Abstract
The HIV care continuum (or treatment cascade) classifies individuals with HIV infection who are diagnosed, linked to care, retained in care, on antiretroviral therapy, and virally suppressed, to assess the effectiveness of health care and treatment from a population-level health perspective. Initially, it was estimated that only approximately 50% of individuals diagnosed with HIV infection are retained in care, and a lower percentage is virally suppressed. In an HIV clinic, retention in care should be addressed from a system perspective but focus on persons on an individual basis, and success in retention of a high proportion of individuals in care is likely to depend on collaboration with surrounding communities, local health departments, and other agencies. Two initiatives to improve engagement and retention in care undertaken at the University of Alabama at Birmingham 1917 Clinic are discussed. This article summarizes a presentation by Michael J. Mugavero, MD, MHSc, at the Ryan White HIV/AIDS Program Clinical Care Conference held in New Orleans, Louisiana, in December 2015.
Keywords: HIV, care continuum, care cascade, adherence, retention in care, antiretroviral therapy
Consider the case of a 21-year-old man diagnosed with HIV infection in June 2009. He entered care at the HIV clinic in August 2009, with a plasma HIV RNA level of approximately 100,000 copies/mL and a CD4+ cell count of 78/μL. He initiated antiretroviral therapy, and his viral load was undetectable in November 2009 and February 2010; his CD4+ cell count increased to 376/μL and 455/μL, respectively, at these visits. From a global perspective of the HIV care continuum, he rapidly and successfully spanned the successive steps from diagnosis through viral suppression. However, he then missed several visits and returned to the clinic in November 2010 with an HIV RNA level of 22,700 copies/mL and a CD4+ cell count of 248/μL, after which he was lost to care for approximately 2 years despite clinic efforts to contact and locate him. At a visit for laboratory evaluation in November 2012, he had an HIV RNA level of 80,300 copies/mL and a CD4+ cell count of 108/μL. It was not until April 2013, 5 months later, that he returned to the clinic for a visit with his practitioner, with an HIV RNA level of 200,000 copies/mL and a CD4+ cell count of 64/μL. He presented with a cough, weight loss, night sweats, and cutaneous Kaposi sarcoma–associated lesions; a chest x-ray showed bilateral opacification of the lungs. There was no response to empiric treatment for Pneumocystis jiroveci pneumonia. He was admitted to the hospital, and a bronchoscopy revealed pulmonary Kaposi sarcoma. This picture is encountered too commonly in clinical practice, in which as many as 50% of individuals diagnosed with HIV infection are lost to care.
The Continuum of HIV Care
The global process of HIV care is often referred to as a continuum, but in practice, it is more of a cascade—from a population-level health perspective—from higher to lower levels of success from the starting point of diagnosis of HIV infection to retention in care and achievement of viral suppression (Figure 1).1 However, individuals routinely shift within the framework of the cascade of HIV care, sometimes engaged in care for a prolonged period and then lost to care or only intermittently engaged in care, retained in care and adherent to antiretroviral therapy then nonadherent, and virally suppressed then unsuppressed (Figure 2).2
Figure 1.
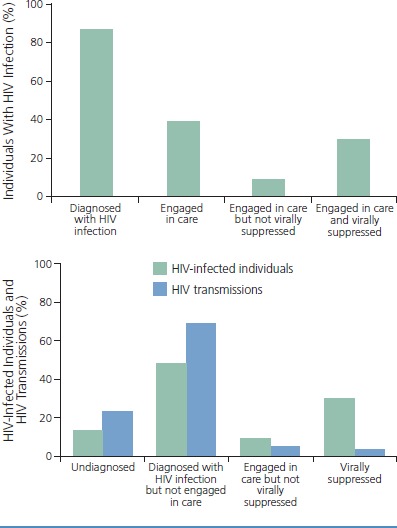
Estimated proportion of individuals diagnosed with HIV infection and engaged in care with or without viral suppression (top), and estimated proportion of HIV transmissions from individuals at different stages of the HIV care continuum (bottom) in the United States and Puerto Rico, 2012. Adapted from American Foundation for AIDS Research.1
Figure 2.

Health Resources and Services Administration continuum of HIV care. Steps toward and potential barriers to full engagement in care for HIV-infected individuals. Adapted from Cheever.2
In practice, an individual does not simply, sequentially move from HIV diagnosis to linkage to care, retention in care, adherence to antiretroviral treatment, and then to viral suppression. Further, as shown in Figure 3,3,4 the continuum of care in practice is dynamic at the population and individual levels and includes the crucial component of reengagement in care, emphasizing the relationships that a clinic must forge with individuals and agencies in the community and public health to coordinate the efforts that must be made to reconnect individuals to care.
Figure 3.

National HIV/AIDS Strategy 2020 goals. Changes from the 2015 goals are underlined. ART indicates antiretroviral therapy. Adapted from Mugavero et al and Ulett et al.3,4
The National HIV/AIDS Strategy 2020 goals are shown in Figure 3.3,4 The goal for linkage to care from time of HIV diagnosis is 85% of HIV-infected individuals within 1 month, an ambitious target. The goals for retention in care and viral suppression are 90% and 80%, respectively, of individuals with diagnosed HIV infection.
Many factors influence the ability to successfully engage individuals in HIV care.3 These factors include individual risk or predisposing factors such as stigma, resilience, insurance status, and others. Beyond the individual, there is the influence of relationships (eg, with a spouse, significant other, or partner, or with peer mentors, practitioners, and clinicians), community-related factors (eg, education level, employment status, and income), health care system–related factors (eg, fragmentation and ease or difficulty of navigation), and health care policy–related factors (eg, the availability of needle-exchange programs and if there are waiting lists for the AIDS Drug Assistance Program). Some of these factors, at each level of this socioecologic framework, are targets for interventions that may improve the success of care.
Engagement in Care
Engagement in care is vital to HIV-infected individuals. Implications of poor engagement at the individual level include delayed receipt of and nonadherence to antiretroviral therapy, inferior CD4+ cell count and viral load outcomes, emergence of resistance to antiretroviral drugs, and increased risk for adverse clinical events and mortality. At the population level, poor engagement in care is a contributor to health care disparities and plays a role in HIV transmission by reducing the chances of changing risk behaviors and missing the opportunity to reduce transmission via effective antiretroviral therapy and accompanying reduction in HIV viral load.
According to one study, in 2009, approximately 90% of cases of HIV transmission in the United States were attributable to HIV-infected individuals who were undiagnosed or diagnosed but not retained in care, with the latter category accounting for approximately twice as many cases as the former (Figure 1).5 In another study, despite efforts in outreach and testing and other initiatives, average CD4+ cell count at presentation increased among HIV-infected individuals by just 1.5/μL per year between 1991 and 2011, with an overall average of 307/μL.6 Thus, identifying HIV-infected individuals and engaging them in care at earlier stages of disease remains a major challenge, with considerable opportunities for improvement in testing approaches to foster earlier identification of infection, prior to disease progression.
Linkage to and Retention in Care
With regard to linkage to care, problems are exemplified by the findings of a 2-year study at the University of Alabama at Birmingham (UAB) 1917 Clinic. Of 522 new patients who called to establish care at the clinic between 2004 and 2006, 160 (30.7%) did not attend a clinic visit within 6 months of the initial call.7 The average time from initial call to scheduled visit was 28 days. This delay from call to scheduled visit was a major hurdle, as much can occur in an individual’s life in 4 weeks that might dispose the individual to miss a scheduled visit. Project CONNECT (Client-Oriented New Patient Navigation to Encourage Connection to Treatment) was developed in response to this hurdle.
In Project CONNECT, the initial hour-long social work visit was uncoupled from the initial hour-long medical visit (at 4 weeks) and scheduled for within 5 days after an individual’s initial phone call to the clinic. In essence, individuals are welcomed into the clinic, interviewed about factors such as housing, disclosure of HIV serostatus to others, substance use, and mental health, and questionnaires that screen for depression, substance use, stigma, and other issues are administered. Social workers are able to identify and address any potential barriers to care before the initial medical visit. Baseline laboratory results are also obtained at this first visit. Prophylaxis is prescribed if needed, for Pneumocystis jiroveci pneumonia and Mycobacterium avium complex, and this information is then available at the first medical visit. Project CONNECT did not increase costs to the clinic, as it represented largely a shifting of tasks and timing in linking a patient to care at the clinic.
Of the first 361 HIV-infected persons who called the UAB 1917 Clinic after implementation of Project CONNECT, 17.7% did not attend their scheduled visit compared with 30.7% before implementation; the odds ratio (OR) for not attending was substantially reduced on unadjusted analysis (OR, 0.48; 95% CI, 0.35-0.68) and on multivariate analysis adjusted for age, race or ethnicity, sex, insurance status, location of residence, and time from call to scheduled visit (OR, 0.54; 95% CI, 0.38-0.76). Currently, the UAB 1917 Clinic achieves a linkage-to-care rate of 85% to 95% of HIV-infected persons who call to schedule a visit, and this has been sustained over time, since the implementation of Project CONNECT, which is recognized by the Centers for Disease Control and Prevention (CDC) as an evidence-informed best practice intervention.
Following an initial HIV clinic visit, missed visits during the first year of care and low CD4+ cell count at time of entry into care are better predictors of long-term mortality among persons initiating outpatient HIV care than are initial viral load or whether antiretroviral therapy is started in the first year. In a study at the UAB 1917 Clinic, hazard ratios (HRs) for mortality among 543 individuals initiating outpatient HIV care between 2000 and 2005 were 2.90 (95% CI, 1.28-6.56) for a missed visit in the first year after diagnosis, 2.70 (95% CI, 1.00-7.30) for a CD4+ count below 200/μL versus at or above this count, and 1.58 (95% CI, 1.12-2.22) for each 10-year increase in age, with initial plasma HIV RNA level and initiation of antiretroviral therapy (alone, not sustained treatment) being nonsignificant in analysis adjusted for sex, race or ethnicity, insurance status, affective mental health status, and alcohol or substance use.7 Similar data have been reported by others.8,9
The effect of missed visits on mortality points to missed visits as a warning sign that factors in individuals’ lives may be interfering with their ability to remain in care. Clinicians—including pharmacists, nurses, social workers, and physicians—should reach out to a patient immediately when there is a missed visit. A missed visit is an easily measured and actionable event with profound prognostic value for untoward clinical events.
Missed visits are more likely among black than white individuals. In a study by Zinski and colleagues involving 10,000 participants at 6 US sites, black participants were more likely to have 2, 3, or 4 missed visits per year than white participants.10 However, when the 2 groups were stratified by number of missed visits, the proportions of participants who achieved viral suppression were similar. Similar findings have been made in other studies.11,12 Such findings indicate that increased efforts to reduce missed visits among black individuals could help to reduce the disparities in virologic suppression and mortality observed between white and black individuals in epidemiologic studies, which aligns with a principal tenet of the National HIV/AIDS Strategy to address and overcome disparities.
Current guidelines for interventions to achieve linkage to and retention in HIV care include monitoring of entry into and retention in care; use of brief, strength-based case management, focusing on the internal assets and strengths of an individual and applying these self-care engagement and adherence behaviors to linkage to care (eg, the Antiretroviral Treatment and Access to Services [ARTAS] model); intensive outreach for retention in care; and use of peer or paraprofessional navigation for retention in care.13 Clinics typically do not have sufficient resources to implement these interventions and often rely on and cooperate with the community and health departments to offer such services.
One program for improving retention in care is highly clinic based and provides clinics with an opportunity to use low-cost interventions shown to have positive results. The UAB 1917 Clinic was 1 of 6 sites that participated in the CDC/Health Resources and Services Administration (HRSA)–cofunded Stay Connected study of this program.14 Phase I of the program included a clinic-wide intervention involving the use of posters and brochures in waiting and exam rooms and brief messages from all clinic staff, all with the message of staying connected, attending visits, and staying in care. Phase II of the program consisted of patient-centered behavioral interventions, including enhanced contact in the form of personal reminder phone calls (not automated) made by the same clinic staff person at 7 and 2 days before each scheduled visit and within 24 to 48 hours after a missed visit. Also in phase II, skill-building learning modules for patients, presented in 2 brief sessions, focused on problem solving, communication with health care practitioners, and organizational skills.
An evaluation that compared preintervention with postintervention results after phase I showed that there was a 3.0% improvement overall in visit adherence, with improvements of 7.6% for individuals new to or reengaged in care, 5.5% for those with detectable viral loads preintervention, and 5.5% for those with CD4+ cell counts below 350/μL preintervention.15 Among participants in phase II who were randomly assigned to the intervention program or standard of care, the interventions increased visit adherence from 67% to 72% overall, from 66% to 70% among black individuals, from 65% to 70% among women, from 65% to 74% among individuals with Medicare benefits, and from 66% to 71% among individuals with Medicaid benefits.16
The CDC continues to build its library of best practices to improve linkage to and retention and reengagement in care.14 HRSA, through the National Resource Center, has also created a resource for training and improving clinic-based retention interventions.17
Adherence to Antiretroviral Therapy
Adherence guidelines indicate that adherence to antiretroviral therapy should be monitored in clinical practice through self-reporting or pharmacy refills and not through measurement of drug concentrations, pill count, electronic devices, or measurement of viral load (as viral load is the biologic correlate of adherence behavior).13,18 To improve or ensure adherence, barriers must be identified before an increase in viral load is observed; viral load is the biologic correlate of the nonadherence behavior and should not be used as a screening tool for adherence to antiretroviral therapy. The goal is to identify suboptimal adherence and intervene before it reaches a level that results in viral load rebound.
What should the clinician do if an individual reports “very good” or “good” adherence on a scale ranging from “very poor” to “excellent”? Available data indicate that self-reported adherence tends to be overestimated. Thus, individuals should be questioned for more specific information if self-reported adherence is anything lower than “excellent” or “perfect.” There is a dose-response relationship between self-reported adherence and risk of virologic failure.19 A practitioner may ask a patient directly how many doses have been missed and then question further based on the patient’s answer. However, it is imperative that practitioners normalize missed doses and doses taken off schedule and avoid leading, penalizing, or pejorative comments related to nonadherence. If a framework for open discussion and trust is established, there is considerably greater likelihood of identifying specific challenges with adherence and troubleshooting with a patient to address their individual circumstances and challenges (eg, evening dose, weekend doses, travel-related nonadherence).
In current adherence guidelines,13 strategies for improving adherence include reminder devices and interactive communication technologies; education and counseling using adherence-related tools; various individual, group, and peer education and counseling; case manager services (eg, assistance obtaining food or housing); and integration of medication management into pharmacy systems.
Returning to the patient referenced above, in April 2013, he returned to the clinic with an HIV RNA level of 200,000 copies/mL and a CD4+ cell count of 64/μL and was hospitalized with pulmonary Kaposi sarcoma. He resumed antiretroviral therapy and underwent chemotherapy with good response. In July 2013, his HIV RNA level was 79 copies/mL and his CD4+ cell count increased to 253/μL. He then missed a visit and was called immediately by his nurse practitioner to find out what had happened and to encourage him to come to the clinic. In December 2013 when he returned to the clinic, prompted by the personal call, his HIV RNA level had rebounded to 525 copies/mL and his CD4+ cell count was 226/μL. Since then, he has maintained adherence to clinic visits. In March 2014, his HIV RNA level was below 20 copies/mL and his CD4+ cell count was 365/μL. He participated in a triathlon in the summer of 2014 and remains in care with sustained viral suppression through September 2016, when he was last seen in clinic.
Summary
Engagement across the continuum of HIV care is dynamic and impacts individual- and population-level health. Systematic monitoring of engagement in care and adherence to antiretroviral therapy is foundational, and the prognostic value of missed visits and the predictive value of any self-reported nonadherence must be recognized. Evidence-based interventions to improve engagement in care and adherence to antiretroviral therapy are amenable to and should be implemented in clinical settings. Ultimately, partnerships between HIV clinics and public health and community agencies are essential to improve outcomes in the continuum of HIV care, particularly at the community-clinical interface of linkage to and reengagement in care.
References
- 1.American Foundation for AIDS Research (amfAR). Curbing the HIV epidemic by supporting effective engagement in HIV care. http://www.amfar.org/uploadedFiles/_amfarorg/Articles/On_The_Hill/2016/DC-2016-Engagement-Policy-Report_081916-October.pdf. Accessed on October 19, 2016.
- 2.Cheever LW. Engaging HIV-infected patients in care: their lives depend on it. Clin Infect Dis. 2007;44(11):1500-1502. [DOI] [PubMed] [Google Scholar]
- 3.Mugavero MJ, Norton WE, Saag MS. Health care system and policy factors influencing engagement in HIV medical care: piecing together the fragments of a fractured health care delivery system. Clin Infect Dis. 2011;52(Suppl 2):S238-S246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ulett KB, Willig JH, Lin HY, et al. The therapeutic implications of timely linkage and early retention in HIV care. AIDS Patient Care STDs. 2009;23(1):41-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skarbinski J, Rosenberg E, Paz-Bailey G, et al. Human immunodeficiency virus transmission at each step of the care continuum in the United States. JAMA Intern Med. 2015;175(4):588-596. [DOI] [PubMed] [Google Scholar]
- 6.Lesko CR, Cole SR, Zinski A, Poole C, Mugavero MJ. A systematic review and meta-regression of temporal trends in adult CD4(+) cell count at presentation to HIV care, 1992-2011. Clin Infect Dis. 2013; 57(7):1027-1037. [DOI] [PubMed] [Google Scholar]
- 7.Mugavero MJ, Lin HY, Allison JJ, et al. Failure to establish HIV care: characterizing the "no show" phenomenon. Clin Infect Dis. 2007; 45(1):127-130. [DOI] [PubMed] [Google Scholar]
- 8.Horberg MA, Hurley LB, Silverberg MJ, Klein DB, Quesenberry CP, Mugavero MJ. Missed office visits and risk of mortality among HIV-infected subjects in a large healthcare system in the United States. AIDS Patient Care STDs. 2013;27(8):442-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Dou Z, Sun K, et al. Association between missed early visits and mortality among patients of china national free antiretroviral treatment cohort. JAIDS. 2012;60(1):59-67. [DOI] [PubMed] [Google Scholar]
- 10.Zinski A, Westfall AO, Gardner LI, et al. The Contribution of Missed Clinic Visits to Disparities in HIV Viral Load Outcomes. Am J Public Health. 2015;105(10):2068-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mugavero MJ, Lin HY, Allison JJ, et al. Racial disparities in HIV virologic failure: do missed visits matter? JAIDS. 2009;50(1):100-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howe CJ, Napravnik S, Cole SR, et al. African American race and HIV virological suppression: beyond disparities in clinic attendance. Am J Epidemiol. 2014;179(12):1484-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson MA, Mugavero MJ, Amico KR, et al. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: evidence-based recommendations from an International Association of Physicians in AIDS Care panel. Ann Intern Med. 2012;156(11):817-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC). Compendium of evidence-based interventions and best practices for HIV prevention. http://www.cdc.gov/hiv/research/interventionresearch/compendium/lrc/index.html. Accessed on October 19, 2016.
- 15.Gardner LI, Marks G, Craw JA, et al. A low-effort, clinic-wide intervention improves attendance for HIV primary care. Clin Infect Dis. 2012;55(8):1124-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardner LI, Giordano TP, Marks G, et al. Enhanced personal contact with HIV patients improves retention in primary care: a randomized trial in 6 US HIV clinics. Clin Infect Dis. 2014;59(5):725-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.AIDS Education and Training Centers National Resource Center. Engagement in care toolkit: introduction and overview. https://aidsetc.org/engagement-toolkit. Accessed on October 19, 2016.
- 18.Gunthard HF, Saag MS, Benson CA, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society–USA panel. JAMA. 2016;316(2):191-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldman BJ, Fredericksen RJ, Crane PK, et al. Evaluation of the single-item self-rating adherence scale for use in routine clinical care of people living with HIV. AIDS Behav. 2013;17(1):307-318. [DOI] [PMC free article] [PubMed] [Google Scholar]


