvirtide, a long-acting parenteral fusion inhibitor. Investigational integrase strand transfer inhibitors (InSTIs) include elvitegravir, recently approved by the US Food and Drug Administration (FDA) as part of a once-daily, single-tablet formulation with cobicistat/tenofovir/emtricitabine; dolutegravir, which maintains some activity against raltegravir- and elvitegravir-resistant mutants; and S/GSK1265744, which also maintains some activity against resistance mutations in the integrase gene and is being developed as a long-lasting parenteral agent. Novel 2-(quinolin-3-yl)acetic acid derivatives (LEDGINs), agents that were originally thought to inhibit the interaction of integrase with its cofactor lens epithelium-derived growth factor p75 (LEDGF/p75), are active against InSTI-resistant mutants and to have additive activity when combined with InSTIs. This article summarizes a presentation by Michael S. Saag, MD, at the IAS–USA live Improving the Management of HCV Disease continuing medical education program held in New York in October 2012.
Investigational antiretroviral drugs include new nucleoside analogue reverse transcriptase inhibitors (nRTIs); nonnucleoside analogue reverse transcriptase inhibitors (NNRTIs); entry inhibitors; fusion inhibitors; integrase strand transfer inhibitors (InSTIs); and 2-(quinolin-3-yl)acetic acid derivatives (LEDGINs), agents that inhibit the interaction of HIV integrase with its cofactor lens epithelium-derived growth factor p75 (LEDGF/p75) through a putative allosteric binding mechanism in the LEDGF/p75 binding pocket of the integrase.
nRTIs
Festinavir (BMS986001) is a thymidine analogue similar to stavudine with less potential for toxicity. The compound has a 50% effective concentration (EC50) for inhibition of mitochondrial DNA (mtDNA) polymerase-γ that is 100-fold less than that of stavudine. Festinavir has no apparent effect on mtDNA in renal proximal tubular cells, muscle cells, or adipocytes, or on cellular adenosine triphosphate (ATP) levels or lactate production, suggesting that it may avoid stavudine-associated metabolic and renal toxicities.
GS-7340 is a prodrug of tenofovir that achieves substantially greater intracellular concentrations than tenofovir alone in the context of much lower plasma concentrations of drug, potentially leading to less renal and bone toxicities. The US-120-0104 study, a 10-day monotherapy trial in treatment-naive patients, compared the antiretroviral effects of tenofovir 300 mg with different doses of GS-7340. The study showed that median time-weighted average reductions in HIV RNA were larger with 8 mg (-0.76 log10 copies/mL; P = .216), 25 mg (-0.94 log10 copies/mL; P = .017), and 40 mg (-1.08 log10 copies/mL; P = .01) of GS-7340 than with a standard 300 mg dose of tenofovir (-0.48 log10 copies/mL).1 Whereas tenofovir plasma concentrations were markedly lower with GS-7340 (area under the concentration time curve [AUC] reduced by 79% to 96%; maximum concentration reduced by 89% to 98%), intracellular concentrations of tenofovir diphosphate with the 25 mg and 40 mg doses of GS-7340 were approximately 7- and 20-fold higher, respectively, than with tenofovir DF 300 mg. The research suggests that the higher plasma levels of tenofovir found with the standard 300 mg formulation may be responsible for most of the adverse renal and bone toxicities associated with the currently approved formulation of the drug.
NNRTIs
The in vitro and preclinical profiles of MK-1439 indicate that it possesses desirable characteristics for any new NNRTI, including once-daily dosing, good absorption, reduced potential for toxicity, the ability to be coformulated with other antiretroviral agents, and activity against resistant virus. With regard to antiretroviral activity, MK-1439 has a 95% inhibitory concentration (IC95) of 20 nM against wild-type virus in vitro, with the IC95 increasing to only 43 nM against virus with the K103N mutation (conferring resistance to nevirapine and efavirenz) and 27 nM against virus with the Y181C mutation (conferring reduced susceptibility to nevirapine, etravirine and rilpivirine, which have IC95 values of 1980 nM, 225 nM and 117 nM against Y181C, respectively, compared with wild-type values of 90 nM, 33 nM and 36 nM, respectively). IC95 against virus containing K103N and Y181C mutations is 55 nM for MK-1439, compared with greater than 21,000 nM for nevirapine, 3220 nM for efavirenz, 624 nM for etravirine, and 374 nM for rilpivirine. The K103N, Y181C, and G190A mutations account for more than 90% of transmitted resistance mutations in the United States. When measured as average fold-change in IC50, the potency shift of MK-1439 against these resistant mutants is less than 3-fold (1.5-, 2.5-, and 2.7-fold, respectively).
Entry Inhibitors
BMS 663068 is an investigational attachment inhibitor. It is a methyl phosphate prodrug of another investigational agent (BMS 626529) that binds directly to the viral gp120 and blocks binding to the CD4 receptor. In a monotherapy study, it produced a 1- to 2-log10 copy/mL decrease in HIV RNA at 8 days overall, but a 2- to 3-log10 copy/mL decrease among susceptible HIV viruses.2,3 This is due to a natural polymorphism conferring resistance to the agent at known envelope sites in a minority proportion of HIV strains, one of which is the M426L substitution. Clinical development of BMS 663068 will require pretreatment screening for the known resistance-associated substitutions, with its use being reserved for patients not harboring such substitutions.
Fusion Inhibitors
Albuvirtide is a 3-maleimimidopropionic acid (MPA)-modified peptide fusion inhibitor, related to enfuvirtide, that binds to the HIV gp41 envelope protein. The drug can irreversibly conjugate with serum albumin, thus prolonging half-life. Although the drug must be given intravenously, its half-life has been reported to be 11 days, indicating that it may be suitable for once-weekly or less frequent dosing intervals. Initial studies indicate that a 320-mg dose produces an approximately 1-log10 copy/mL reduction in HIV RNA level. Injection site reactions have been observed at higher doses in phase I evaluation.
InSTIs
Cobicistat-Boosted Elvitegravir
Cobicistat-boosted elvitegravir was recently approved by the US Food and Drug Administration (FDA) as part of a once-daily, single-tablet formulation with tenofovir/emtricitabine. The effects of elvitegravir/cobicistat/emtricitabine/tenofovir (elvitegravir-based group; n = 749) were compared with those of once-daily efavirenz/emtricitabine/tenofovir (efavirenz-based group; n = 375) and once-daily ritonavir-boosted (/r) atazanavir plus tenofovir/emtricitabine (atazanavir-based group; n = 355) in an analysis combining a phase II study (elvitegravir-based group vs efavirenz-based group) and 2 phase III studies (elvitegravir-based group vs efavirenz-based group and elvitegravir-based group vs atazanavir-based group). Overall, 82% to 85% of patients had asymptomatic HIV infection, 1% to 2% were hepatitis B virus (HBV) HBsAg positive and 3% to 5% were hepatitis C virus (HCV) seropositive, 32% to 40% had HIV RNA levels greater than 100,000 copies/mL, and mean CD4+ cell counts were 375/μL to 386/μL, with 41% to 47% of patients having CD4+ cell counts of 350/μL or below. Median estimated glomerular filtration rate (GFR) was 114 mL/min to 115 mL/min.
Any demonstrations of superiority in efficacy in this analysis must be interpreted with caution, due to the pooling of data. The analysis generally showed that elvitegravir/cobicistat/emtricitabine/tenofovir produces results very similar to efavirenz/emtricitabine/tenofovir and atazanavir/r plus tenofovir/emtricitabine. The snapshot analysis of the primary endpoint of viral load less than 50 copies/mL at week 48 showed virologic success in 89% of elvitegravir-based group patients, 84% of efavirenz-based group patients (difference of 5.1%; 95% confidence interval [CI], 0.7%-9.4%; P = .016), and 87% of atazanavir-based group patients (difference of 1.9%; 95% CI, -2.3%-6.1%; P = .37). Virologic non-suppression was observed in 6%, 7%, and 5% of patients, respectively, and fewer patients in the elvitegravir-based group had no week 48 data available (5%, 9%, and 8%, respectively). Responses by viral load and CD4+ cell count subgroups are shown in Figure 1. Mean increases in CD4+ cell count at week 48 were 224/μL with the elvitegravir-based group, 203/μL with the efavirenz-based group (P = not statistically significant), and 211/μL with the atazanavir-based group (P = not statistically significant).
Figure 1.
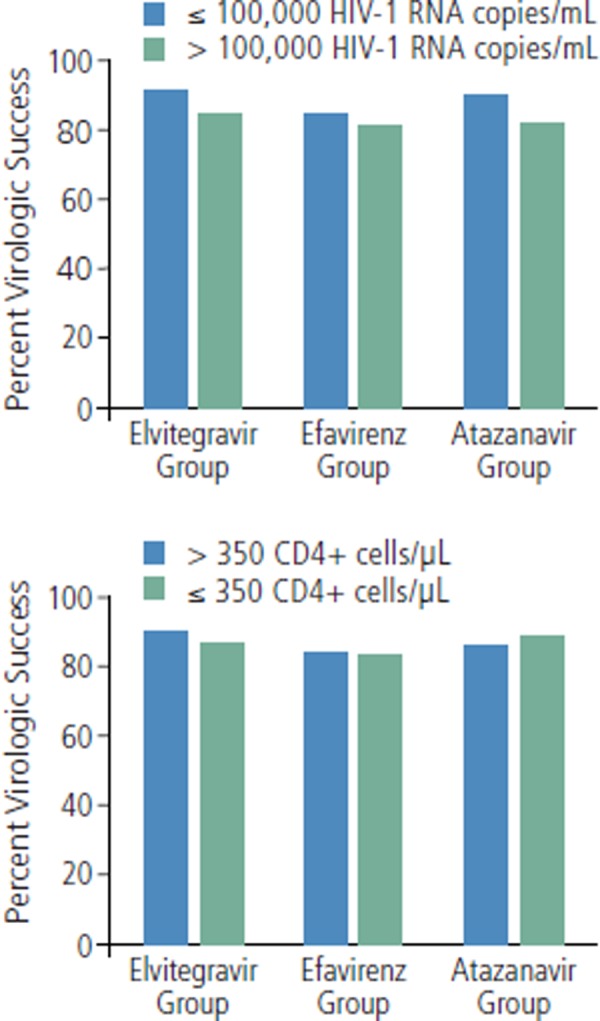
Virologic success at 48 weeks (defined as HIV RNA below 50 copies/mL) according to baseline viral load (top) and CD4+ cell count (bottom) in studies comparing elvitegravir/cobicistat/emtricitabine/tenofovir (elvitegravir group) with efavirenz/emtricitabine/tenofovir (efavirenz group) or atazanavir/ritonavir plus tenofovir/emtricitabine (atazanavir group). Adapted with permission from Ward et al.9
Given the general equivalence of these regimens in terms of antiretroviral activity, differences in tolerability, drug interaction profiles and toxicity profiles are likely to govern selection of the regimens for particular patients. Adverse events in this analysis are shown in Table 1. Rash, depression, fatigue, abnormal dreams, and dizziness were somewhat more common in the efavirenz-based group than in the elvitegravir-based group, whereas diarrhea and headache were somewhat more common in the elvitegravir-based group.
Table 1.
Common Adverse Events and Adverse Events Resulting in Treatment Discontinuation in Studies Comparing Elvitegravir-Based, Efavirenz-Based, and Atazanavir-Based Regimens
| Adverse Event | Rate of Occurrence by Treatment Regimen | |||
|---|---|---|---|---|
| Elvitegravir-Based (n = 749) | Efavirenz-Based (n = 375) | Atazanavir-Based (n = 355) | ||
| Grade 2 to 4 Adverse Eventsa | ||||
| Diarrhea | 6% | 4% | 8% | |
| Rash events | 4% | 9% | 4% | |
| Headache | 4% | 2% | 3% | |
| Bronchitis | 4% | 2% | 3% | |
| Upper respiratory infection | 4% | 4% | 3% | |
| Depression | 3% | 7% | 3% | |
| Nausea | 3% | 3% | 3% | |
| Insomnia | 2% | 3% | 2% | |
| Fatigue | 2% | 4% | 4% | |
| Herpes zoster | 2% | 1% | 3% | |
| Abnormal dreams | 0.4% | 4% | 0.3% | |
| Dizziness | 0.4% | 3% | 1% | |
| Adverse Events Leading to Treatment Discontinuation | ||||
| Renal events | 0.8% | 0% | 0.3% | |
| Rash events | 0.1% | 1.1% | 1.1% | |
| Nausea | 0.3% | 0% | 1.1% | |
| Diarrhea | 0.3% | 0% | 0.3% | |
| Pyrexia | 0.3% | 0.3% | 0% | |
| Fatigue | 0.3% | 0.3% | 0.6% | |
Elvitegravir-based group received elvitegravir/cobicistat/emtricitabine/tenofovir; efavirenzbased group received efavirenz/emtricitabine/tenofovir; atazanavir-based group received atazanavir/ritonavir plus tenofovir/emtricitabine.
Affecting at least 2.5% of patients in any group.
Adapted with permission from Benson et al.8
It is important to note that cobicistat produces an increase in serum creatinine via inhibition of secretion of creatinine through the renal proximal tubules. Because this increase is not associated with an actual reduction in glomerular filtration rates (true creatinine clearance), this effect of cobicistat results in reduction in estimated GFR without change in actual GFR by direct measurement. This effect is to be distinguished from the proximal tubular dysfunction associated with tenofovir. Median serum creatinine increased over the first 2 to 4 weeks of treatment in patients taking elvitegravir/cobicistat/emtricitabine/tenofovir, likely predominantly reflecting the effect of cobicistat, and remained fairly constant thereafter (Figure 2). Thus a 0.1 mg/dL to 0.15 mg/dL increase in serum creatinine over the first 2 weeks to 4 weeks in patients receiving cobicistat is expected. Greater increases should prompt suspicion of other renal toxicity that is likely having an actual impact on GFR. If estimated GFR drops below 70 mL/min and approaches 60 mL/min, substituting another regimen for elvitegravir/cobicistat/emtricitabine/tenofovir should be considered.
Figure 2.
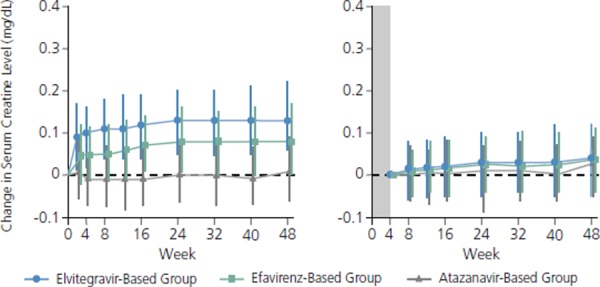
Changes in serum creatinine level (mg/dL) from baseline (left) and from week 4 (right) in studies comparing treatment with elvitegravir/cobicistat/emtricitabine/tenofovir (elvitegravir-based group) with efavirenz/emtricitabine/tenofovir (efavirenz-based group) or atazanavir/ritonavir plus tenofovir/emtricitabine (atazanavir-based group). Adapted with permisssion from Benson et al.8
Potential advantages of elvitegravir/cobicistat/emtricitabine/tenofovir include lesser effects in altering cytochrome P450 3A4 (CYP3A4) and cytochrome P450 2D6 (CYP2D6) metabolism and reduced adverse effects on lipids. In the pooled analysis, elvitegravir/cobicistat/emtricitabine/tenofovir was associated with statistically significantly smaller increases in total cholesterol levels (10 mg/dL vs 19 mg/dL; P < .001) and low-density lipoprotein (LDL) cholesterol levels (10 mg/dL vs 17 mg/dL; P < .001) than efavirenz/emtricitabine/tenofovir (no difference vs atazanavir/r plus tenofovir/emtricitabine) and a statistically significantly smaller increase in triglyceride levels (8 mg/dL vs 23 mg/dL; P = .006) than atazanavir/r plus tenofovir/emtricitabine (no difference vs efavirenz/emtricitabine/tenofovir). Elvitegravir/cobicistat/emtricitabine/tenofovir was also associated with a statistically significantly smaller increase in high-density lipoprotein (HDL) cholesterol levels (5 mg/dL vs 8 mg/dL; P = .002) than efavirenz/emtricitabine/tenofovir (no difference vs atazanavir/r plus tenofovir/emtricitabine).
Resistance to elvitegravir is similar to that seen with raltegravir, with the exception of a T66I mutation that is not typically observed with raltegravir. Patients in whom raltegravir fails are unlikely to respond to elvitegravir. Resistance mutations can be anticipated in patients who experience virologic failure on elvitegravir-containing regimens. In 1 of the studies included in the pooled analysis, major InSTI resistance mutations were found in 6 patients in the elvitegravir-based group, including E92Q and T66I in 1 patient each and Q148R and N155H in 2 patients each. Important nRTI-associated resistance mutations were found in 4 patients in the elvitegravir-based group in whom treatment failed, including M184V/I in 4 patients and K65R in 1 patient. No major protease inhibitor (PI)-associated or nRTI-associated resistance mutations were found in patients in the atazanavir-based group.
Cobicistat versus Ritonavir as a Booster
In a study comparing cobicistat-boosted atazanavir (cobicistat group, n = 350) with atazanavir/r (ritonavir group, n = 350), each was administered with emtricitabine/tenofovir in treatment-naive patients with 5000 HIV RNA copies/mL or higher, any CD4+ cell count, and estimated GFR of at least 70 mL/min. Virologic success rates at the 48-week snapshot analysis were 85% in the cobicistat group versus 87% in the ritonavir group, with virologic nonsuppression occurring in 6% versus 4% of patients, respectively, and absence of week 48 data for 9% of patients in both groups (cobicistat group noninferior; difference of -2.2%; 95% CI, -7.4%-3.0%).4 Overall adverse events and adverse events leading to discontinuation were similar in the 2 groups (see Table 2): 5 of 6 cobicistat group patients and 2 of 5 ritonavir group patients who discontinued treatment due to renal abnormalities did so due to proximal tubulopathy. Consistent with the activity of cobicistat in inhibiting renal secretion of creatinine, there was an early increase in serum creatinine and reduction in estimated GFR in the cobicistat group, with the differences remaining statistically significant at 48 weeks compared with the ritonavir group (median increase in creatinine clearance 0.13 mg/dL vs 0.09 mg/dL; P < .001; median decrease in estimated GFR -13 mL/min vs -9 mL/min; P < .001). Increases in total cholesterol, LDL cholesterol, and triglyceride levels were nonsignificantly greater and the increase in HDL cholesterol level was nonsignificantly smaller in the ritonavir group than in the cobicistat group.
Table 2.
Common Adverse Events and Those Resulting in Treatment Discontinuation in a Study Comparing Atazanavir Boosted by Cobicistat with Atazanavir Boosted by Ritonavir
| Cobicistat-Boosted (n = 344) | Ritonavir-Boosted (n = 348) | |
|---|---|---|
| Adverse Events Affecting ≥ 10% of Subjects | ||
| Bilirubin-relateda | 41% | 36% |
| Nausea | 18% | 16% |
| Diarrhea | 15% | 20% |
| Headache | 11% | 16% |
| Nasopharyngitis | 11% | 15% |
| Upper respiratory infection | 10% | 8% |
| Adverse Events Leading to Treatment Discontinuation (n) | ||
| Study drug discontinuation due to any adverse event | 7.3% (25) | 7.2% (25) |
| Bilirubin-relateda | 3.5% (12) | 3.2% (11) |
| Renal abnormalities | 1.7% (6) | 1.4% (5) |
| Rash | 0.3% (1) | 0.6% (2) |
| Dermatitis allergic | 0.6% (2) | 0 |
Bilirubin-related adverse events include jaundice, ocular icterus, hyperbilirubinemia, and increased blood bilirubin level. Adapted with permission from Gallant et al.4
Dolutegravir
Dolutegravir, which currently is in phase III development and available through an expanded access program, retains activity in patients in whom raltegravir has failed with virus harboring raltegravir and elvitegravir resistance mutations. The recent SPRING-1 phase II study compared 3 doses (10 mg, 25 mg, or 50 mg once daily) of dolutegravir (n = 155) with efavirenz plus a dual nRTI backbone (either abacavir plus lamivudine or tenofovir plus emtricitabine as selected by the provider) in patients without InSTI-related resistance mutations at baseline.5 Through 96 weeks of treatment, virologic response rates were higher in all dolutegravir groups than in the efavirenz group. It has been repeatedly observed that reduction in viral load is quite rapid with InSTI-containing regimens; the causes and potential clinical implications of the faster decline remain unclear. No InSTI-associated resistance mutations were observed in the dolutegravir groups through week 96. Adverse events leading to treatment discontinuation occurred in 3% of patients taking a dolutegravir regimen and in 10% of patients taking the efavirenz regimen. Dolutegravir, without cobicistat boosting, also inhibits creatinine secretion, resulting in 0.1 mg/dL to 1.5 mg/dL increases in serum creatinine without affecting actual GFR.
The likely dose of dolutegravir for treatment-naive patients will be 50 mg once daily, with 50 mg twice daily being used for patients in whom other InSTI therapy has failed. In the VIKING Cohort II functional monotherapy study in patients with raltegravir-resistant virus, the primary endpoint of HIV RNA levels below 400 copies/mL or a minimum 0.7-log10 copy/mL decline in viral load was achieved in 78% of 27 patients receiving 50 mg dolutegravir once daily and 96% of 24 patients receiving 50 mg dolutegravir twice daily. Analysis by mutational pathway showed virologic response in 33% of 9 patients in the once-daily group with virus having the Q148 mutation plus at least 1 other mutation, compared with response in 100% of 11 patients in the twice-daily group with virus having Q148 and another mutation. Virologic response was achieved in 100% of 18 patients in the once-daily group and in 92% of 13 patients in the twice-daily group with virus having resistance pathways other than Q148. Viral load was reduced below 400 copies/mL in 41% of the once-daily group and in 54% of the twice-daily group. IAS–USA Topics in Antiviral Medicine
In a noninferiority trial comparing dolutegravir 50 mg once daily plus abacavir/lamivudine (n = 414) with efavirenz/emtricitabine/tenofovir (n = 419) in treatment-naive patients, the dolutegravir regimen produced a statistically significantly higher response rate than the efavirenz regimen at 48 weeks (viral load less than 50 copies/mL in 88% vs 81% of patients, P = .003).6 Median time to response was statistically significantly shorter in the dolutegravir group than in the efavirenz group (28 days vs 84 days, P < .0001). The mean increase in CD4+ cell count was statistically significantly greater in the dolutegravir group than in the efavirenz group (267/μL vs 208/μL; P < .001). Virologic failure with dolutegravir treatment was not accompanied by emergence of resistance mutations in these treatment-naive patients, in contrast to the resistance emergence that has been observed with raltegravir and elvitegravir. Dolutegravir treatment was associated with a small increase in serum creatinine, due to inhibition of proximal tubule enzyme systems similar to what is seen with cobicistat, that continued for 48 weeks. The efavirenz/emtricitabine/tenofovir combination was associated with higher rates of discontinuation due to adverse events (10% vs 2% with dolutegravir) and a higher incidence of liver function abnormalities (9% vs 2%, respectively) than dolutegravir.
S/GSK1265744
S/GSK1265744 is being investigated as an oral agent and as a long-lasting parenteral agent (using nanosuspension technology) given via intramuscular (IM) or subcutaneous (SC) injection. In 10-day monotherapy studies, the agent produced a greater than 2.5-log10 reduction in viral load. Like dolutegravir, S/GSK1265744 retains substantial activity against raltegravir- and elvitegravir-resistant mutants. When given by IM or SC injection, S/GSK1265744 produces plasma concentrations that remain over protein-adjusted IC90 values for wild-type virus for up to 24 weeks following administration of 800 mg IM (Figure 3).
Figure 3.
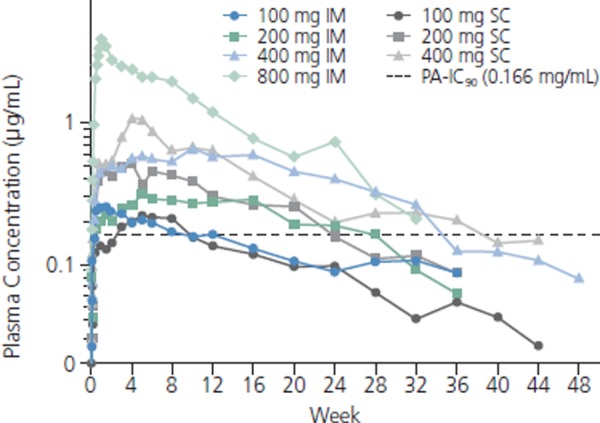
Plasma S/GSK1265744 concentrations over time after intramuscular (IM) or subcutaneous (SC) administration. PA-IC90 indicates protein-adjusted 90% inhibitory concentration. Adapted with permission from Spreen et al.10
LEDGINs
As noted, LEDGF/p75 is a cofactor of HIV integrase, acting to tether the provirus to the cellular genome.7 By binding to the LEDGF/p75 binding site on the integrase, LEDGINs (eg, CX05168, CX05045, and CX14442) putatively inhibit LEDGF/p75 binding and strand transfer activity of the integrase, thus inhibiting provirus formation. Further, it has been shown that the viral particles produced in the presence of these agents have impaired infectivity. LEDGINs are active against a wide range of InSTI-resistant mutants and exhibit additive to synergistic activity when combined with InSTIs.
References
References
- 1.Ruane P, DeJesus E, Berger D, et al. GS-7340 25 mg and 40 mg demonstrate superior efficacy to tenofovir 300 mg in a 10-day monotherapy study of HIV-1+ patients. [Abstract 103.] 19th Conference on Retroviruses and Opportunistic Infections (CROI) March 5-8, 2012; Washington, DC. [Google Scholar]
- 2.Zhou N, Ray N, Healy M, et al. Genotypic and phenotypic correlates of virologic response to the attachment inhibitor BMS-626529 in a short-term monotherapy study with its prodrug BMS-663068. [Abstract 6.] International Workshop on HIV & Hepatitis Virus Drug Resistance June 5-9, 2012; Sitges, Spain. [Google Scholar]
- 3.Nettles RE, Schurmann D, Zhu L, et al. Pharmacodynamics, safety, and pharmacokinetics of BMS-663068, an oral HIV-1 attachment inhibitor in HIV-1-infected subjects. J Infect Dis. 2012;206(7):1002–1011.. [DOI] [PubMed] [Google Scholar]
- 4.Gallant J, Koenig E, Andrade-Villanueva J, et al. Cobicistat versus ritonavir as pharmacoenhancers in combination with atazanavir plus tenofovir disoproxil fumarate/emtricitabine: phase 3 randomized, double blind, active-controlled trial, week 48 results. [Abstract TUAB0103.] 19th International AIDS Conference (IAS) July 22-27, 2012; Washington, DC. [Google Scholar]
- 5.Stellbrink HJ, Reynes J, Lazzarin A, et al. Dolutegravir in combination therapy exhibits rapid and sustained antiviral response in ARV-naive adults: 96-week results from SPRING-1 (ING112276). [Abstract 102LB.] 19th Conference on Retroviruses and Opportunistic Infections (CROI) March 5-8, 2012; Washington, DC. [Google Scholar]
- 6.Walmsley S, Antela A, Clumeck N, et al. Dolutegravir (DTG; S/GSK1349572) + abacavir/lamivudine once daily statistically superior to tenofovir/emtricitabine/efavirenz: 48-week results - SINGLE (ING114467). [Abstract H-556b.] 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) September 9-12, 2012; San Francisco, CA. [Google Scholar]
- 7.Christ F, Shaw S, Demeulemeester J, et al. Small-molecule inhibitors of the LEDGF/p75 binding site of integrase block HIV replication and modulate integrase multimerization. Antimicrob Agents Chemother. 2012;56(8):4365-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benson P, Mayer C, Morales-Ramirez J, et al. Renal safety profile of cobicistat-boosted elvitegravir or atazanavir plus emtricitabine/tenofovir DF in HIV patients. [Abstract H-891.] 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) September 9-12, 2012; San Francisco, CA. [Google Scholar]
- 9.Ward D, Crofoot G, Shamblaw D, et al. Efficacy and safety of elvitegravir/cobicistat/emtricitabine/tenofovir DF from an integrated analysis of phase 2 and 3 clinical trials. [Abstract H-555.] 52nd Interscience Conference on Antimicrobials and Chemotherapy (ICAAC) September 9-12, 2012. San Francisco, CA. [Google Scholar]
- 10.Spreen W, Ford SL, Chen S, et al. Pharmacokinetics, safety and tolerability of the HIV integrase inhibitor s/gsk1265744 long acting parenteral nanosuspension following single dose administration to healthy adults. [Abstract TUPE040.] 19th International AIDS Conference (IAS) July 22-27, 2012; Washington, DC. [Google Scholar]


