Abstract
HIV infection is associated with increased cardiovascular disease (CVD), and increased rates of myocardial infarction and stroke have been observed in HIV-infected individuals. After traditional risk factors that are more common among people living with HIV infection (such as smoking and diabetes) are accounted for, the excess risk for CVD persists. Recent studies suggest that increased immune activation and inflammation may contribute to excess risk for CVD in the context of HIV infection. Imaging studies in the HIV-infected population have found inflamed, noncalcified plaque that is vulnerable to rupture. Statin therapy may represent a potentially useful primary prevention strategy for CVD in HIV-infected individuals, as this class of drugs lowers lipid levels and may simultaneously reduce immune activation and inflammation. REPRIEVE (Randomized Trial to Prevent Vascular Events in HIV) is a large, multicenter study funded by the National Institutes of Health. REPRIEVE will test whether pitavastatin, a newer statin that does not have substantial interactions with antiretroviral drugs, can prevent vascular events over time among HIV-infected individuals who do not have known CVD. This study is now open to enrollment at sites throughout the United States and abroad and will hopefully provide definitive data on this important question.
Keywords: HIV, REPRIEVE trial, cardiovascular disease, CVD
Cardiovascular Disease in the HIV-Infected Population
The use of more potent antiretroviral therapy has increased survival among people living with HIV infection. Nonetheless, even as the prevalence of opportunistic infections is declining, the prevalence of non–AIDS-related comorbidities, including cardiovascular disease (CVD), remains increased among HIV-infected individuals compared with uninfected individuals.1 A consistent body of evidence demonstrates that people living with HIV infection are 50% to 100% more likely to develop CVD than people without HIV infection, even when additional risk factors are controlled for, such as hypertension, cholesterol, and smoking status.2 Most recently, data on 82,459 patients in the Veterans Aging Cohort Study found that patients with HIV infection had a 1.5-times higher risk of acute myocardial infarction than the general population.3
Imaging studies have shed light on the mechanisms of CVD in HIV-infected individuals. Findings from initial studies that used computed tomography (CT) angiography suggested an increased prevalence of coronary plaque in almost 60% of HIV-infected participants compared with 30% of an uninfected control group who had similar traditional risk factors. Moreover, lesions were more often noncalcified and had more high-risk morphologic features, including low attenuation and positive remodeling, 2 features that suggest high-risk plaque that is vulnerable to rupture.4,5 In studies that used 18fluorine-2-deoxy-D-glucose positron emission tomography (18F-FDG-PET) imaging, increased arterial inflammation was observed among HIV-infected individuals matched for traditional coronary risk factors.6 Taken together, these studies suggest a unique morphology of high-risk inflamed plaque among HIV-infected individuals. Of note, subclinical CVD may occur among HIV-infected individuals with low or moderate traditional risk who may not be well identified by conventional risk scoring.7 Indeed, high-risk plaque is substantially associated with increased immune activation, more so than other risk factors, suggesting that immune activation and inflammation may contribute to the unique pathophysiology of CVD in HIV-infected individuals.
Development of a successful primary prevention strategy is crucial for the HIV-infected population. In this regard, a number of different strategies may be useful to prevent CVD in HIV-infected individuals. In the SMART (Strategies for Management of Antiretroviral Therapy) study, consistent use of antiretroviral therapy in individuals with CD4+ cell counts below 350/μL resulted in a decrease in AIDS-related adverse events and in CVD events.8 In the START (Strategic Timing of Antiretroviral Treatment) study, although AIDS-related events were reduced, utilization of early, CD4+ cell count–guided antiretroviral therapy did not reduce CVD events. However, relatively few CVD events were observed among antiretroviral therapy–naive individuals who may have had somewhat shorter durations of HIV infection.9 Taken together, these data suggest that suppressive antiretroviral therapy based on stricter CD4+ cell count thresholds will likely result in decreased rather than increased CVD rates, particularly as newer antiretroviral regimens with fewer metabolic effects are utilized. However, CVD rates currently remain increased among HIV-infected individuals, even those taking antiretroviral therapy who are virally suppressed, suggesting that other strategies are needed.
Lifestyle modification and treatment of traditional risk factors, including those for hypertension and diabetes, are important initial strategies. In addition, smoking cessation will prevent heart disease and is an important strategy in the large group of HIV-infected individuals who continue to smoke. However, a key question is whether these strategies targeting individual traditional risk factors are sufficient? The answer remains unknown, but it is interesting to speculate that other strategies may be needed, as the risk of hypertension and diabetes, although increased among HIV-infected individuals, does not fully account for the marked increase in CVD in this population.1 Moreover, substantial data have emerged that suggest that excess CVD risk is associated with immune activation among HIV-infected individuals.
Statins
Statins may be particularly useful in the HIV-infected population, as they have the advantage of lowering low-density lipoprotein (LDL) cholesterol, a known traditional CVD risk marker, and of reducing immune activation and inflammation. Statins have been shown to consistently lower mortality rates among HIV-uninfected individuals in association with reductions in LDL cholesterol levels.10 The JUPITER (Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin) trial included uninfected participants without known CVD and with reasonably controlled LDL cholesterol levels (<130 mg/dL). Participants in the JUPITER trial were required to have evidence of inflammation, as documented by increased C-reactive protein. In JUPITER, rosuvastatin use was shown to reduce future CVD events by 44%, more than would be expected by lowering of LDL cholesterol level alone.11
Among HIV-infected individuals, initial studies have begun to assess the potential utility of statin therapy. The general safety and efficacy of statins on liver, muscle, and LDL cholesterol were examined in a large cohort study by Silverberg and colleagues.12 In the study, statin use reduced LDL cholesterol levels by 26% and resulted in a 2% or lower prevalence of substantial muscle or liver adverse effects.
Funderberg and colleagues demonstrated the effects of rosuvastatin on improving key indices of immune activation among HIV-infected individuals, including indices of monocyte (soluble CD14, CD14dimCD16+TF+ subsets) and T-cell (CD4+CD38+HLA-DR+) activation.13 In addition, preliminary double-blind, placebo-controlled trials suggest that statins may improve high-risk plaque morphology in HIV-infected individuals by reducing noncalcified plaque volume, high-risk coronary plaque features, and markers of vascular inflammation (Figure 1).14
Figure 1.
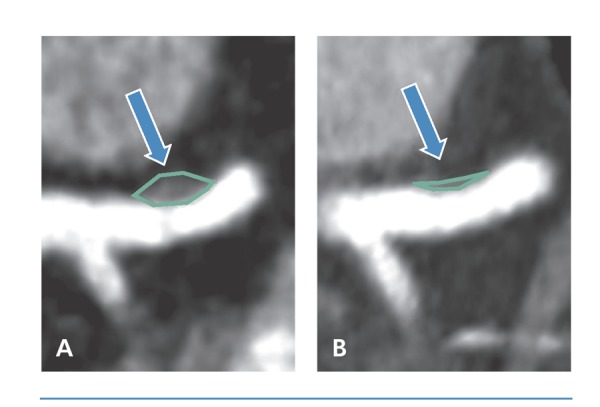
Noncalcified plaque in the proximal left anterior descending coronary artery in an individual before (A) and after (B) 12 months of therapy with atorvastatin.
Despite the emerging data on statin efficacy in HIV-infected individuals, key questions must be answered before this class of drugs can be recommended as a prevention strategy for CVD in the context of HIV infection. The small initial studies performed to date among HIV-infected participants have not yet investigated the effects of statins on actual CVD events but rather on plaque indices and inflammatory markers. Thus, it remains to be established if the robust effects seen on plaque in the context of HIV infection will translate to a reduction in CVD events. In addition, safety and tolerability concerns must be fully addressed. Some statins (eg, simvastatin and, to a lesser extent, atorvastatin) may interact with protease inhibitors through metabolic pathways involving cytochrome P450 3A.15 Some newer statins, including pitavastatin, are glucuronidated and are not known to interact with currently available antiretroviral drugs. Whereas some studies have suggested that certain statins increase glucose level, pitavastatin was not shown to have an adverse effect on diabetes or glucose level in the large, recently completed INTREPID (HIV-Infected Patients and Treatment With Pitavastatin vs Pravastatin for Dyslipidemia) study conducted among HIV-infected individuals.16
The REPRIEVE Trial
Given the crucial need for prevention of CVD events among HIV-infected individuals and the encouraging data to date on statins in this population, the National Institutes of Health (NIH) recently launched a large trial to definitively assess the efficacy of statins as a primary prevention strategy for CVD in this at-risk population. The REPRIEVE (Randomized Trial to Prevent Vascular Events in HIV) trial was launched in April 2015 and is the largest clinical trial of HIV-related CVD to date. The REPRIEVE trial will enroll 6500 HIV-infected participants who have no known clinical history or symptoms of CVD. Participants will be randomly assigned to receive daily pitavastatin 4 mg or a placebo for an average of 4 years (Figure 1), depending on date of entry into the trial. The trial is being conducted in collaboration with 100 plus sites from the AIDS Clinical Trials Group (ACTG) network and other NIH Division of AIDS networks in the United States and internationally, including sites in Canada, Thailand, and Brazil.
REPRIEVE is a collaborative study funded primarily by the NIH National Heart, Lung, and Blood Institute with additional support from the National Institute of Allergy and Infectious Diseases. In addition, the NIH Office of AIDS Research has contributed substantially to the project by providing additional funding. Reflecting the multidisciplinary nature of the REPRIEVE trial, the core team of researchers consists of specialists in metabolic aspects of HIV disease, cardiology, radiology, and AIDS care. The drug, pitavastatin, along with some additional funding is being donated to the trial by the manufacturer (Kowa Pharmaceuticals, Montgomery, AL). Pitavastatin was selected for REPRIEVE because it has had fewer interactions with antiretroviral drugs than other statins and effectively lowers LDL cholesterol levels in people living with HIV infection.17
Trial Design
The REPRIEVE trial will investigate the crucial question of whether HIV-infected individuals with low or moderate risk of heart disease, estimated using traditional risk assessment paradigms,18 will benefit from statin therapy. To be enrolled in the trial, participants must be between age 40 years and 75 years, taking an antiretroviral regimen, have no known CVD or substantial kidney or liver disease, and have a CD4+ cell count higher than 100/μL (Box). There is no specific viral load cut off for the trial, although it is anticipated that most patients taking antiretroviral therapy will demonstrate undetectable viremia (see www.REPRIEVEtrial.org for inclusion criteria, participating sites, and other information). The primary end point of REPRIEVE will be prevention of major adverse cardiovascular events, including CVD-related mortality, myocardial infarction, stroke, unstable angina, peripheral artery disease, and cardiac revascularization. The REPRIEVE trial will also investigate the effects of statin therapy on secondary outcomes, such as all-cause mortality, time to diagnosis of non–AIDS-defining cancer (excluding basal cell and squamous cell carcinomas of the skin), AIDS-defining events, initiation of dialysis or renal transplantation, cirrhosis, or hepatic decompensation requiring hospitalization. It is hypothesized that by decreasing inflammation and immune activation, statins may improve these related comorbidities.
In addition to the main study, REPRIEVE will include a substudy of 800 participants to investigate the mechanism of action of pitavastatin, particularly in reducing immune activation and inflammation. Biomarkers will be investigated and cardiac CT angiography will be performed to assess the effect of pitavastatin on noncalcified plaque volume and high-risk arterial morphologic features in the context of a large, randomized, placebo-controlled trial. Effects on specific biomarkers of monocyte activation, endothelial activation, arterial inflammation, and coagulation will be investigated and related to the primary study outcomes. Two ancillary studies have also been funded by the NIH: 1) to evaluate the longitudinal effects of statin therapy on renal function in HIV disease; and 2) to explore sex-specific mechanisms of CVD risk and statin-induced risk reduction among participants in the REPRIEVE trial.
Current Status
The REPRIEVE trial was launched in April 2015, with its first participant recruited approximately 6 months after the grant was awarded, a major triumph given the complex regulatory hurdles associated with launching a trial of this magnitude. As of November 2015, approximately 600 patients are enrolled in REPRIEVE. Continued recruitment efforts are needed in order to meet the NIH-mandated goal of completing recruitment within the first 2.5 years of the trial. Participants in the main trial will visit their local site once every 4 months for the duration of the study. Participants in the substudy will also have blood drawn to assess biomarkers and undergo CT angiography at baseline and at 2 years.
Goals
The REPRIEVE trial represents a new paradigm in studies of comorbidities in HIV-infected individuals. As HIV-infected individuals are living longer with fewer AIDS-related complications, understanding the mechanism and treatment strategies for prevention of comorbidities becomes increasingly important. Unlike prior shorter-term studies that assessed biomarkers, the REPRIEVE trial will assess effects on hard end points. Therefore, data will need to be captured over a longer time period. Without such studies, whether primary prevention strategies save lives in the HIV-infected population will remain unknown. The REPRIEVE trial was designed to be a simple study with straightforward clinical assessments using participant histories and minimal blood work. Importantly, REPRIEVE will provide information to all enrolled participants on lifestyle modifications to improve CVD risk. Clinical equipoise remains regarding the ability of long-term statin use to prevent HIV-related CVD, and many clinicians are waiting for results from the REPRIEVE trial to inform their practice. As the HIV-seropositive population ages, now is a crucial time to study this relationship.
Figure 2.
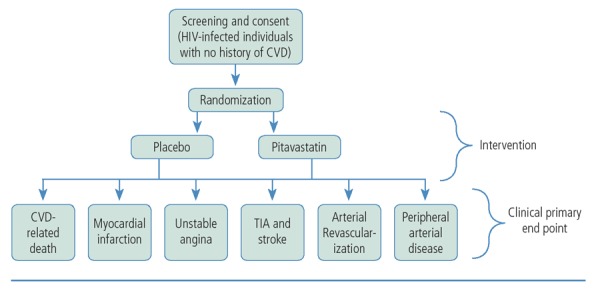
Schema of the REPRIEVE (Randomized Trial to Prevent Vascular Events in HIV) study in which participants will be randomly assigned to receive daily pitavastatin 4 mg or a placebo for an average of 4 years. CVD indicates cardiovascular disease; MI, myocardial infarction; TIA, transient ischemic attack.
Box. Eligibility Criteria for Participants in the REPRIEVE Trial
-
–
Must have HIV infection
-
–
Must be between age 40 years and 75 years
-
–
Must have been taking antiretroviral therapy for at least 6 months
-
–
Must have a CD4+ cell count greater than 100/μL
-
–
Must have no history of cardiovascular disease (eg, heart attack or stroke)
-
–
Must not be currently taking a statin
-
–
Must have low or moderate risk of cardiovascular disease according to the 2013 American College of Cardiology/American Heart Association risk calculator (http://www.cvriskcalculator.com)
Abbreviations: REPRIEVE, Randomized Trial to Prevent Vascular Events in HIV.
Summary
Tailored strategies to prevent heart disease are urgently needed in the HIV-infected population in whom the risk of CVD remains increased even as rates of traditional AIDS-related morbidities decrease. The NIH has recognized this important gap in knowledge of HIV disease. On a larger scale, the REPRIEVE trial has the potential to advance understanding of how inflammation should be incorporated into CVD risk prediction analyses, which are not generally accounted for in these measures. Results from the REPRIEVE trial may also provide important insights into other inflammatory conditions in which increased CVD rates are seen. Results from the REPRIEVE trial should become available in the next 5 years to 6 years and may influence future US guidelines for lowering the risk of heart-related disease among an aging HIV-infected population that is subject to an increasing prevalence of comorbidities related to chronic inflammation.
References
References
- 1.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92(7):2506-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitka M. Exploring statins to decrease HIV-related heart disease risk. JAMA. 2015;314(7):657-659. [DOI] [PubMed] [Google Scholar]
- 3.Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173(8): 614-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lo J, Abbara S, Shturman L, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS. 2010;24(2):243-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zanni MV, Abbara S, Lo J, et al. Increased coronary atherosclerotic plaque vulnerability by coronary computed tomography angiography in HIV-infected men. AIDS. 2013;27(8):1263-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Subramanian S, Tawakol A, Burdo TH, et al. Arterial inflammation in patients with HIV. JAMA. 2012;308(4):379-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zanni MV, Fitch KV, Feldpausch M, et al. 2013 American College of Cardiology/American Heart Association and 2004 Adult Treatment Panel III cholesterol guidelines applied to HIV-infected patients with/without subclinical high-risk coronary plaque. AIDS. 2014; 28(14):2061-2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355(22): 2283-2296. [DOI] [PubMed] [Google Scholar]
- 9.Lundgren JD, Babiker AG, Gordin F, et al. Initiation of antiretroviral therapy in early asymptomatic HIV Infection. N Engl J Med. 2015;373(9):795-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010; 376(9753):1670-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195-2207. [DOI] [PubMed] [Google Scholar]
- 12.Silverberg MJ, Leyden W, Hurley L, et al. Response to newly prescribed lipid-lowering therapy in patients with and without HIV infection. Ann Intern Med. 2009;150(5):301-313. [DOI] [PubMed] [Google Scholar]
- 13.Funderburg NT, Jiang Y, Debanne SM, et al. Rosuvastatin reduces vascular inflammation and T-cell and monocyte activation in HIV- infected subjects on antiretroviral therapy. JAIDS. 2015;68(4): 396-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo J, Lu MT, Ihenachor EJ, et al. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Lancet HIV. 2015;2(2):e52-e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fichtenbaum CJ, Gerber JG, Rosenkranz SL, et al. Pharmacokinetic interactions between protease inhibitors and statins in HIV seronegative volunteers: AIDS Clinical Trials Group (ACTG) study A5047. AIDS. 2002;16:569-577. [DOI] [PubMed] [Google Scholar]
- 16.Aberg JA, Sponseller CA, Kryzhanovski VA, Kartman CE, Thompson MA. Neutral effects of pitavastatin 4 mg and pravastatin 40 mg on blood glucose and HbA1c levels over 12 weeks: prespecified safety analysis from INTREPID (HIV-Infected Patients and Treatment With Pitavastatin vs Pravastatin for Dyslipidemia), a phase 4 trial. The Endocrine Society's 95th Annual Meeting and Expo. June 15-18, 2013; San Francisco, California. [Google Scholar]
- 17.Malvestutto CD, Ma Q, Morse GD, Underberg JA, Aberg JA. Lack of pharmacokinetic interactions between pitavastatin and efavirenz or darunavir/ritonavir. JAIDS. 2014;67(4):390-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2889-2934. [DOI] [PubMed] [Google Scholar]


