Abstract
All patients with HIV infection should be screened for hepatitis B virus (HBV) infection. Preventive HBV vaccination is less effective in HIV-infected patients than in those without HIV infection. Emtricitabine, lamivudine, and tenofovir disoproxil fumarate (tenofovir) each have activity against HIV and HBV. In HBV/HIV-coinfected patients, if HBV or HIV treatment is needed, it should be initiated with tenofovir and emtricitabine or tenofovir and lamivudine as the nucleoside analogue reverse transcriptase inhibitor backbone of a fully suppressive antiretroviral regimen. If HBV treatment is needed and tenofovir cannot be used safely, entecavir is recommended in addition to a fully suppressive antiretroviral regimen. Initiation of treatment for HBV infection is based on the presence of cirrhosis and on HBV DNA level, alanine aminotransferase level, and biopsy results. Current HBV treatments are associated with low functional cure rates. This article summarizes a presentation by Kenneth E. Sherman, MD, PhD, at the IAS–USA continuing education program held in San Francisco, California, in March 2015.
Keywords: HIV, HBV, hepatitis B virus, HBV/HIV coinfection, HBV vaccine, HBV treatment
It is estimated that 350 million people worldwide have chronic hepatitis B virus (HBV) infection and that 4 million to 8 million people are coinfected with HBV and HIV. HBV/HIV coinfection has been shown to result in higher rates of liver-related morbidity and mortality. For example, in the D:A:D (Data Collection on Adverse Events of Anti-HIV Drugs) study, HBV infection was associated with an increased risk of liver-related death among HIV-infected participants (relative risk, 3.73).1
Characteristics of HBV
HBV is a hepadnavirus with a partially double-stranded DNA and its own reverse transcriptase for viral replication. The viral genome encodes 4 primary open reading frames involved in protein synthesis: P (polymerase), Pre-S and S (surface glycoproteins), Pre-C and C (core; structural protein of the nucleocapsid), and X (regulatory protein; the gene is thought to have transactivating function and may be involved in the development of liver cancer). The genes have markedly overlapping reading frames, leading to a high conservation of gene function and considerable potential for therapeutic disruption of the virus life cycle.
During HBV infection, the virus binds to the hepatocyte surface using sodium taurocholate receptors. It enters the cell and uncoats, releasing a relaxed coil DNA that moves to the cell nucleus. In the nucleus, it is transformed into covalently closed circular DNA (cccDNA), which is then transcribed into messenger RNA, which encodes the translation of surface and X proteins and, as pregenomic RNA, the translation of the hepatitis B e antigen (HBeAg), polymerase, and core proteins. When replication occurs at high levels, HBeAg is secreted. The components of pregenomic RNA and polymerase are packaged in the formation of a new capsid, followed by the reverse transcription of pregenomic RNA into relaxed coil DNA, the envelopment of the core in surface antigens, and the budding of virus from the cell. In most cases of HBV infection, there is excess production of surface proteins and secretion of subviral particles (vs infectious particles).
HBV infection slowly progresses over several decades. The initial phase of infection is characterized by immune tolerance, with a normal or near-normal level of alanine aminotransferase (ALT) and a high level of HBV DNA. This is followed by the immune clearance phase, or HBeAg-positive chronic hepatitis, during which the immune response to infection results in liver damage, with ALT and HBV DNA levels fluctuating with variations in immune response. If the immune response controls infection, the HBV-infected person becomes a chronic carrier, with reduced HBV DNA and a lower ALT level. Chronic carriers are subject to spontaneous reactivation of infection (HBeAg-negative chronic hepatitis). Some individuals have occult HBV infection; these individuals’ test results are negative for HB surface antigen (HBsAg) and HB surface antibody (anti-HBs), are often positive for HB core antibody (anti-HBc) immunoglobulin G (IgG), and are intermittently positive for HBV DNA in serum and in the liver. Chronic HBV infection may be associated with HBsAg mutations that result in reduced production of surface antigens. Occult HBV infection may result in the reemergence of acute HBV infection in individuals who have undergone a liver transplant and in HIV-infected individuals, and is also associated with increased risk of hepatocellular carcinoma.
HBV Prevention
Efforts to prevent HBV infection include modification of risk behaviors, vaccination, and preexposure prophylaxis. Modification of risk behaviors has not proved to be a highly effective prevention strategy. A study in Amsterdam, the Netherlands, reported an increase in risk encounters during an HBV vaccination campaign.2 However, preexposure prophylaxis for high-risk persons (especially those who have not been successfully vaccinated) could be an effective strategy, particularly given the activity of drugs such as tenofovir against both HBV and HIV infections.
Compared with an efficacy rate of approximately 95% in HIV-seronegative persons, efficacy rates of HBV vaccine in HIV-infected persons range from approximately 20% to 70%.3 In cases in which vaccination with a standard vaccine dose failed, occult HBV infection should be considered and testing for HBV DNA should be performed. A double-dose booster of the HBV vaccine should be given and anti-HBs titers should be measured at 1 month; if titer results are negative for anti-HBS, the full 3 or 4 dose series of double-dose vaccination should be completed. If prior double-dose vaccination has failed, then occult HBV infection should be considered and a double-dose booster can be given.
In one study of an HBV vaccine, 437 HIV-infected individuals were randomly assigned to receive 1 of 3 regimens. A protective level of response was considered to be 10 mIU/mL or higher of anti-HBs.4 Among individuals who received 3 standard doses of HBV vaccine of 20 μg intramuscularly (IM) at baseline, 4 weeks, and 24 weeks, 65% achieved a minimally protective level of anti-HBs, compared with 82% of those who received 4 doses of 40 μg IM at baseline, 4 weeks, 8 weeks, and 24 weeks and 77% of those who received 4 doses of 4 μg intradermally at baseline, 4 weeks, 8 weeks, and 24 weeks. Geometric mean titers of anti-HBs were highest among persons who received 4 doses of 40 μg IM, followed by those who received 4 doses of 4 μg intradermally. Patients with CD4+ cell counts of less than 350/μL have lower response rates to the standard vaccine series. Vaccination of all individuals, particularly those who engage in high-risk behaviors, regardless of CD4+ cell count is currently recommended. Repeat vaccination can be performed for those who do not exhibit protective immunity (defined as anti-HBS >10 mIU/mL).
Treatment
There are several outcomes of HBV treatment. Suppression generally refers to reduction of HBV DNA to below detectable levels. Seroconversion can refer to conversion from HBeAg-positive status to an HBeAg-negative status, or to conversion to anti-HBe–positive status from an HBeAg-negative status. Durable seroconversion occurs when a patient’s test results are negative for HBeAg and positive for anti-HBe for 1 year after completion of treatment. A functional cure is characterized by undetectable HBsAg. A true cure occurs when HBV cccDNA is cleared; this is exceedingly rare in HBV-infected individuals, but is the ultimate aim of treatment. However, functional cure can prevent acute liver injury, reduce fibrotic progression to cirrhosis, and reduce the risk of developing liver cancer.
US Food and Drug Administration (FDA)-approved drugs for the treatment of HBV infection are lamivudine, adefovir, entecavir, telbivudine, tenofovir, interferon alfa, and peginterferon alfa. Effective drugs that are not FDA approved for use as HBV treatment include the fixed-dose combination of tenofovir and emtricitabine or emtricitabine alone.
Current US Department of Health and Human Services (DHHS) and IAS–USA guidelines for use of antiretroviral therapy to treat HIV/HBV coinfection include the following5,6:
Prior to initiation of antiretroviral therapy, all individuals who test positive for HBsAg should be tested for HBV DNA using a quantitative assay to determine the level of HBV replication.
Because emtricitabine, lamivudine, and tenofovir disoproxil fumarate (tenofovir) have activity against HIV and HBV, if HBV or HIV treatment is needed, antiretroviral therapy should be initiated with the combination of tenofovir and emtricitabine or of tenofovir and lamivudine as the nucleoside analogue reverse transcriptase inhibitor (nRTI) backbone of a fully suppressive antiretroviral regimen.
If HBV treatment is needed and tenofovir cannot be used safely, then entecavir in addition to a fully suppressive antiretroviral regimen is the recommended alternative treatment option.
Of note, although not yet included in the guidelines referenced above, for individuals who experience nephrotoxic effects attributable to tenofovir, substitution with the investigational nucleos(t)ide analogue reverse transcriptase inhibitor tenofovir alafenamide fumarate may be an option when and if it is FDA approved.
For individuals with HBV/HIV coinfection, treatment of both infections should be initiated following an initial workup and evaluation, in accordance with current DHHS guidelines.5 For those who choose to defer therapy for HIV infection, a representative schema for deciding when to treat HBV infection is shown in Figure 1.7 Treatment should be started for individuals with cirrhosis whose test results are positive for HBsAg. In HBV-infected patients without cirrhosis, treatment should be started for those with HBV DNA levels above 20,000 IU/mL and ALT levels above normal limits. Liver biopsy should be considered for those with normal ALT levels, and treatment should be started for those with inflammation and fibrosis confirmed via biopsy. Among patients whose test results are negative for HBeAg and who have HBV DNA levels above 2000 IU/mL, treatment should be started for those with ALT levels above normal limits; biopsy should be considered for those with normal ALT levels, and treatment should be initiated if biopsy results show inflammation and fibrosis. The lower HBV DNA level threshold for treatment in patients whose test results are negative for HBeAg reflects the fact that many such patients have HBV mutations that result in disease that is more difficult to treat.
Figure 1.
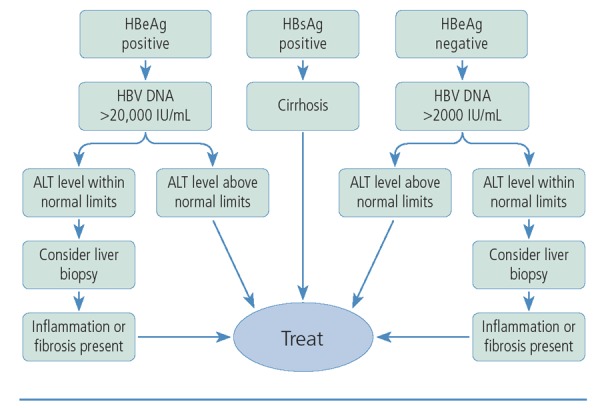
Treatment algorithm for hepatitis B virus infection. ALT indicates alanine aminotransferase; HBeAg, hepatitis B e antigen; HB-sAg, hepatitis B surface antigen; HBV, hepatitis B virus. Adapted from Keeffe et al.7
Patients receiving treatment for HBV infection should be tested every 6 months for HBV DNA. Those receiving tenofovir should have creatinine and urinalysis testing performed every 6 months (more frequently for those with creatinine clearance <60 mL/min). If HBV DNA level is greater than 1000 IU/mL after 1 year, the patient’s HBV resistance profile should be checked and, if cirrhosis is present, the regimen should be intensified; adherence to and malabsorption of drug should also be considered.
A comparison of characteristics of available anti-HBV drugs is shown in Table 1. Rates of functional cure of HBV infection (ie, loss of HBsAg) with established treatments are low, with rates of approximately 8% to 15% in those receiving an extended regimen of peginterferon alfa or peginterferon alfa plus lamivudine and rates of 0% to 10% in those receiving extended treatment with an nRTI-based regimen.8
Table 1.
Comparison of Efficacy, Resistance, and Cost of Drugs for Treatment of Hepatitis B Virus Infection
| Drug | Efficacy | Resistance | Cost |
|---|---|---|---|
| Lamivudine | +++ | ++++ | + |
| Adefovir | ++ | ++ | +++ |
| Entecavir | ++++ | ++ | +++ |
| Telbivudine | ++++ | ++ | +++ |
| Tenofovir | ++++ | + | +++ |
| Peginterferon alfa | ++++ | + | ++ |
HBV mutations are common, owing to the low fidelity of HBV polymerase. Most of the mutations associated with resistance to anti-HBV drugs appear on the tyrosine-methionine-aspartic acid-aspartic acid (YMDD) motif within the HBV polymerase. Persons with viral breakthrough caused by a mutation emerging during treatment with a single agent tend to experience disease flares. Persons with advanced disease sometimes develop hepatic failure and die. If the ineffective single agent is not stopped in the setting of viral breakthrough, compensatory mutations develop over time that render the virus resistant to virtually all available agents. Rates of treatment failure and emergence of resistance are higher in individuals with HBV/HIV coinfection than in those with HBV monoinfection, when lamivudine is the primary treatment for HBV infection. The risk of these events has declined with the use of more potent drugs, including tenofovir and entecavir. As seen with the management of HIV infection through antiretroviral treatment, adherence to HBV antiviral treatment is a cornerstone of effective care for HBV infection.
Path to Cure
A substantial proportion of hepatitis C virus (HCV) infections can now be cured. However, HBV is a more complicated virus to cure. As opposed to an RNA virus that replicates only in the cytoplasm, HBV is a DNA virus that effectively forms its own minichromosome in the nucleus of the host cell, which is passed through divisions from generation to generation of hepatocytes. Strategies to improve rates of functional cure and ultimately achieve true cure include exploitation of other viral targets, development of more potent nucleos(t)ide analogue inhibitors, therapeutic vaccination, and immune modulation.
There has been little progress in the development of more potent nRTIs. With regard to other viral targets, entry inhibitors such as myrcludex B that block sodium taurocholate receptors are being evaluated and have shown promise. Cyclophilin inhibitors such as alisporivir, which was evaluated for the treatment of HCV infection, interfere with host factors necessary to viral replication. Clinical trials of such approaches are likely to provide information in the near future. It has been hypothesized that zinc finger endonucleases could be used to target HBV cccDNA; however, targeting endonucleases to the hepatocyte nuclei and determining whether endonuclease activity can be focused on cccDNA remain challenging. Other nucleases, including transcription activator-like effector nuclease and homing endonuclease, are also being investigated.
In addition to targeted approaches, immunologic therapies also show promise. One basic strategy for therapeutic vaccination involves reduction of HBV replication via nucleos-(t)ide analogue inhibitor treatment, achieving HBsAg seroconversion via protein prime vaccination, and achieving viral clearance via recombinant vector boost vaccination.9 A number of vaccination options have been attempted but have met with little success thus far, including vaccines that carry proteins or peptides (eg, HBV recombinant subunits or pieces of C or pre-S regions) that might improve immune response; use of modified vaccinia vectors; direct placement of DNA into cells that produce pre-C, C, and S elements to increase level of immune response; use of peptide-pulsed dendritic cells; and use of adoptive T-cell transfer.
The use of targeted molecular immunogens is one approach being investigated. For example, one study examined a yeast-based immunotherapy platform that expresses X, surface, and core HBV antigens. As shown in Figure 2, T-cell response—measured by production of gamma interferon—to S and C antigens and to X, S, and C antigens was augmented in uninfected persons, but was also greater in patients with chronic HBV infection who were receiving adefovir.10
Figure 2.
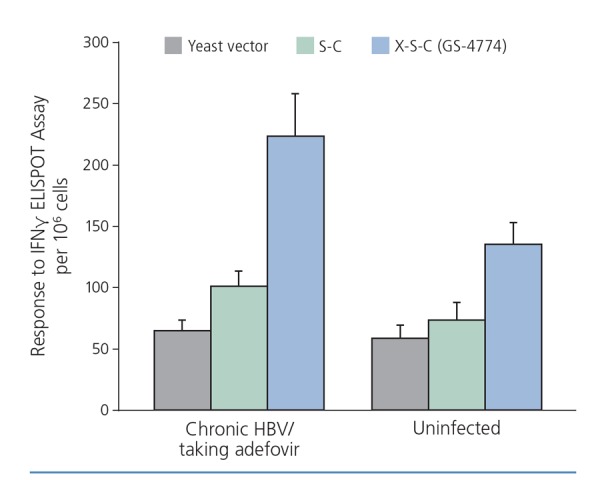
Immune response to yeast-based immunotherapy platform expressing X (regulatory protein), S (surface), and C (core) hepatitis B virus (HBV) antigens in uninfected individuals and in those with chronic HBV infection who are taking adefovir. ELISPOT indicates enzyme-linked immunospot; IFN, interferon. Adapted from King et al.10
Other approaches being evaluated involve use of inhibitors of immune checkpoint molecules such as programmed cell death 1 (PD-1) and programmed cell death ligand 1 (PD-L1), cytotoxic T-lymphocyte–associated protein 4 (CTLA-4), T-cell immunoglobulin mucin 3 (TIM-3), B7-H3, lymphocyte-activation gene 3 (LAG-3), and OX40. These molecules are responsible for reducing T-cell activation in the aftermath of HBV infection so that immune response does not continue unabated. In the setting of chronic persistent HBV infection, PD-1 and PD-L1 are upregulated, resulting in the reduction of specific immune response and likely leading to immune tolerance. Monoclonal antibodies that inhibit PD-1 or PD-L1 have been found to promote resurgence of immune response. Studies in vitro have shown a marked increase in HBV-specific CD8+ T cells with blockade of PD-1, PD-L1, and PD-L2.11
Another approach to augmenting immune response with the hope of clearing the virus is to use Toll-like receptor (TLR) agonists, a treatment option that is also being investigated as a potential HIV cure. TLR agonists, such as the investigational drug GS-9620, induce responses in interferon-stimulated genes. In a study in which HBV-infected chimpanzees received 3 weekly doses of GS-9620 for 4 weeks, HBV DNA levels in serum and liver were reduced.12 A study in woodchucks showed statistically significant reductions in cccDNA levels with use of GS-9620.13 Such findings indicate that TLR agonists might be useful in combination with nRTIs to spare patients the adverse effects associated with interferon alfa.
Conclusion
All patients with HIV infection should be screened for HBV infection and evaluated for HBV treatment candidacy. HBV vaccination is a crucial element of prevention, but current vaccination methods are imperfect. The goal of HBV treatment is to achieve complete suppression of viral replication. The decision to treat HBV infection is closely linked with the decision to treat HIV infection. Effective HBV treatment decreases fibrosis and the risk for hepatocellular carcinoma. Long-term suppression of HBV rarely leads to functional cure (an HBsAg-negative state), and other modalities are needed in order to clear the HBV cccDNA reservoir.
References
- 1.Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166(15):1632-1641. [DOI] [PubMed] [Google Scholar]
- 2.Xiridou M, Wallinga J, Dukers-Muijers N, Coutinho R. Hepatitis B vaccination and changes in sexual risk behaviour among men who have sex with men in Amsterdam. Epidemiol Infect. 2009; 137(4):504-512. [DOI] [PubMed] [Google Scholar]
- 3.Sun HY, Sheng WH, Tsai MS, Lee KY, Chang SY, Hung CC. Hepatitis B virus coinfection in human immunodeficiency virus-infected patients: a review. World J Gastroenterol. 2014;20(40):14598-14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Launay O, Van der Vliet D, Rosenberg AR, et al. Safety and immunogenicity of 4 intramuscular double doses and 4 intradermal low doses vs standard hepatitis B vaccine regimen in adults with HIV-1: a randomized controlled trial. JAMA. 2011;305(14):1432-1440. [DOI] [PubMed] [Google Scholar]
- 5.Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadoles-centgl.pdf. Accessed on April 15, 2015.
- 6.Gunthard HF, Aberg JA, Eron JJ, et al. Antiretroviral treatment of adult HIV infection: 2014 recommendations of the International Antiviral Society–USA panel. JAMA. 2014;312(4):410-425. [DOI] [PubMed] [Google Scholar]
- 7.Keeffe EB, Dieterich DT, Han SH, et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol. 2008;6(12):1315-1341. [DOI] [PubMed] [Google Scholar]
- 8.Kwon P, Lok AS. Hepatitis B therapy. Nat Rev Gastroenterol Hepatol. 2011;8(5):275-284. [DOI] [PubMed] [Google Scholar]
- 9.Kutscher S, Bauer T, Dembek C, Sprinzl M, Protzer U. Design of therapeutic vaccines: hepatitis B as an example. Microb Biotechnol. 2012;5(2):270-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King TH, Kemmler CB, Guo Z, et al. A whole recombinant yeast-based therapeutic vaccine elicits HBV X, S and Core specific T cells in mice and activates human T cells recognizing epitopes linked to viral clearance. PLoS One. 2014;9(7):e101904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sherman A, Trehanpati N, Daucher M, et al. Augmentation of hepatitis B virus-specific cellular immunity with programmed death receptor-1 /programmed death receptor-L1 blockade in hepatitis B virus and HIV/hepatitis B virus coinfected patients treated with adefovir. AIDS Res Hum Retroviruses. 2013;29(4):665-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lanford RE, Guerra B, Chavez D, et al. GS-9620, an oral agonist of Toll-like receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology. 2013;144(7):1508-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menne S, Tumas DB, Liu KH, et al. Sustained efficacy and seroconversion with the Toll-like receptor 7 agonist GS-9620 in the woodchuck model of chronic hepatitis B. J Hepatol. 2015;62(6):1237-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]


