Abstract
As HIV-infected patients are living longer, non–AIDS-defining cancers are increasing in number and now constitute the majority of cancers diagnosed in the HIV-infected population. The excess incidence of Hodgkin lymphoma and head and neck and liver cancers has been increasing among HIV-infected individuals. Breast and lung cancers appear to occur earlier in the HIV-infected population; Hodgkin lymphoma appears to have a later onset, reflecting the fact that most cases in the HIV-infected population are related to Epstein-Barr virus infection, which is generally seen in older rather than younger individuals. Mortality from Hodgkin lymphoma and lung and prostate cancers is higher among HIV-infected individuals than HIV-uninfected individuals. The greater risk of cancer in the HIV-infected population may be due to a number of factors, including more rapid immunosenescence. At a minimum, age- and sex-appropriate cancer screenings should be performed in all HIV-infected patients, and patients should be counseled on measures to reduce cancer risk. This article summarizes a presentation by Ronald T. Mitsuyasu, MD, at the IAS–USA continuing education program held in San Francisco, California, in March 2013.
Keywords: anogenital cancers, cancer incidence, cancer mortality, cancer screening, HIV, Hodgkin lymphoma, immunosenescence, lung cancer, NADCs
HIV-infected individuals are living longer as a result of more effective and better tolerated antiretroviral treatment and fewer risks of opportunistic diseases. The overall incidence of AIDS-defining cancers (ADCs) among HIV-infected individuals has decreased, reflecting lower rates of such cancers as Kaposi sarcoma and non-Hodgkin lymphoma (NHL) in patients aged 40 years or younger. Concurrently, the number of non–AIDS-defining cancers (NADCs) among HIV-infected individuals has increased owing in large part to diagnoses in patients older than 40 years of age. Thus, the number of NADCs diagnosed each year in the HIV-infected population has now surpassed the number of ADCs.1
Increase in Non–AIDS-Defining Cancers
Data from HIV and cancer matched registries in the United States have shown that among the HIV-infected population, NADCs accounted for 31.4% of all cancers reported from 1991 to 1995 and that this proportion increased to 58% from 1996 to 2002.2 Standardized incidence ratios were used to compare NADC incidence in the HIV-infected population with that in the general population, with data indicating an increase in the standardized incidence ratio for some NADCs and stable values for others from the earlier to the later period. Standardized incidence ratios increased for laryngeal cancer (from 1.8 to 2.7), liver cancer (from 0 to 3.7), and Hodgkin lymphoma (from 2.8 to 6.7) but remained stable for lung cancer (from 2.6 to 2.6) and anal cancer (from 10.0 to 9.1).1
As shown in Figure 1, in the United States between 1991 and 2005, Kaposi sarcoma and NHL cases decreased in number and incidence in the HIV-infected population; anal and prostate cancers increased in number and incidence; cervical, liver, and colorectal cancers and Hodgkin lymphoma cases increased in number but maintained a relatively stable incidence; and lung cancers increased in number but decreased somewhat in incidence.1 Factors contributing to the increase in cancer cases among HIV-infected individuals include the approximately 4-fold increase in the size of the HIV-infected population over this time period, higher smoking rates and earlier smoking initiation among HIV-infected individuals, increasing number of HIV-infected patients who are 50 years of age or older, increased incidence of cancer with advancing age, and higher risk of developing cancers among immunodeficient individuals.
Figure 1.

Number and incidence rates of AIDS-defining cancers (ADCs) and non–AIDS-defining cancers (NADCs) in the United States, 1991 to 2005. Adapted from Shiels et al.1
Epidemiologic data raise the important question of whether HIV infection itself may be associated with an increased risk of developing cancer at an earlier age than in the general population. A study by the National Cancer Institute (NCI) using data from HIV and cancer matched registries indicates that this is the case for some cancers but not others.3 Figure 2 shows the observed number and age distribution of cancer cases in the HIV-infected and general populations in the United States between 1996 and 2007 and the expected cancer rate in the general population when modeled to have the same age distribution as the HIV-infected population.3
Figure 2.
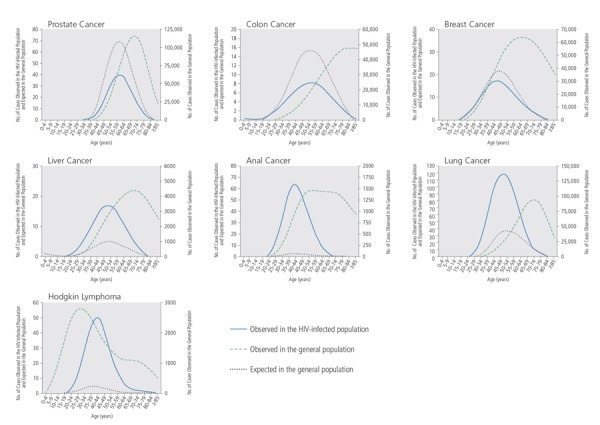
Observed and expected cases of cancer, by age, in the HIV-infected and general populations in the United States, 1996 to 2007. Expected cases in the general population are modeled to match the age distribution of the HIV-infected population. Adapted from Shiels et al.3
The curve for the HIV-infected population is left-shifted compared with the curve for the expected rate in the age-adjusted general population for some of these cancers, such as for liver and lung cancers. The curve for Hodgkin lymphoma in HIV-infected individuals, however, is right-shifted, indicating that this cancer develops more commonly at later ages in the HIV-infected population than in the general population. The pattern observed for Hodgkin lymphoma in the HIV-infected population may reflect the fact that nearly all cases of this cancer in HIV-infected persons are related to Epstein-Barr virus (EBV) infection, which occurs more frequently in older individuals, whereas cases in the general population reflect cancers in both non–EBV-related Hodgkin lymphoma, which is more common in younger persons, and EBV-related Hodgkin lymphoma, which is more common in older persons.
Higher Cancer Mortality in HIV-Infected Patients
A retrospective analysis assessed survival rates for incident prostate, anal, lung, and colorectal cancers, and Hodgkin lymphoma in 22,081 HIV-infected and 230,069 HIV-uninfected individuals enrolled in Kaiser Permanente in California.4 Participants were matched for age, sex, clinic, and initial year of follow-up. The study showed that HIV-infected persons were diagnosed with anal, lung, and colorectal cancers at a somewhat earlier mean age than their HIV-uninfected counterparts; were diagnosed with more advanced stages of lung cancer and Hodgkin lymphoma; and had statistically significantly poorer survival rates for Hodgkin lymphoma and lung and prostate cancers.
Pathogenesis of NADCs
Although numerous factors are likely to be involved in the development of NADCs, many NADCs are associated with a viral pathogenesis. Anal cancer and oral squamous carcinoma are associated with human papillomavirus (HPV); Hodgkin lymphoma and pediatric leiomyosarcoma with EBV; Merkel cell carcinoma (which has a relative risk of approximately 20-fold in HIV-infected versus HIV-uninfected individuals) with Merkel cell virus, a newly described DNA virus; and hepatocellular carcinoma with hepatitis B and C virus (HBV; HCV) infections. Among ADCs, Kaposi sarcoma is associated with human herpesvirus 8 (HHV-8), NHL with EBV and HHV-8, and invasive cervical carcinoma with HPV infection.
It is known that decreased immune surveillance and increased immune activation play a major role in cancer development. There is also a growing body of data, much of it from in vitro cell line studies, to suggest that HIV may have a direct role in perhaps activating cellular oncogenes or proto-oncogenes and inhibiting tumor suppressor genes. The NCI is conducting a large study comparing gene expression profiles in tumor specimens for a variety of cancers in HIV-infected and HIV-uninfected persons,5 and the findings should provide a clearer picture of which genes might be affected by HIV. Further, HIV has been found to induce genetic instability as manifested, for example, by a 6-fold higher number of microsatellite alterations in HIV-associated than in non–HIV-associated lung cancer.6 Susceptibility to the effects of certain carcinogens may be greater in HIV-infected than in HIV-uninfected individuals, possibly due to the endothelial abnormalities associated with HIV infection, which may be permissive for tumor growth.
HIV-associated immunosenescence may also be associated with increased cancer risk. A number of reports indicate that the aging phenotype of immune markers first defined in Scandinavians aged 80 years to 90 years is also common in younger HIV-infected individuals. Thus, increased CD8+CD28- and CD4+CD28-cells, shortened telomeres, increased CD31-cells (especially in the CD45RA+ naive T cell population), and increased CD56+CD57+ cells are seen in HIV-infected patients compared with age-matched, HIV-uninfected patients. Such findings support the notion that HIV-infected persons experience more rapid immunologic aging, putting them at an increased risk for cancer.7-9
Predictors for NADCs in HIV-infected patients include advancing age (HR, 1.99 per 10 years; P < .001), white versus black race (HR, 1.56; P = .02), lower most-recent CD4+ cell count, smoking and other lifestyle behaviors, history of HBV infection, and socioeconomic disadvantage and limited access to care. A recent report indicates a close correlation between lower CD4+ cell counts within the last 10 years and risk of virus-associated cancers. Antiretroviral therapy has been shown to be protective against ADCs (odds ratio, 0.21; P < .001) but not against NADCs.10-12
Cancer Screening
HIV-infected patients should be screened for cancer at earlier ages than HIV-uninfected patients given the differences in risk between the 2 groups, and screening should be performed more frequently for some cancer types. Guidelines from the American Cancer Society, NCI, and US Preventive Services Task Force indicate that cervical cancer screening should begin within 3 years of first vaginal intercourse or at 21 years of age and be performed every 1 year to 2 years.13-15 For patients aged 30 years to 70 years with 3 normal Papanicolaou (Pap) test results, screening may be performed every 3 years. Prostate cancer screening should be discussed with a physician at age 50 years and a yearly digital rectal examination and individualized determination of prostate-specific antigen (PSA) testing performed. Breast cancer screening should consist of a clinical breast examination every 3 years beginning at age 20 years, yearly clinical breast examinations beginning at age 40 years, and yearly mammograms beginning at age 50 years. Colon cancer screening should begin at age 50 years and include flexible sigmoidoscopy every 5 years or colonoscopy every 10 years to age 75 years and yearly fecal occult blood testing. For other cancers, screening measures consist of periodic health examinations after age 20 years, with health counseling and oral, skin, lymph node, testicle, ovary, and thyroid examinations. Additional screening tests should be performed based on family history and other known cancer risk factors.
Routine cancer screening in HIV-infected patients appears to be conducted less frequently than age-appropriate standard-of-care screening is conducted in the general population for breast cancer (67% vs 79%) and colon cancer (56% vs 78%), and prostate biopsies are also performed less frequently in HIV-infected patients when indicated.16-18 Some of this deficiency in cancer screening may reflect concerns over potentially higher false-positive rates among HIV-infected patients. For example, the NLST (National Lung Screening Trial) found a 20% reduction in lung cancer mortality among older smokers in the general popu-lation who were screened using low-dose helical computed tomography (CT) scan compared with those who underwent x-ray screening.19 The false-positive rate was high, however, and might prove to be even higher in HIV-infected patients in whom pulmonary abnormalities are frequently encountered.
Lung Cancer Screening
A Veterans Affairs Aging Cohort prospective substudy, EXHALE (Examinations of HIV Associated Lung Emphysema), compared rates of abnormal findings on a single, low-dose CT scan in 160 HIV-infected and 139 HIV-uninfected patients (85% and 81% of patients, respectively, were current or former smokers; P = .5).20 There were statistically significantly more men in the HIV-infected group than the HIV-uninfected group (98% vs 88%; P = .001), and a small difference between the 2 groups in age (median age 54 years vs 52 years; P = .03). There was no difference in racial or ethnic distribution (12% white, 72% black, and 16% Hispanic vs 20% white, 64% black, and 16% Hispanic; P = .41), pack-years of smoking (median 26 years vs 22 years; P = .4), or presence of chronic obstructive pulmonary disease, emphysema, or chronic bronchitis (22% vs 24%; P = .4). CD4+ cell count was <200/μL in 14% and >200/μL in 86% of the HIV-infected group, and 84% of the HIV-infected group was on antiretroviral therapy.
There were no statistically significant differences between the HIV-infected and HIV-uninfected groups in presence of pulmonary nodules (48% vs 48%; P = .9), median number of nodules (2 vs 1; P = .2), suspicion of cancer (4% vs 3%; P = .8), pleural effusion (0% vs 0.7%; P = .5), ground glass infiltrates (15% vs 14%; P = .9), bronchiectasis (6% vs 6%; P = .8), or granulomas (24% vs 18%; P = .2). Borderline statistically significant differences were observed between the HIV-infected and HIV-uninfected groups in emphysematous changes (41% vs 30%; P = .05) and lymphadenopathy (13% vs 6.5%; P = .1). Follow-up was recommended for 23% of the HIV-infected group versus 30% of the HIV-uninfected group (P = .2) and resulted in a lung cancer diagnosis in 3 subjects in the HIV-infected group and 1 subject in the HIV-uninfected group (2% vs 0.7%). It remains unclear whether routine CT screening for lung cancer would provide much benefit. At present, it appears that CT findings would be contaminated by the presence of many abnormalities, perhaps leading to additional evaluation that might not necessarily assist in early diagnosis and could ultimately prove to be harmful.
Anogenital Dysplasia Screening
Anal cancer is particularly common in HIV-infected men who have sex with men, although it also develops in HIV-infected women, and it is one of several cancers with an increasing incidence in the antiretroviral therapy era. Currently, there are no national or international guidelines for anal cancer screening other than the New York State Department of Health AIDS Institute clinical guidelines for anal dysplasia and cancer,21 which do not provide guidance on what to do in the case of positive screening results. Many experts recommend yearly cervical and anal Pap smears, with colposcopy or high-resolution anoscopy and biopsy of any suspicious lesions, and follow-up every 6 months in patients with abnormalities. A number of cervical cancer screen-and-treat programs are now operating in resource-limited settings.22,23
AMC-052 (AIDS Malignancy Consortium Protocol 052) evaluated anal cancer screening as part of an HPV vaccination protocol and showed that a large percentage of HIV-infected men tested negative for HPV types associated with cancer,24 indicating that vaccination would benefit this population. At 7 months, after 3 vaccine doses, geometric mean anti-HPV antibody levels were 357 mMU/mL, 525 mMU/mL, 1139 mMU/mL, and 181 mMU/mL for HPV types 6, 11, 16, and 18, respectively. Although these antibody levels are not as high as those achieved in HIV-uninfected men, they are still likely to be protective.
With regard to dysplasia treatment, a 16-week randomized trial evaluated the use of topical imiquimod (thrice weekly), topical 5-fluorouracil (5-FU; twice weekly), and electrocautery (monthly) in 156 HIV-infected men with anal intra epithelial neoplasia (AIN), 63% of whom had high-grade AIN (HGAIN).25 Using a modified intent-to-treat analysis, at 4 weeks after completing treatment, complete response rates were 24% in those treated with imiquimod, 17% in those treated with 5-FU, and 39% in those treated with electrocautery (P = .027). Relapse rates at 6 months were 19%, 38%, and 14%, and at 48 weeks were 47%, 50%, and 43%, respectively, suggesting an advantage to electrocautery in terms of complete response rates, but relapses were equally high in all groups by 48 weeks. Serious, grade 3 or 4 adverse events were more common in the imiquimod treatment group than in the 5-FU or electrocautery treatment groups (43%, 27%, and 18%, respectively; P = .019).
The AMC has been examining the use of infrared coagulation techniques pioneered by Palefsky and colleagues at the University of California San Francisco and has observed higher response rates (62%) and a 1-year recurrence or persistence rate of less than 38%.26 Although optimal treatment is still being identified, it seems clear that effective early intervention in AIN can reduce anal cancer incidence.
Cancer Prevention
Smoking cessation is a high-priority measure for cancer prevention in any population, as is the use of sunscreen and avoiding overexposure to the sun. In HIV-infected patients, obtaining a complete family history for malignancies is important and a high index of suspicion for cancer should be maintained. Plasma HIV RNA levels should be maximally suppressed with effective antiretroviral therapy, and HCV or HBV should be treated in individuals with active HIV/HCV or HIV/HBV coinfections. Other measures for cancer prevention in HIV-infected patients include vaccination against HBV and HPV. Annual cervical and anal Pap tests, and regular screening with high-resolution anoscopy may be indicated. Breast, prostate, and colon cancer screening should be performed according to relevant guidelines for the general population.
Conclusion
As the HIV-infected population ages with persistent immune abnormalities, cancers are increasing in number. The risk of NADCs is high, with lung, anal, and liver cancers and Hodgkin lymphoma accounting for most new cases. The risk for colon, breast, and prostate cancers is lower in the HIV-infected population than in the general population. Hodgkin lymphoma incidence is similar overall, but higher in older HIV-infected individuals, and may reflect the lack of a younger age peak, which is seen in the general US population, as almost all cases of Hodgkin lymphoma in the HIV-infected population appear to be related to EBV infection. At a minimum, age- and sex-appropriate cancer screening should be performed in all patients, and patients should be counseled on measures to reduce cancer risk. Only through prospective clinical trials can HIV-specific cancer prevention strategies be effectively evaluated.
References
- 1.Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103(9):753-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1): 187-194. [DOI] [PubMed] [Google Scholar]
- 3.Shiels MS, Pfeiffer RM, Engels EA. Age at cancer diagnosis among persons with AIDS in the United States. Ann Intern Med. 2010;153(7):452-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silverberg MJ, Chao CR, Leyden W, et al. Cancer stage, age at diagnosis and survival comparing HIV(+) and HIV(-) individuals with common non–AIDS-defining cancers [Abstract 903]. 19th Conference on Retroviruses and Opportunistic Infections (CROI) March 5-8, 2012; Seattle, Washington. [Google Scholar]
- 5.National Cancer Institute. Collecting and Studying Tissue Samples From Patients With HIV-Associated Malignancies. http://www.cancer.gov/clinicaltrials/search/view?cdrid=729843&version=HealthProfessional. Accessed on February 11, 2014.
- 6.Wistuba II, Behrens C, Gazdar AF. Pathogenesis of non–AIDS-defining cancers: a review. AIDS Patient Care STDS. 1999;13(7):415-426. [DOI] [PubMed] [Google Scholar]
- 7.Boucher N, Dufeu-Duchesne T, Vicaut E, Farge D, Effros RB, Schachter F. CD28 expression in T cell aging and human longevity. Exp Gerontol. 1998;33(3):267-282. [DOI] [PubMed] [Google Scholar]
- 8.Effros RB, Fletcher CV, Gebo K, et al. Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clin Infect Dis. 2008;47(4):542-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rickabaugh TM, Kilpatrick RD, Hultin LE, et al. The dual impact of HIV-1 infection and aging on naive CD4 T-cells: additive and distinct patterns of impairment. PLoS One. 2011;6(1):e16459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crum-Cianflone N, Hullsiek KH, Marconi V, et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS. 2009;23(1):41-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Llibre JM, Falco V, Tural C, et al. The changing face of HIV/AIDS in treated patients. Curr HIV Res. 2009;7(4):365-377. [DOI] [PubMed] [Google Scholar]
- 12.Reekie J, Kosa C, Engsig F, et al. Relationship between current level of immunodeficiency and non-acquired immunodeficiency syndrome-defining malignancies. Cancer. 2010;116(22):5306-5315. [DOI] [PubMed] [Google Scholar]
- 13.American Cancer Society. American Cancer Society Guidelines for the Early Detection of Cancer. http://www.cancer.org/healthy/findcancerearly/cancerscreening-guidelines/american-cancer-society-guide-lines-for-the-early-detection-of-cancer. Accessed on February 11, 2014.
- 14.National Cancer Institute. Screening and Testing to Detect Cancer. http://www.cancer.gov/cancertopics/screening. Accessed on February 11, 2014.
- 15.US Preventive Services Task Force. Recommendations of the US Preventive Services Task Force. http://www.uspre-ventiveservicestaskforce.org/recommen-dations.htm#cancer. Accessed on February 11, 2014.
- 16.Preston-Martin S, Kirstein LM, Pogoda JM, et al. Use of mammographic screening by HIV-infected women in the Women's Interagency HIV Study (WIHS). Prev Med. 2002;34(3):386-392. [DOI] [PubMed] [Google Scholar]
- 17.Reinhold JP, Moon M, Tenner CT, Poles MA, Bini EJ. Colorectal cancer screening in HIV-infected patients 50 years of age and older: missed opportunities for prevention. Am J Gastroenterol. 2005;100(8):1805-1812. [DOI] [PubMed] [Google Scholar]
- 18.Hsiao W, Anastasia K, Hall J, et al. Association between HIV status and positive prostate biopsy in a study of US veterans. ScientificWorldJournal. 2009;9:102-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sigel K, Wisnivesky J, Shahrir S, et al. Findings in asymptomatic HIV-infected patients undergoing chest computed tomography testing: implications for lung cancer screening. AIDS. 2014;28(7):1007-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.New York State Department of AIDS Health Institute. Anal Dysplasia and Cancer. http://www.hivguidelines.org/clinicalguidelines/adults/anal-dysplasia-and-cancer/. Accessed on February 13, 2014.
- 22.Chiao EY, Giordano TP, Palefsky JM, Tyring S, El Serag H. Screening HIV-infected individuals for anal cancer precursor lesions: a systematic review. Clin Infect Dis. 2006;43(2):223-233. [DOI] [PubMed] [Google Scholar]
- 23.Goldie SJ, Kuntz KM, Weinstein B, Freed-berg KA, Welton ML, Palefsky JM. The clinical effectiveness and cost-effectiveness of screening for anal squamous intraepithelial lesions in homosexual and bisexual HIV-positive men. JAMA. 1999; 281(19):1822-1829. [DOI] [PubMed] [Google Scholar]
- 24.Wilkin T, Lee JY, Lensing SY, et al. Safety and immunogenicity of the quadriva-lent human papillomavirus vaccine in HIV-1-infected men. J Infect Dis. 2010; 202(8):1246-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richel O, de Vries HJ, van Noesel CJ, Dijkgraaf MG, Prins JM. Comparison of imiquimod, topical fluorouracil. Lancet Oncol. 2013;14(4):346-353. [DOI] [PubMed] [Google Scholar]
- 26.Stier EA, Goldstone SE, Berry JM, et al. Infrared coagulator treatment of high-grade anal dysplasia in HIV-infected individuals: an AIDS malignancy consortium pilot study. JAIDS. 2008;47(1):56-61. [DOI] [PubMed] [Google Scholar]


