Abstract
Access to newer therapies for the treatment of hepatitis C virus (HCV) infection is limited by the costs of these treatments. Newer HCV regimens have been shown to be cost-effective in early stages and late stages of the disease, but payers in the United States may refuse to reimburse for treatment of early disease because of budget constraints. Approaches that can maximize patients’ access to appropriate therapy include having dedicated staff to handle prior authorizations and appeals, keeping records of successful approaches to prior authorizations and appeals and sharing these approaches with colleagues, and communicating with patients so that they will not be lost to appropriate health care. This article summarizes a presentation by Benjamin P. Linas, MD, MPH, at the IAS–USA continuing education program, Management of Hepatitis C Virus in the New Era: Small Molecules Bring Big Changes, held in Atlanta, Georgia, in September 2015.
Keywords: HIV, HCV, hepatitis C, HCV therapy, payers, insurance, cost-effectiveness, early HCV disease
Practitioners are becoming experienced with the high cost of newer treatments for hepatitis C virus (HCV) infection and with payers limiting access to therapy. However, practitioners can be advocates for patients in their care in this environment.
The illustrative case below is likely familiar to many who treat individuals with HCV disease. A 45-year-old man is infected with HCV genotype 1a. He does not have cirrhosis and has not been treated for his HCV infection before. He has a history of injection drug use but does not report any recent drug use. He has an HCV RNA level of 4.5 million IU/mL, a platelet count of 250 x 103/μL, and a serum albumin level of 3.8 g/dL. His alanine aminotransferase and aspartate aminotransferase levels are each approximately 90 U/L. He has an international normalized ratio of 1.1 and a transient elastography score of 2.1 kPa. His physician recommends an oral, interferon alfa–free therapy, with no preference among the appropriate treatment options, and requests prior authorization.
The payer denies authorization of treatment on the basis of early stage disease, and an appeal is unsuccessful. This individual and his physician may assume they have no alternative but to wait and perhaps follow-up with annual transient elastography evaluation. The physician might argue that the treatment is medically appropriate for this individual, that the American Association for the Study of Liver Diseases (AASLD)/Infectious Diseases Society of America (IDSA) HCV Guidance recommends treatment, or that treatment for HCV during the early stages of the disease is less costly than treatment at later stages.1 However, arguing with payers about the rationale for therapy may not affect their decision. Payers are familiar with these arguments, and their approach to authorization of treatment incorporates sophisticated thought and strategy regarding costs and markets.
Insurance Payers
The landscape of payers includes pharmaceutical manufacturers who develop and market drugs, pharmacy benefit managers who are often intermediaries between pharmaceutical manufacturers and insurance companies, private insurance companies, and government health programs (eg, Medicaid, Medicare).
Pharmaceutical companies in the United States determine the list price of their products based on proprietary information. Negotiations about price come thereafter. Pharmacy benefit managers are large, stand-alone companies that recruit payers (health insurers) and gather large groups of patients. With these groups as leverage, pharmacy benefit managers negotiate with pharmaceutical manufacturers for price discounts on treatments in exchange for exclusivity. Thus, pharmacy benefit managers may be negotiating on behalf of insurance companies and government health programs. Not all Medicaid programs use pharmacy benefit managers. Medicare is prohibited by law from negotiating drug prices. Private insurers may but are not required to work with pharmacy benefit managers.
Thus, there is great heterogeneity in how payers decide on the final cost of medicines. In the United States, there is no single person or entity that determines the price of treatment, and there is generally no single price. Pricing is the end product of negotiations and is dependent on who does the negotiating, how much leverage the negotiator has, and the personalities in the room at the time of negotiation. For example, among pharmacy benefit managers who represent numerous payers, one representative may express excitement over the negotiated price for a medication while their counterpart negotiates for 20% lower cost; both may be happy to receive a “good” price, although they are paying vastly different costs.
Negotiated price discounts are typically characterized by nondisclosure agreements. In exchange for exclusively offering their product, pharmaceutical manufactures agree to sell the medications to representatives at a discounted price. However, representatives cannot reveal the initial price of the medication to others. This strategy, which eliminates transparency, allows manufacturers to extract the best possible price out of each pool. From a purely business perspective, nondisclosure agreements are rational and can maximize profit.
Are Newer HCV Therapies a Good Use of Resources?
A cost-effectiveness analysis quantifies the value of treatment, seeks to maximize the impact of treatment, and aims to improve public health. The goal in determining cost-effectiveness is not to save money. Cost-effectiveness analysis first asks how much money is available to set a budget. The goal is to spend all of the budget, but also to spend it well in order to benefit most from the available resources. Cost-effectiveness analysis is aimed at maximizing population-level benefits of medical therapies in that, although it does not seek to minimize cost, it requires an explicit decision about willingness to pay.
Cost-effectiveness analysis uses 2 outcomes: cost (eg, in US dollars) and effectiveness. Effectiveness is often denominated in terms of quality-adjusted life-years (QALYs) gained, although it can also be quantified as simple life-expectancy gains. QALYs are an attempt to integrate some measure of quality of life and duration of life, recognizing that both are important. Measures of cost and effectiveness are used to derive the incremental cost-effectiveness ratio (ICER), a measure of additional resources used to pay for newer versus older treatments divided by the additional benefit (eg, in QALY gained) expected with newer versus older treatments. An ICER might be, for example, $42,000 per QALY gained, meaning that the cost of an additional QALY is $42,000. Society must determine what it is willing to pay to extend QALYs.
The concept of willingness to pay may be controversial in cost-effectiveness analysis, because willingness to pay requires an explicit valuation of a QALY, which may imply that life is not infinitely valuable. In truth, however, resources are always limited and society routinely makes decisions about the value of saving a life. Whenever a safety policy or intervention, such as expanded emergency response systems or improved roads, is deferred for cost reasons, the implication is that society cannot afford to pay to potentially save the life of the next person who would benefit from those services. Cost-effectiveness analysis highlights this kind of decision making about willingness to pay.
Cost-effectiveness analysis typically identifies society’s willingness to pay to save a QALY by assessing ICERs for health care interventions that are routinely performed in the health care system. For example, if the ICER for a routinely used radiologic procedure is $75,000 per QALY gained, then society is willing to pay at least $75,000 per QALY gained, as evidenced by the fact that the procedure is routinely used. Reported estimates of ICERs in the United States, inflated to the 2015 currency year, include $31,500 per QALY gained for antiretroviral therapy for HIV infection,2 $47,700 per QALY gained for statin treatment in primary prevention of cardiovascular events,3 $81,900 per QALY gained for implantable defibrillators,4 and $187,000 per QALY gained for dialysis in seriously ill adults.5 Some may conclude that approximately $50,000 per QALY gained is the maximum amount that those in the United States are willing to pay. Others believe that the acceptable ICER threshold is approximately $100,000 per QALY gained. However, an argument can be made that the ICER threshold in the United States is or should be higher, incorporating ICERs such as those noted for dialysis.
Numerous studies of the cost-effectiveness of the newer treatments for HCV infection have been published. ICERs for interferon alfa–free regimens have an estimated range of $9700 to $79,000 per QALY gained for individuals with HCV genotype 1 disease, with cirrhosis and without cirrhosis; $34,000 to $238,000 per QALY gained and $27,000 to $281,000 per QALY gained for individuals with HCV genotype 2 disease, with cirrhosis and without cirrhosis, respectively; and $51,000 to more than $383,000 per QALY gained and $51,000 to more than $500,000 per QALY gained for individuals with HCV genotype 3 disease, with cirrhosis and without cirrhosis, respectively. The wide range in ICERs partly reflects that the approach to determining drug cost varies between analyses. Some use the list price as their base-case analysis, and others use some negotiated discount price. All of these cost-effective analyses, however, include sensitivity analyses on drug cost.
Thus, ICERs for treating HCV genotype 1 disease and the lower range of estimates for treating HCV genotypes 2 and 3 disease are within the $100,000 per QALY gained threshold that may be considered cost-effective. It should be noted that the reported estimates for HCV genotype 2 disease reflect drug prices that have been markedly reduced in the recent past (eg, from $84,000 per year to $50,000-$60,000 per year).
Figure 1 shows a cost-effectiveness study sensitivity analysis of the relationship between ICERs for treatment in early HCV disease and assumptions in the parameters of treatment effectiveness, patient age, fibrosis progression rate, and quality of life.6 The ICER of HCV treatment for patients with early stage HCV disease is dependent on quality-of-life assumptions. If early stage HCV disease has very little or no impact on quality of life, then the ICER of therapy for patients with early stage disease far exceeds $100,000 per QALY. More so than other factors, quality of life leads payers to conclude that early stage treatment is not cost-effective. For late-stage disease, quality of life is more clearly affected by treatment, and ICERs are lower.
Figure 1.
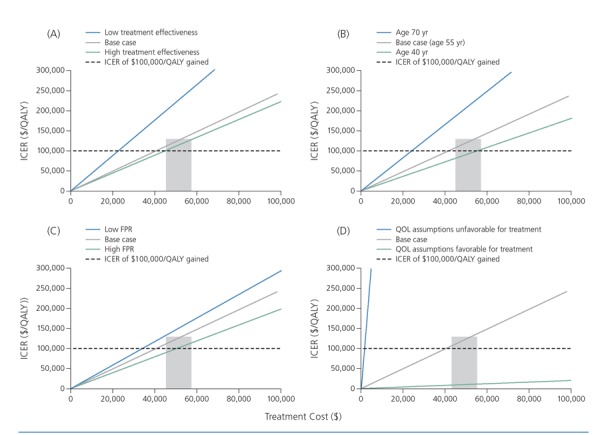
Incremental cost-effectiveness ratios (ICERs) for hepatitis C therapy in early stage disease according to treatment effectiveness (A), age (B), fibrosis progression rate (FPR) (C), and quality of life (QOL) (D). The y axis in each graph is the ICER for treating an individual with early stage disease compared with waiting for later-stage disease. The dashed black line indicates an ICER of $100,000 per quality-adjusted life-year (QALY) gained, posited as a reasonable cost-effectiveness threshold. The grey boxes represent a range of prices that most payers are paying for a new hepatitis C regimen. The solid grey lines in each graph represent a base-case assumption about the 4 parameters shown. The base case generally intersects $100,000 per QALY gained within or near the grey box, indicating that treatment is cost-effective at current prices in base cases of early disease. However, the assumptions regarding QOL include a nearly vertical line, representing almost no effect of early disease. Adapted from Leidner et al.6
Overall, interferon alfa–free regimens increase cost, but they also increase quality-adjusted life expectancy. Thus, interferon alfa–free treatments are cost-effective in early and late-stage HCV disease.
Barriers to Coverage of Cost-Effective Treatment
Cost limits access to HCV treatments, despite the cost-effectiveness of these medications. Figure 2 shows Medicaid restrictions on reimbursement of treatment with sofosbuvir in the United States, according to fibrosis stage and duration of abstinence from recreational drug use.7 Recently, several states announced that they would lift all restrictions for HCV treatment. It is possible that the trend will continue and that limited access to treatment will become a historical artifact, but at this time the large majority of states limits access to HCV treatment. Many Medicaid programs limit treatment to individuals with Metavir stage F3 fibrosis or worse. In many states, a period of abstinence from recreational drug use of up to 1 year is required and must be confirmed through documented urine test results before HCV treatment can be initiated. This restriction may be applied to marijuana and alcohol use in addition to injection drug use by some. Restrictions on type of practitioner are also being used to limit access to HCV treatment. In some states, only subspecialists (eg, hepatology or infectious diseases specialists) are permitted to treat HCV infection, although many treatment-naive individuals without cirrhosis could be treated by physicians other than subspecialists. Many individuals may also be limited to onetime access to treatment, with no retreatment permitted in the case of later reinfection.
Figure 2.
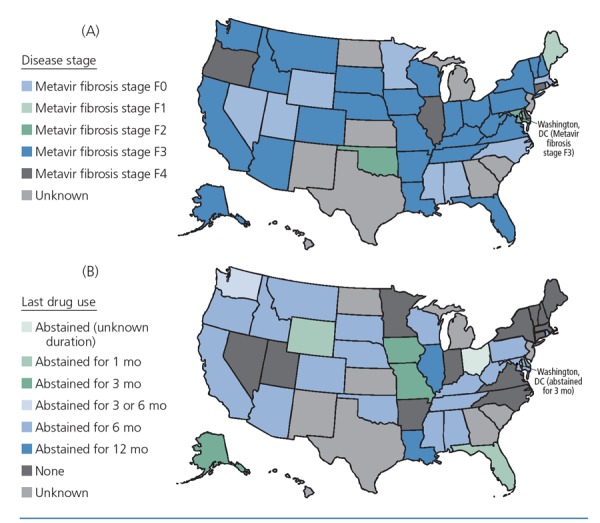
Medicaid restrictions on reimbursement for treatment with sofosbuvir, by disease stage (A) and time since last recreational drug use (B). These maps reflect policy current in late 2015. Ongoing litigation is changing Medicaid policy on a state-by-state basis. Adapted from Barua et al.7
aRecent negotiation or litigation has resulted in Washington, Massachusetts, and Connecticut dropping all treatment restrictions.
The primary reason that cost limits access to treatment is that cost-effectiveness does not equal cost-savings or affordability. Cost-effectiveness indicates that the best possible out come has been achieved using the available resources. Affordability is concerned with budget impact—quantification of the cost to a specific budget over the short term—and is unconcerned with epidemiologic or clinical outcomes. Assessment of budget impact includes no explicit consideration of outcomes other than impact on cost of treatment.
There are core differences between cost-effectiveness and budget impact and affordability. Budget impact and affordability are what policymakers use to set budgets and access (eg, the cost of treating all HCV-infected individuals under their plan). Budget impact analysis is done from the payer’s perspective, has a short time horizon, and incorporates poor outcomes only with regard to their impact on cost within that short time horizon. In contrast, cost-effectiveness analysis takes a societal perspective, uses a lifetime horizon in estimates, and directly incorporates poor outcomes into calculation of the ICER. It can take into account all costs important to society (eg, for medicines, hospitalization, productivity, and patient expenses), and estimates can include costs that would be avoided by curing HCV infection (eg, an expensive liver transplant that an HCV-infected individual might need in 20 years).
What is the budget impact of treating HCV infection with newer regimens? Taking a simple approach, a 5-year budget impact analysis on the cost of treating all patients with HCV genotype 1 infection used feasible assumptions for how quickly people would seek care and arrived at a figure of approximately $120 billion.8 Assumptions included list (nondiscounted) prices of the newer regimens, and it is likely that prices being paid now are discounted due to a more competitive market place. Thus, a more realistic estimate may be substantially lower. However, discounted prices vary and, along with the budget impact, are not well known. Cost of care is subject to location, insurance carrier, and coverage plan. However, the disparities in access to treatment owing to cost and budget impact are clear.
One example of this comes from the Massachusetts Department of Corrections (DOC). Most DOC facilities do not routinely screen for HCV infection, although it is widely known that there is a high prevalence of infection within the corrections system. In the Massachusetts DOC, there are approximately 1500 confirmed cases of HCV infection in the system, but in actuality there are likely 3000 cases. Not all of the 1500 individuals with confirmed HCV infection will be able to initiate treatment with new regimens immediately; thus, a conservative approach would be that 10% of these individuals will be treated each year, with the price of treatment likely discounted to approximately $50,000 per treatment course. Thus, in the first year, treatment costs would total $7.5 million (150 individuals at $50,000 per individual). Although the cost may not seem high in the context of government spending, the entire yearly pharmacy budget for the system is $11 million. Thus, even with the assumptions that only half of HCV-infected individuals will be treated, that only 10% will be treated each year, and that cost of treatment is highly discounted, the system would still be spending two-thirds of its entire annual budget on HCV treatment.
Navigating the Current Coverage Landscape
Insurance companies consider budget impact. Thus, cost-effectiveness and prevention of cirrhosis are insufficient motivation for insurance companies to cover treatment.
To best link individuals to treatment, a dedicated person should be assigned to process and manage prior authorizations (eg, a nurse, physician assistant, pharmacist, or case manager). The prior authorization process is designed to limit access to treatment and can be exhausting; thus, someone within the clinic should be dedicated to handling this process. Working with a specialty pharmacy can be helpful. Tracking data (ie, monitoring successes and failures) is important. If a good success rate is not achieved, then the process for obtaining prior authorizations should be reassessed. Also, if a particular insurer is routinely rejecting coverage, presenting them with data showing that they reject coverage more frequently than other insurers may motivate them to reconsider their decision.
Practitioners should share successful approaches to appeals with their colleagues. Appeals may be based on factors such as extrahepatic manifestations, HIV/HCV coinfection, potential pregnancy, and AASLD/IDSA HCV Guidance.1 Practitioners must decide how much effort they will put into an appeal if success seems unlikely. In some cases, such as those in which an individual has Metavir stage F0 fibrosis and the payer is known to deny all such cases without consideration of compelling mitigating factors, it may be prudent for practitioners to allocate time to other cases for which appeals may have a better chance of success.
Communicating with individuals about coverage and denial or deferral of coverage is crucial, and the difference between a denial and a deferral should be made clear. It should also be made clear that if treatment cannot be initiated it is because of lack of coverage, not because the physician has decided against treatment. Patients should be made aware that the practitioner’s goal is to provide appropriate treatment and that such treatment will be provided as soon as possible. For all individuals, and particularly those who will not be able to initiate treatment immediately, a focus on liver health is important. One goal of HCV treatment is to keep an individual’s liver healthy, to prevent cirrhosis and liver cancer. Individuals who are denied treatment because their disease is in the early stages should be reminded that disease is in its early stages and assured that they will be closely monitored.
Currently, there are no guidelines on management options for those who are waiting for disease progression that meets payer criteria for coverage. Practitioners may follow up with patients every 6 months to ensure they do not become lost to follow-up. Some practitioners perform frequent staging with various staging modalities to increase the likelihood of obtaining results that will convince payers to approve treatment coverage.
References
- 1.AASLD/IDSA. HCV guidance: recommendations for testing, managing, and treating hepatitis C. http://hcvguidelines.org/. Accessed on July 31, 2016.
- 2.Freedberg KA, Losina E, Weinstein MC, et al. The cost effectiveness of combination antiretroviral therapy for HIV disease. N Engl J Med. 2001;344(11):824-831. [DOI] [PubMed] [Google Scholar]
- 3.Pletcher MJ, Lazar L, Bibbins-Domingo K, et al. Comparing impact and cost-effectiveness of primary prevention strategies for lipid-lowering. Ann Intern Med. 2009;150(4):243-254. [DOI] [PubMed] [Google Scholar]
- 4.Sanders GD, Bayoumi AM, Sundaram V, et al. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N Engl J Med. 2005;352(6):570-585. [DOI] [PubMed] [Google Scholar]
- 5.Hamel MB, Phillips RS, Davis RB, et al. Outcomes and cost-effectiveness of initiating dialysis and continuing aggressive care in seriously ill hospitalized adults. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. Ann Intern Med. 1997;127(3):195-202. [DOI] [PubMed] [Google Scholar]
- 6.Leidner AJ, Chesson HW, Xu F, Ward JW, Spradling PR, Holmberg SD. Cost-effectiveness of hepatitis C treatment for patients in early stages of liver disease. Hepatology. 2015;61(6):1860-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barua S, Greenwald R, Grebely J, Dore GJ, Swan T, Taylor LE. Restrictions for Medicaid reimbursement of sofosbuvir for the treatment of hepatitis C virus infection in the United States. Ann Intern Med. 2015;163(3):215-223. [DOI] [PubMed] [Google Scholar]
- 8.Chhatwal J, Kanwal F, Roberts MS, Dunn MA.Cost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Ann Intern Med. 2015;162(6):397-406. [DOI] [PMC free article] [PubMed] [Google Scholar]


