Abstract
HIV infection and antiretroviral therapy each appear to increase cardiovascular disease risk. Increased risk may be attributable to the inflammatory effects of HIV infection and dyslipidemia associated with some antiretroviral agents. The prevalence of cardiovascular disease is increasing as patients live longer, age, and acquire traditional coronary heart disease (CHD) risk factors. In general, any additional cardiovascular risk posed by HIV infection or antiretroviral therapy is of potential concern for patients who are already at moderate or high risk for CHD. Long-term and well-designed studies are needed to more accurately ascertain to what degree HIV infection and antiretroviral therapy affect long-term cardiovascular disease risk. Management of dyslipidemia to reduce CHD risk in HIV-infected patients is much the same as in the general population, with the cornerstone consisting of statin therapy and lifestyle interventions. Smoking cessation is a major step in reducing CHD risk in those who smoke. This article summarizes a presentation by James H. Stein, MD, at the IAS–USA live continuing medical education activity held in New York City in March 2012.
Cardiovascular Disease Is Caused by the Usual Suspects
The leading cause of death of people in the United States is cardiovascular disease, mostly heart attack and stroke, followed closely by cancer. Data from 7 years ago show that the leading cause of non-HIV-related death in HIV-infected men taking antiretroviral therapy in New York City was cardiovascular disease.1 As HIV-infected patients live longer and grow older as a result of effective antiretroviral therapy, the prevalence of cardiovascular disease will increase. There should be increased emphasis on preventing such disease now.
The major risk factors for heart disease—high blood pressure (BP), high total cholesterol level, diabetes mellitus, cigarette smoking, and aging—are well known. A key study from the past 10 years is the analysis of risk by Greenland and colleagues using 2 to 3 decades of follow-up of people with heart disease from 3 large longitudinal cohorts: CHA (Chicago Heart Association) Detection Project in Industry, the FHS (Framingham Heart Study), and MRFIT (Multiple Risk Factor Intervention Trial).2 The analysis showed that, depending on sex and age group, between 86% and 100% of persons dying from coronary heart disease (CHD) had at least 1 major CHD risk factor: systolic BP at least 140 mmHg or diastolic BP at least 90 mmHg, total cholesterol 240 mg/dL or higher, cigarette smoking, or diabetes. More than 96% of persons dying from CHD had at least 1 risk factor at higher than favorable levels, eg, systolic BP of 120 mmHg or higher or diastolic BP of 80 mmHg or higher or use of antihypertensive medication; total cholesterol level of 200 mg/dL or above or use of cholesterol medication; current cigarette smoking; or diabetes.
The attributable contribution of major risk factors to death from CHD is well over 90%, and more than 80% of that risk is attributable to lifestyle-related risk factors—elevated BP, high cholesterol level, diabetes, and smoking. Little can be done to reduce age-related or sex-related risk, but interventions are available for lifestyle-related CHD risk.
Is Coronary Heart Disease Risk Different for HIV-Infected Persons?
Recently, Dr Stein has been approached by people expressing such opinions as “People with HIV are at greatly increased risk of heart disease” and “Since we know that HIV is a coronary risk equivalent to diabetes, should all patients with HIV have their low density lipoprotein (LDL) cholesterol levels below 50 mg/dL?” However, the data do not support the idea that HIV is as powerful a risk factor as the conventional major risk factors for CHD.
The D:A:D (Data Collection in Adverse Effects of Anti-HIV Drugs) study is one of the most useful observational studies examining risk factors for CHD in HIV-infected individuals. It showed that risk for myocardial infarction (MI) was statistically significantly increased by such traditional risk factors as older age, male sex, greater body mass index (BMI), family history of CHD, current or former smoking status, and history of a cardiovascular event.3 It also showed that HIV protease inhibitor (PI) use was associated with increased cardiovascular risk; however, on adjusted analysis, much of this risk was attributable to the dysmetabolic effects of PI treatment—ie, increased risk of diabetes mellitus, hypertension, and dyslipidemia. This finding emphasizes the fact that traditional risk factors pose risk for CHD in HIV-infected persons and HIV-seronegative persons alike.
Reports of the association of antiretroviral therapy with risk of heart disease were accompanied by concerns of an epidemic of CHD in the HIV-infected population. However, what appears to be occurring is an increased prevalence of CHD among HIV-infected patients in association with aging of patients and increased exposure to traditional risk factors, much as is seen in patients with other chronic inflammatory conditions such as rheumatoid arthritis, systemic lupus erythematosus, and inflammatory bowel disease.
Figure 1 shows all-cause mortality and measures of cardiovascular disease among more than 36,000 HIV-infected patients, most of them men, from Veterans Affairs hospitals.4 The use of potent antiretroviral therapy resulted in a dramatic decline in mortality. At the same time, rates of hospitalization for cardiovascular or cerebrovascular disease remained relatively stable. These rates would not likely have remained stable if an epidemic of cardiovascular disease were occurring in patients receiving antiretroviral therapy. Although there may have been a slight increase in risk of cardiovascular disease among HIV-infected patients over the past decade, it is very small compared with the benefits of antiretroviral therapy on all-cause mortality.
Figure 1.
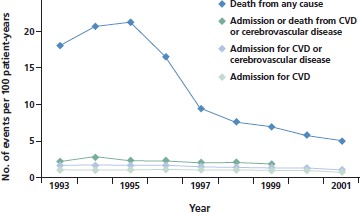
All-cause mortality and cardiovascular outcomes in HIV-infected patients in a Veterans Affairs population. Decrease in deaths is attributed to the introduction of potent antiretroviral therapy around 1996; no increase in cardiovascular disease (CVD) risk was observed from 1993 to 2001. Adapted from Bozzette et al.4
If there is any increase in risk of cardiovascular disease associated with antiretroviral therapy, it appears to be counterbalanced by the beneficial antiinflammatory effects of antiretroviral therapy on blood vessels. In ACTG (AIDS Clinical Trials Group) study 5152s, researchers randomized 82 antiretroviral therapy-naive patients to receive a PI-sparing regimen, a nucleoside analogue reverse transcriptase inhibitor (nRTI)-sparing regimen, or a nonnucleoside analogue reverse transcriptase inhibitor (NNRTI)-sparing regimen.5 Short-term antiretroviral treatment significantly improved endothelial function (measured as brachial artery flow-mediated dilation) to a similar degree in all 3 groups despite substantial differences among the groups in lipid level changes. The only factor examined in the study that predicted improvement in arterial function was viral suppression. The greater the reduction in HIV RNA level, the greater the improvement in endothelial function, irrespective of the antiretroviral therapy regimen used.
This finding is consistent with one of the findings of the SMART (Strategies for Management of Antiretroviral Therapy) study, which compared a CD4+ cell count–guided “drug conservation strategy” (treatment interruption) with a viral suppression strategy (continuous antiretroviral therapy). The data showed that the treatment interruption strategy was associated with a 2.6-fold increase in risk of progression or death. Analysis of cardiovascular outcomes showed that treatment interruption was associated with a 57% increase in risk for the composite endpoint of MI, percutaneous coronary intervention/coronary artery bypass grafting (PCI/CABG), or cardiovascular death (P = .05). When peripheral vascular disease, congestive heart failure, and coronary artery disease requiring medication were added to the composite endpoint, the strength of the statistical association increased (49% increase in risk, P = .03).6,7 These findings emphasize that viral activation and associated inflammation are deleterious for blood vessels. Effective antiretroviral treatment thereby reduces cardiovascular risk. Over time, exposure to some antiretroviral medications may slightly increase CHD risk more than others.
The D:A:D study indicates that cumulative exposure to the PIs indinavir and ritonavir-boosted (/r) lopinavir was associated with an increased risk of MI at a relative rate of approximately 1.1 per year.8 This slight increase in risk probably does not matter for patients who are at otherwise low risk for CHD and must be assessed in light of the risk of poorly suppressed HIV infection. For patients at moderate or high risk, such an elevation in risk should be taken into account when considering antiretroviral therapy options. Similarly, the study found that recent or current use of abacavir was associated with increased risk of MI at a relative rate of approximately 1.6. Again, this finding should be considered when selecting an antiretroviral regimen for patients with preexisting moderate or high CHD risk.
Whether HIV infection or antiretroviral therapy increases cardiovascular risk beyond traditional risk factors remains difficult to determine as of 2012. The main predictor of heart disease is age. Most patients living with HIV infection today are relatively young (ie, 30 years old to 50 years old) and thus are at relatively low short-term cardiovascular risk from this perspective. The attempt to determine whether there is something unique about HIV infection that increases risk beyond traditional factors is confounded by the fact that antiretroviral therapy can affect lipid levels, BP, and diabetes risk, and may consequently affect cardiovascular disease risk.
An important and sometimes overlooked consideration in parsing cardiovascular disease risk factors in HIV-infected individuals is the high frequency of cigarette smoking in this population. The rate of smoking among HIV-infected individuals is approximately 2 to 3 times higher than that in the general population. After a personal history of heart disease and increased age, cigarette smoking is the strongest predictor of CHD events in HIV-infected individuals. It is very difficult to isolate the potential contributions of HIV infection or antiretroviral therapy to CHD risk in relatively small studies of a relatively young population with a substantial proportion of cigarette smokers.
Our current state of knowledge with regard to cardiovascular risk and direct effects of HIV infection can best be summarized as “Most of what we think we know today, we don’t really know for certain.” The majority of research into the association of CHD risk and HIV infection has been small observational studies, which are subject to biases and potential confounding factors. As a result, the studies that have provided the data linking HIV infection to cardiovascular risk are somewhat inconclusive. Many of the studies have been conducted with a younger population that has experienced few cardiovascular events and have included limited follow-up periods, making it difficult to ascertain the potential contribution of risk factors to disease endpoints. Studies must often use surrogate markers and may not have well-matched control groups. It is likely to take at least 10 more years of research and concomitant accrual of cardiovascular events to robustly determine whether HIV infection or antiretroviral therapy themselves increase CHD risk. Until the research is more conclusive, humility regarding “what we know” is rational.
The Partners Health System Cohort study, one of the best studies of MI risk in HIV infection to date, provides an example of the problems in current studies of CHD risk in HIV-infected patients.9 The cohort is derived from a large data registry of 3851 HIV-infected persons and 1,044,589 HIV-uninfected persons who were followed up for approximately 4.5 years. This cohort is extremely young (mean age, 38 years) for purposes of studying cardiovascular risk and the follow-up period is extremely short for examining cardiovascular risk. The acute MI rate was 1.1% per year in HIV-infected persons versus 0.7% per year in uninfected persons, yielding a statistically significant 53% increase in risk (P < .0001) associated with HIV infection. However, more HIV-infected persons had hypertension, dyslipidemia, and diabetes mellitus (P < .001 for each).
These findings raised a number of questions. Was the increased risk in HIV-infected persons attributable to HIV infection itself or to the other risk factors identified? How many of the HIV-infected patients who had an MI might have had such risk factors and had an MI if they were not HIV-infected? A multivariate analysis adjusting for age, sex, race, hypertension, dyslipidemia, and diabetes found that HIV-infection status was associated with a 75% increase in risk for MI (relative risk 1.75; P < .0001), with this increase being much smaller than the increase in risk associated with older age or dyslipidemia. The data thus suggest that HIV infection alone is associated with increased CHD risk. However, problems with the study include the fact that it was not specifically designed to examine MI risk in HIV infection, and as such it does not have complete covariate data. For example, cigarette smoking was not included in the risk models, raising the question of how much it may have contributed to the risk attributed to HIV-infected status. Further, because complete lipid and BP data were not available, lipid levels and BP were analyzed as dichotomous variables (considering only the presence or absence of hypertension or dyslipidemia, not specific levels of either) rather than as continuous variables, which would have provided a more accurate assessment of the contribution of these factors to overall risk. The net effect of these limitations is to introduce misclassification bias, whereby the effects of risk factors may be underestimated and the effects of HIV infection may be overestimated. For such a study to produce more conclusive results, researchers would need to know subjects’ specific lipid levels and BPs, as well as smoking status; and the cohort would need 10 or more years of follow-up. This would provide better data on whether HIV infection is a substantial independent predictor of MI.
Based on the information that is available today, it appears that any long-term CHD risk increase in HIV-infected patients is relatively small. This increase is likely contributed to by the effects of certain antiretroviral drugs. Some HIV PIs may increase risk through dyslipidemia or other metabolic effects and abacavir may increase the short-term cardiovascular risk. Effects of persistent viral infection likely also contribute to the risk increase via persistent inflammation, viremia, and immune activation. These mechanisms reduce the ability of blood vessels to dilate and generate an anticoagulant surface, which may facilitate the plaque erosion and rupture that can cause acute coronary syndromes. For now, any absolute risk associated with antiretroviral therapy is most important in selecting treatment regimens for patients who are already at moderate to high CHD risk.
Management of Dyslipidemia to Reduce Coronary Heart Disease Risk
The guiding principle of CHD risk assessment and management is that a patient’s absolute CHD risk determines the intensity of interventions. Table 1 shows the current National Cholesterol Education Program (NCEP) Adult Treatment Panel guidelines for LDL cholesterol goals and treatment thresholds according to 10-year risk of a CHD event. Risk is determined by counting risk factors and calculating the Framingham Risk Score. The categorical risk factors that modify LDL cholesterol goals are age (men ≥ 45 years, women ≥ 55 years), family history of premature CHD (CHD in male first-degree relative < 55 years of age or in female first-degree relative < 65 years of age), cigarette smoking, hypertension (BP ≥ 140/90 mmHg or use of antihypertensive medication), and low high-density lipoprotein (HDL) cholesterol level (< 40 mg/dL).
Table 1.
Current National Cholesterol Education Program (NCEP) Low-Density Lipoprotein (LDL) Cholesterol Goals According to Coronary Heart Disease (CHD) Risk
| CHD Risk Category | CHD Status or Risk Factors (10-Year Risk of CHD Event) | Goal LDL Level (mg/dL) | LDL Threshold for Evaluation Treatment (mg/dL) |
| High (Very high) | CHD or risk equivalent (> 20%/10 years) | < 100 (< 70) | ≥ 100* |
| Moderately high | 2+ risk factors (10%-20%/10 years) | < 130 | ≥ 130* |
| Moderate | 2+ risk factors (< 10%/10 years) | < 130 | ≥ 160* |
| Low | 0-1 risk factor | < 160 | ≥ 190* |
Adapted from NCEP Adult Treatment Panel III.13
Also consider treatment if LDL level is below goal but above the goal for the next higher risk level.
Non-HDL cholesterol level (total cholesterol minus HDL cholesterol) is a secondary target of therapy when triglyceride levels are above 200 mg/dL, with the non-HDL cholesterol level goal being 30 mg/dL above the LDL cholesterol goal for each risk category (eg, the non-HDL cholesterol goal is < 130 mg/dL when the LDL cholesterol goal is < 100 mg/dL). Non-HDL cholesterol is a measure of all cholesterol values in atherogenic lipoproteins and is actually a more accurate predictor of cardiovascular events than LDL cholesterol.
The triglyceride level is a primary target of lipid-lowering therapy only when the triglyceride level exceeds 500 mg/dL, due to associated risk of pancreatitis. High triglyceride levels are associated with other cardiovascular risk factors (eg, low HDL cholesterol level, hypertension, and insulin resistance), although meta-analyses have indicated that an elevated triglyceride level is also an independent risk factor, albeit with a somewhat low odds ratio. Because LDL cholesterol level becomes a less accurate measure of atherogenic lipoproteins when the triglyceride level is elevated, non-HDL cholesterol is used as a target in the setting of elevated triglyceride levels.
The ACTG/Infectious Diseases Society of America-HIV Medicines Association (IDSA-HIVMA) guidelines for lowering lipid level to reduce CHD risk were published in 2003 and are similar to the NCEP guidelines. According to the ACTG/IDSA-HIVMA guidelines, lipid levels should be measured in HIV-infected patients prior to starting antiretroviral therapy and 3 to 6 months after starting therapy. Risk assessment is the same as in NCEP guidelines, as is the recommendation for intervention for modifiable nonlipid risk factors, including diet and smoking. If target lipid levels are not achieved with lifestyle modification, lipid-lowering therapy and modification of the antiretroviral regimen should be considered. New guidelines from the NCEP and IDSA-HIVMA are expected in the next year or two.
The LDL cholesterol level goal is below 70 mg/dL and below 100 mg/dL in persons at very high risk (eg, someone with CHD who smokes or has metabolic syndrome) and high risk, respectively, and below 130 mg/dL in those at moderately high or moderate risk. There is an option to treat persons with moderately high risk until the LDL cholesterol level falls below 100 mg/dL. It should be noted that an LDL cholesterol level of 130 mg/dL is not typical for non-Westernized humans and other primates and therefore is atherogenic. It is likely that many persons at moderately high risk with such an LDL cholesterol level will develop CHD. Thus, it is advisable to treat such persons until the LDL cholesterol level is below 100 mg/dL.
If there is a single take-home message about treating dyslipidemia to reduce CHD risk, it is to put patients on statin therapy. Statin therapy is very effective in lowering LDL cholesterol and non-HDL cholesterol levels and reducing cardiovascular risk. The recent Cholesterol Treatment Trialists’ (CTT) Collaborators meta-analysis involved more than 160,000 subjects from randomized trials comparing statin therapy with no statin therapy and comparing high-dose statin therapy with low-dose statin therapy. The analysis showed that over the course of 5 years every 39 mg/dL reduction in LDL cholesterol level on statin therapy was associated with a 10% reduction in all-cause mortality; a 20% reduction in CHD mortality; and a 21% reduction in major cardiovascular events, including a 26% reduction in MI or CHD death, a 24% reduction in PCI/CABG, and a 15% reduction in stroke.10 These reductions were consistent across subgroups (eg, diabetes or no diabetes, old age or young age, hypertension or no hypertension), and no increase in risk of death from other causes (eg, cancer) was observed, including among subjects with very low LDL cholesterol level.
However, recent meta-analyses indicate that statin treatment is associated with increased risk of diabetes mellitus.11,12 A 9% risk increase with statin therapy versus placebo was observed, with the excess risk being predicted by older age, not baseline BMI or LDL cholesterol level. The number needed to harm (NNH) for 1 case of diabetes mellitus was estimated at 225 patients over 4 years. However, it also was estimated that 9 cardiovascular disease events are prevented for every case of diabetes attributable to statin therapy. Similarly, a 12% increase in risk of diabetes was found for high-dose versus low- or moderate-dose statin therapy, but the risk of diabetes was again outweighed by the reduction in risk for cardiovascular events. The mechanism of the association between statin therapy and diabetes is unclear.
The relative dose equivalences among available statins for lowering LDL cholesterol are shown in Table 2. Most patients at risk for CHD require large reductions to achieve safer levels of LDL cholesterol and thus most will receive higher doses of the more potent statins—simvastatin, atorvastatin, and rosuvastatin. The highest dose of simvastatin studied (80 mg) is associated with increased risk of rhabdomyolysis and should be avoided. Because simvastatin and lovastatin interact with cytochrome P450 3A4 (CYP3A4) inhibitors, as is the case with most PIs, these statins should avoided with PIs that inhibit CYP3A4. Simvastatin use is widely promoted in managed care settings. If it is used, the maximum dose should be 10 mg per day in patients on cardiac medications such as amiodarone, diltiazem, and verapamil, or 20 mg per day in patients receiving medications such as amlodipine, ranolazine, niacin, or fibrates.
Table 2.
Approximate Dose Equivalences of Selected Statins for Lowering Low-Density Lipoprotein Cholesterol
| Atorvastatin | Fluvastatin | Lovastatin | Pravastatin | Rosuvastatin | Simvastatin | |
| 20 mg | 10 mg | 10 mg | 5 mg | |||
| 5 mg 10 mg | 40 mg 80 mg | 20 mg 40 mg | 20 mg 40 mg | 5 mg | 10 mg 20 mg | |
| 20 mg | 80 mg | 80 mg | 10 mg | 40 mg | ||
| 40 mg | 20 mg | 80 mg | ||||
| 80 mg | 40 mg | |||||
Adapted from Stein JH and McBride PE.14
For triglyceride levels below 500 mg/dL, LDL cholesterol and non-HDL cholesterol should be targeted to reduce CHD risk. As noted, for higher triglyceride levels, the primary goal is to treat the hypertriglyceridemia to prevent pancreatitis. Fibrates are the preferred initial therapy in this setting. Dietary intervention can have a dramatic effect on triglyceride levels. Patients should restrict saturated fats and trans-fats, emphasize intake of omega-3 fatty acids and monounsaturated fats, limit simple carbohydrates and calories, and reduce alcohol intake.
Patients with combined dyslipidemia should be put on statin therapy and if therapy does not achieve lipid goals, lifestyle intervention should be added. There is no evidence that any medication add-on to statin therapy further reduces cardiovascular risk, despite evidence that such measures as fibrates, niacin, and fish oils are associated with varying degrees of risk reduction on their own.
Although lowering lipid levels to reduce cardiovascular risk is focused on statin therapy and lifestyle intervention, controlling virus levels is crucial as well. Controlling viral load and stopping smoking would reduce cardiovascular risk for many HIV-infected patients.
References
- 1.Sackoff JE, Hanna DB, Pfeiffer MR, Torian LV. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med. 2006;145(6):397-406. [DOI] [PubMed] [Google Scholar]
- 2.Greenland P, Knoll MD, Stamler J, et al. Major risk factors as antecedents of fatal and nonfatal coronary heart disease events. JAMA. 2003;290(7):891-897. [DOI] [PubMed] [Google Scholar]
- 3. D:A:D Study Group, Friis-Møller N, Reiss P, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356(17):1723-1735. [DOI] [PubMed] [Google Scholar]
- 4.Bozzette SA, Ake CF, Tam K, Chang SW, Louis TA. Cardiovascular and cerebrovascular events in patients treated for human immunodeficiency virus infection. N Engl J Med. 2003;348(8):702-710. [DOI] [PubMed] [Google Scholar]
- 5.Torriani FJ, Komarow L, Parker RA, et al. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: The ACTG (AIDS Clinical Trials Group) Study 5152s. J Am Coll Cardiol. 2008;52(7):569-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355(22):2283-2296. [DOI] [PubMed] [Google Scholar]
- 7.Phillips AN, Carr A, Neuhaus J, et al. Interruption of antiretroviral therapy and risk of cardiovascular disease in persons with HIV-1 infection: exploratory analyses from the SMART trial. Antivir Ther. 2008;13(2):177-187. [DOI] [PubMed] [Google Scholar]
- 8.Worm SW, Sabin C, Weber R, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J Infect Dis. 2010;201(3):318-330. [DOI] [PubMed] [Google Scholar]
- 9.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92(7):2506-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cholesterol Treatment Trialists' (CTT) Collaboration, Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375(9716):735-742. [DOI] [PubMed] [Google Scholar]
- 12.Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011;305(24):2556-2564. [DOI] [PubMed] [Google Scholar]
- 13.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227-239. [DOI] [PubMed] [Google Scholar]
- 14.Stein JH and McBride PE. 2008 Guidelines for the Diagnosis and Management of Dyslipidemia for Adults >18 Years of Age. UW Health. http://www.unity-health.com/intranet/groups/pubweb/documents/unity_nativefile/005442.pdf. Accessed September 26, 2012.


