Abstract
Investigational strategies to attempt HIV cure or remission include very early initiation of antiretroviral therapy to limit the latent HIV reservoir and preinfection vaccination. In the setting of viral suppression, strategies include reactivation of latently infected cells (eg, through “shock” therapy with histone deacetylase inhibitors or other agents); use of broadly neutralizing antibodies, therapeutic vaccines, immunotoxins, or other immune-based therapies to kill latently infected cells; and gene editing to induce target cell resistance (eg, by eliminating the CC chemokine receptor 5 [CCR5] coreceptor). Improved ability to detect and quantify very low levels of virus is needed. This article summarizes a presentation by Jintanat Ananworanich, MD, PhD, at the IAS–USA continuing education program held in New York, New York, in October 2014.
Keywords: HIV, cure, remission, viral reservoir, latent infection
HIV cure research currently focuses on 2 goals: complete eradication of HIV from the body and HIV remission. During remission, virus can still be detected at low levels in cells or through ultrasensitive testing in plasma, but antiretroviral therapy is not necessary unless continued monitoring reveals increased viremia.
Cases of Remission and Effects of Antiretroviral Therapy in Infants With Early Acute HIV Infection
Timothy R. Brown, also referred to as the Berlin patient, remains the only known person in whom HIV appears to be eradicated. Brown stopped taking antiretroviral therapy approximately 7 years ago and has had no detectable virus capable of replicating since. The mechanism of the possible eradication in this case included bone marrow transplantation with cells lacking the CC chemokine receptor 5 (CCR5) coreceptor, rendering the cells resistant to HIV infection. Two other patients, referred to as the Boston patients, experienced HIV remission 3 months and 7 months, respectively, after receiving bone marrow transplantation with cells that included the CCR5 coreceptor. The HIV-infected Mississippi baby, born to an HIV-infected mother who did not receive antiretroviral therapy, began antiretroviral therapy at 30 hours after birth. HIV infection was confirmed and the infant achieved viral suppression 1 month after starting treatment. Treatment was stopped after 18 months because the child was lost to follow-up care, and no virus was detected for a subsequent 27 months. In mid-2014, the child’s plasma HIV RNA level rebounded to 10,000 copies/mL to 16,000 copies/mL, and it was subsequently determined that HIV RNA level had increased to 9 copies/mL 2 weeks before the rebound.1-4
There have been several other cases of infants who received antiretroviral therapy during early acute HIV infection, resulting in the absence of detectable virus. An HIV-infected baby in California who received antiretroviral treatment at 4 hours after birth continued therapy for 14 months with no detectable cellular or plasma virus. Four HIV-infected Canadian babies who received antiretroviral treatment within their first 24 hours have remained on treatment for 2.5 years to 7 years with no detectable virus. Another Canadian baby who received antiretroviral treatment within 24 hours of birth had no detectable virus during 3 years of treatment but exhibited viral rebound 2 weeks to 3 weeks after stopping treatment. Similarly, an HIV-infected baby in Milan began antiretroviral treatment 12 hours after birth and had no detectable virus for 3 years while receiving treatment but exhibited viral rebound 2 weeks to 3 weeks after stopping treatment.5-9
In general, it is believed that earlier initiation of antiretroviral therapy and the longer it is maintained improve the chance of limiting the viral reservoir and achieving HIV remission. However, among the infants who eventually exhibited rebound after stopping antiretroviral therapy, time to viral rebound was longest for the Mississippi baby despite a later start of therapy (30 hours) than the Canadian baby (<24 hours) or the Milan baby (12 hours) and a shorter duration of therapy (18 months, 3 years, and 3 years, respectively). The baseline HIV RNA level of the Mississippi baby (19,812 copies/mL) was between that of the Canadian baby (808 copies/mL) and the Milan baby (152,560 copies/mL). The Mississippi baby’s longer remission period might be partially explained by the shorter duration of antiretroviral therapy before an HIV RNA level of less than 50 copies/mL was achieved (1 month of therapy for the Mississippi baby vs 6 months for the Canadian baby and 3 months for the Milan baby).
Testing performed while the Mississippi baby was in remission and was not taking antiretroviral therapy and the Milan baby and Canadian baby were taking antiretroviral therapy showed that whereas all 3 had negative test results for HIV DNA in peripheral blood, replication-competent virus, and anti-HIV antibody, the Canadian baby and the Milan baby displayed evidence suggestive of ongoing viral replication. The Milan baby had detectable HIV-specific T cells and a high percentage of activated T cells, and the Canadian baby had detectable cell-associated HIV RNA levels; these findings were not present in the Mississippi baby.2,5,8
These cases of transient HIV remission also show how current tools are limited in their ability to detect low numbers of HIV-infected cells. Acute HIV infection is associated with low levels of HIV DNA, which are further reduced by antiretroviral therapy during early acute infection to levels that may be undetectable. Currently, whether virus is present or in what amount it is present cannot be determined below the detection limits of current assays. Potential methods for measuring ongoing viral replication in the reservoir include measurement of cell-associated HIV RNA, single-copy HIV RNA, and replication-competent virus using viral outgrowth assays. Recently, it has been shown that the replication-competent HIV reservoir may be 60 times greater than what is currently measured by viral outgrowth assay, as there are viruses that are intact but not induced by this method.10 Investigators are examining the use of inducible HIV RNA assays to activate HIV-infected cells, in an attempt to measure reservoirs capable of replicating.
Is Early Antiretroviral Therapy Crucial to Limiting HIV Persistence?
Initiation of antiretroviral therapy in macaques 3 days after establishment of simian immunodeficiency virus (SIV) infection resulted in undetectable proviral DNA levels in peripheral blood but not in the lymph nodes or gut, with DNA levels at these sites declining during 6 months of treatment (Figure 1).11 Viral rebound was observed when antiretroviral treatment was stopped, suggesting that seeding of the viral reservoir begins very early and that initiation of treatment at 3 days in this animal model is not early enough to prevent it.
Figure 1.
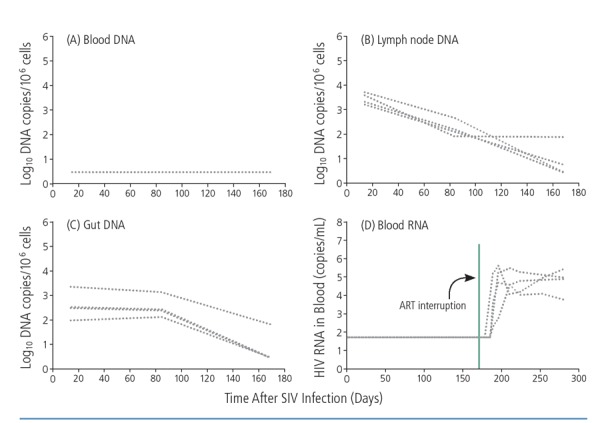
Simian immunodeficiency virus (SIV) proviral DNA was detected in the lymph nodes (B) and gut (C) but not in peripheral blood (A) after antiretroviral therapy (ART) was initiated in macaques 3 days after infection. All macaques had detectable blood HIV RNA after interruption of ART (D). Adapted from Whitney et al.11
The size and composition of the latent HIV reservoir are affected by early antiretroviral therapy. In basic CD4+ cell differentiation, stimulation of naive CD4+ cells by antigens causes them to differentiate into memory CD4+ cells that consist of stem cell, central, transitional, and effector memory CD4+ cells. The shorter-lived transitional and effector memory cells are more likely to differentiate into terminally differentiated cells and then die, whereas central memory cells are longer lived and constitute a latently infected cellular reservoir during HIV infection. During chronic HIV infection, the latent reservoir is large and central memory CD4+ cell infections still constitute a major part of the latently infected cell pool even after years of antiretroviral therapy. The reservoir size is much smaller during acute HIV infection, with years of antiretroviral therapy resulting in a marked decrease in latently infected cells.
Levels of integrated HIV DNA were examined among HIV-infected patients in Thailand who started antiretroviral therapy within 2 weeks of infection, within 3 weeks to 4 weeks of infection, or during chronic infection, and had plasma viral loads below detection limits at 2 years.12 Starting treatment within the first 2 weeks of infection resulted in much lower levels of integrated HIV DNA in all CD4+ cell subsets than when antiretroviral therapy was initiated during chronic HIV infection, and persistence of the viral reservoir was intermediate among patients starting antiretroviral therapy at 3 weeks to 4 weeks after infection.
Studies in the VISCONTI (Virological and Immunological Studies in Controllers After Treatment Interruption) cohort of posttreatment controllers— a group of 14 patients in France who started antiretroviral therapy early and exhibited control of viremia after interrupting treatment for 6 years or more—showed that early treatment could skew the distribution of latently HIV-infected cells to shorter-lived transitional memory cells that may be more rapidly cleared by the immune system (Figure 2).13
Figure 2.
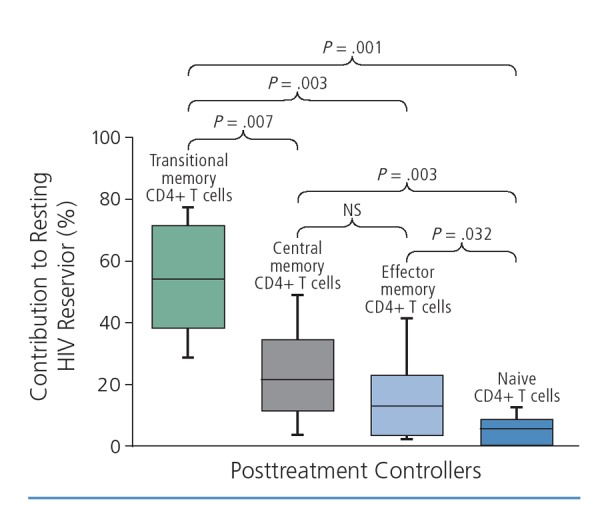
The HIV reservoir skewed to shorter-lived transitional memory cell subsets in the VISCONTI (Virological and Immunological Studies in Controllers After Treatment Interruption) cohort of posttreatment controllers. NS indicates not a statistically significant difference. Adapted from Saez-Cirion et al.13
Strategies to Eliminate HIV Persistence
One HIV remission and cure strategy currently under investigation is preinfection vaccination. In a study of 16 macaques, a cytomegalovirus (CMV)-vector SIV vaccine given prior to SIV infection did not prevent infection but did result in control of viremia in 9 macaques and in SIV eradication in 8 macaques, with no evidence of virus in organs or blood in the latter group.14 This response likely reflects the ability of the replicating CMV vector to generate ongoing immune responses. In responding animals, the vaccine was able to generate a very rapid early mucosal immune response that contained the virus before drastic systemic spread of infection. Further, immune response was not limited to immunedominant epitopes, with the broadness of response preventing viral escape.
A prime-boost adenovirus 26 and modified vaccinia virus Ankara (MVA) vaccine resulted in control of SIV viremia in 3 of 8 macaques. Control of viremia was associated with a broad CD8+ cell response and neutralizing antibody response.15 This vaccine for HIV will be investigated in patients starting antiretroviral therapy during early acute HIV infection, to determine whether it may help them achieve HIV remission.
Another remission strategy, in the setting of suppressed HIV viral load, is the use of “shock” therapy to activate latently infected cells to produce virus. In a study using the histone deacetylase (HDAC) inhibitor romidepsin as shock therapy, reactivation of HIV was observed but was not accompanied by a reduction in HIV DNA level, indicating that few, if any, HIV-infected cells were killed after reactivation.16
Strategies to kill latently HIV-infected cells include use of broadly neutralizing antibodies and vaccines. Broadly neutralizing antibodies bind cell-free virus and might clear infected cells. In one study, administration of 1 or 2 doses of the broadly neutralizing monoclonal antibody PGT121 to macaques with low baseline chimeric SIV/HIV (SHIV) viral load resulted in remission of virus for more than 1 year, long after PGT121 was no longer detectable in the blood.17 Numerous such monoclonal antibodies are being investigated for use in humans. A study of monoclonal antibody VRC01 administered during acute HIV infection is planned, and the AIDS Clinical Trials Group (ACTG) network is planning a study of this antibody in chronically HIV-infected patients.
Immunotoxins may also be used to kill HIV-infected cells, particularly in tissue. When this strategy was previously assessed as monotherapy it was ineffective, but it has since produced promising results when used in combination with antiretroviral therapy. A study in humanized mice showed a marked reduction in HIV RNA-positive cells in tissue when a combination of an immunotoxin with a pseudomonas endotoxin and antiretroviral therapy was used (Figure 3).18
Figure 3.
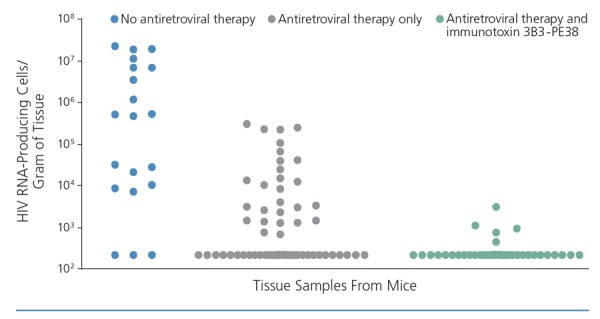
Effects of immunotoxin 3B3-PE38 combined with antiretroviral therapy on HIV RNA in tissue in mice. Adapted from Denton et al.18
Inducing resistance to HIV infection in cells has been examined by using gene therapy to eliminate CCR5 coreceptor. In one study, HIV-infected patients underwent leukapheresis and their cells underwent gene editing with zinc finger nucleases to remove CCR5 genes. Modified cells that no longer expressed CCR5 were then proliferated and reinfused into the same patient. HIV DNA levels were reduced but interruption of antiretroviral therapy resulted in viral rebound in all patients. However, viral rebound was followed by spontaneous control of virus in 1 patient who was heterozygous for the CCR5 gene and who exhibited the highest level of engraftment of the modified cells (see Tebas et al, 201419). Studies are currently examining additional doses of the modified cells and use of chemotherapy preconditioning to improve engraftment.
Summary
HIV cure strategies currently being examined in human studies (Figure 420) begin with minimizing the HIV reservoir through early antiretroviral therapy and use of broadly neutralizing antibodies. Once viral load is suppressed, latently infected cells can be reactivated (eg, with HDAC inhibitors or activation of toll-like receptors or protein kinase C) and immune-based therapies (eg, broadly neutralizing antibodies, therapeutic vaccines, or anti–programmed cell death [PD] 1 and anti–PD ligand 1 agents) can be used to kill HIV-infected cells, or cells can be made resistant to HIV (eg, transfusion with CCR5-negative cells). Achieving meaningful HIV remission or cure will almost certainly require combined treatments rather than single approaches.
Figure 4.
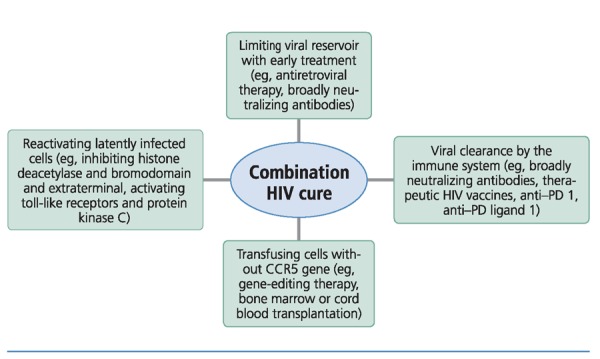
Different HIV cure strategies currently used in human studies that may need to be combined in order to achieve HIV remission. CCR5 incidates CC chemokine receptor 5; PD, programmed cell death.20
Testing to determine if HIV remission or cure has been achieved is challenging. Treatment cessation is the ultimate test of HIV remission, and ensuring safety requires frequent monitoring of viral load and a low threshold for restarting treatment.21 In some planned studies, viral load will be measured every 3 days to 7 days and treatment will be restarted with viral recrudescence. Many of the drugs being tested for HIV cure are cancer drugs with potential toxic efftects and many require intravenous infusion. Studies of the various HIV cure strategies will be a major burden to patients, requiring frequent follow-up, blood draws, and tissue sampling. Because there are many proposed treatments in the pipeline and because combination treatments will be needed, novel study designs to quickly move from demonstrating safety of individual therapy to assessing combination therapy for efficacy will be crucial. It will also be crucial to optimize the tools used to measure the HIV reservoir in tissue and blood.
In conclusion, SIV and HIV eradication has thus far been achieved via a CMV vector vaccine that maintained an effector T–cell response against SIV and via bone marrow transplantation utilizing CCR5-negative cells (the Berlin patient1). Early antiretroviral therapy is currently the most effective strategy to limit establishment of the HIV reservoir. Complete eradication of HIV will be difficult or impossible to achieve in the near future. HIV remission is a more attainable goal that will require testing of combination therapies to reduce the size of the HIV reservoir and boost HIV-specific immunity. In this early stage of HIV cure research, there will be many disappointments, but it is important to iteratively learn from these and steadily move the field forward.
References
References
- 1.Hutter G, Nowak D, Mossner M, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360(7):692;698. [DOI] [PubMed] [Google Scholar]
- 2.Persaud D, Gay H, Ziemniak C, et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med. 2013;369(19):1828;1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henrich TJ, Hanhauser E, Marty FM, et al. Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: report of 2 cases. Ann Intern Med. 2014;161(5):319;327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luzuriaga K, Gay H, Ziemniak C, et al. Viremic relapse after HIV-1 remission in a perinatally infected child. N Engl J Med. 2015;372(8):786;788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giacomet V, Trabattoni D, Zanchetta N, et al. No cure of HIV infection in a child despite early treatment and apparent viral clearance. Lancet. 2014;384(9950):1320. [DOI] [PubMed] [Google Scholar]
- 6.Rainwater-Lovett K, Luzuriaga K, Persaud D. Very early combination antiretroviral therapy in infants: prospects for cure. Curr Opin HIV AIDS. 2015;10(1):4;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bitnun A, Samson L, Chun TW, et al. Early initiation of combination antiretroviral therapy in HIV-1-infected newborns can achieve sustained virologic suppression with low frequency of CD4+ T cells carrying HIV in peripheral blood. Clin Infect Dis. 2014;59(7):1012;1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brophy J, Chun TW, Samson L et al. Impact of early initiation of combination antiretroviral therapy on measures of virus in peripheral blood of vertically HIV-1-infected children [Abstract TUAB0206LB]. 20th International AIDS Conference July 20-25, 2014; Melbourne, Australia. [Google Scholar]
- 9.Persaud D, Deveikis A, Gay H, et al. Very early combination antiretroviral therapy in perinatal HIV infection: two case studies [CROI abstract 75LB]. In Special Issue: Abstracts From the 2014 Conference on Retroviruses and Opportunistic Infections. Top Antivir Med. 2014;(e-1):37;38. [Google Scholar]
- 10.Ho YC, Shan L, Hosmane NN, et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell. 2013;155(3):540;551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitney JB, Hill AL, Sanisetty S, et al. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature. 2014;512(7512):74;77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ananworanich J, Vandergeeten C, Chom-chey N, et al. Early ART intervention restricts the seeding of the HIV reservoir in long-lived central memory CD4 T cells [Abstract 47]. 20th Conference on Retroviruses and Opportunistic Infections (CROI) March 3-6, 2013; Atlanta, Georgia. [Google Scholar]
- 13.Saez-Cirion A, Bacchus C, Hocqueloux L, et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 2013;9(3):e1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansen SG, Piatak M, Jr., Ventura AB, et al. Immune clearance of highly pathogenic SIV infection. Nature. 2013;502 (7469):100;104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barouch DH, Liu J, Li H, et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature. 2012;482(7383):89;93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kjaer A, Sogaard OS, Graverson ME, et al. The HDAC inhibitor romidepsin is safe and effectively reverses HIV-1 latency in vivo as measured by standard clinical assays [TUAA0106LB]. 20th International AIDS Conference July 20-25, 2014; Melbourne, Australia. [Google Scholar]
- 17.Barouch DH, Whitney JB, Moldt B, et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 2013;503(7475):224;228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denton PW, Long JM, Wietgrefe SW, et al. Targeted cytotoxic therapy kills persisting HIV infected cells during ART. PLoS Pathog. 2014;10(1):e1003872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tebas P, Stein D, Tang WW, et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med. 2014;370(10):901;910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ananworanich J, Fauci AS. HIV cure research: a formidable challenge. Journal of Virus Education. 2015;1:1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothenberger MK, Keele BF, Wietgrefe SW, et al. Large number of rebounding/founder HIV variants emerge from multifocal infection in lymphatic tissues after treatment interruption. Proc Natl Acad Sci USA. 2015;112(10):E1126-E1134. [DOI] [PMC free article] [PubMed] [Google Scholar]


