Abstract
Preexposure prophylaxis (PrEP) with tenofovir and emtricitabine has been shown to be effective and cost-effective in preventing acquisition of HIV infection. However, PrEP has not yet been widely adopted in the clinical practice setting. Data thus far, although imperfect, do not indicate an increase in risk behaviors in the setting of PrEP, although potential risk compensation outside of the clinical trial setting should be further examined. Substantial work remains to implement and support PrEP use, including identification of optimal settings for providing and managing PrEP, identification of methods to ensure optimal adherence to treatment, and employment of strategies to deliver PrEP to populations at greatest risk. This article summarizes a presentation made by Raphael J. Landovitz, MD, MSc, at the 2015 Conference on Retroviruses and Opportunistic Infections held from February 23 to February 26 in Seattle, Washington.
Keywords: HIV, PrEP, preexposure prophylaxis, tenofovir, emtricitabine, adherence, risk compensation, PrEP uptake, resistance
Despite the availability of a robust portfolio of prevention tools, there are still approximately 6000 new HIV infections daily worldwide (Figure 1).1 Preexposure prophylaxis (PrEP) is an important tool in the fight against HIV, but much work remains to increase PrEP uptake and optimize the preventive yield of this strategy.
Figure 1.
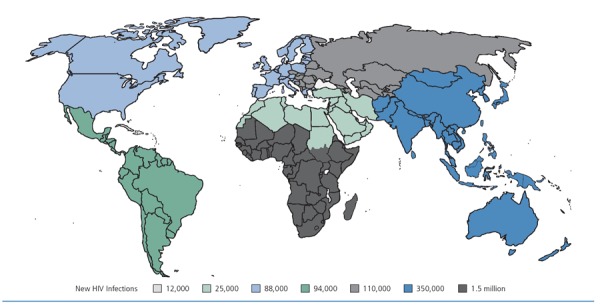
Annual new HIV infections in 2013. Adapted from the Joint United Nations Program on HIV/AIDS.1
PrEP With Tenofovir and Emtricitabine
Fixed-dose tenofovir and emtricitabine (tenofovir/emtricitabine) was approved by the US Food and Drug Administration (FDA) for use in HIV prevention in July 2012 and remains the only available FDA-approved regimen for PrEP.2
Tenofovir/emtricitabine possesses a number of advantageous characteristics for PrEP, including a relatively high barrier to resistance, rapid concentration in genital and rectal tissues, and a long intracellular half-life. Use in nonhuman primate models suggests that tenofovir/emtricitabine confers greater protection than tenofovir alone, that tenofovir-based PrEP confers protection against HIV exposure via various mechanisms of action, and that oral tenofovir concentrates less well in cervicovaginal tissues than in rectal tissues—a finding with important implications for forgiveness of nonadherence to PrEP in the context of vaginal HIV exposure.3-5 These models also indicate that intermittent dosing might be protective, suggesting the feasibility of on-demand use for some populations.6
In addition to being potent inhibi tors of viral reverse transcriptase, tenofovir/emtricitabine may reduce inflammation and immune activation. A study by Castillo-Mancilla and colleagues showed decreased HLA-DR and CD38 expression in CD8+ lymphocytes and decreased levels of soluble CD14 and soluble CD27 in uninfected volunteers who received tenofovir/emtricitabine daily for 30 days followed by a washout period of more than 30 days.4
PrEP was developed based on the observation that a crucial aspect of the efficacy of postexposure prophylaxis (PEP) is the time from HIV exposure to the first dose of antiretroviral medication. Efficacy is maximized as the time between exposure and PEP initiation approaches zero. The time sensitivity of PEP efficacy suggested that minimizing the time between exposure and PEP initiation could be achieved by having antiretroviral medication already present in relevant tissues by the time the HIV exposure occurred. This is similar to malaria prevention strategies, which may include use of a chemoprophylactic agent before, during, and after an unintended exposure to the infectious agent. Such preclinical and clinical observations led to human trials and, ultimately, to 5 phase III randomized controlled trials of oral PrEP.
The first such study, the iPrEx (Chemoprophylaxis for HIV Prevention in Men) study, enrolled a global population of men who have sex with men (MSM) and transgender women, randomly assigning participants to receive oral tenofovir/emtricitabine or a placebo daily and providing comprehensive HIV prevention services to each group. In 2010, the results from the iPrEX study were published, demonstrating a 42% reduction in incident HIV infections among individuals who were administered a daily oral regimen of tenofovir/emtricitabine.7,8 Chronologically, the next milestone in PrEP development was the FEM-PrEP (Preexposure Prophylaxis Trial for HIV Prevention Among African Women) trial, which was designed similarly to the iPrEx study and showed a 6% effectiveness rate of PrEP among uninfected women in Kenya, South Africa, and Tanzania.9
The Centers for Disease Control and Prevention (CDC)-sponsored TDF2 trial evaluating tenofovir/emtricitabine in heterosexual men and women in Botswana showed a 62% overall protection rate, including 80% in men and 49% in women.10 Importantly, the subset analysis in women did not show a statistically significant difference in PrEP effect compared with placebo in this study. The Partners PrEP trial conducted among HIV-serodiscordant heterosexual couples in Kenya and Uganda provided the first statistically significant demonstration of PrEP effectiveness for both men and women, with a protective efficacy rate of 63% in women and 71% in men with tenofovir alone, and 66% in women and 84% in men with tenofovir/emtricitabine.11 The VOICE (Vaginal and Oral Interventions to Control the Epidemic) trial evaluating oral and vaginal tenofovir gel and tenofovir/emtricitabine in women showed no preventive benefit of any of the oral or topical PrEP strategies.12
The discrepant results of the trials are at least partly explained by differences in adherence. There is a strong correlation between preventive effectiveness and levels of active drug detected in plasma and cellular samples from PrEP study participants (Figure 2). Studies of single-dose PrEP have shown that levels of tenofovir in rectal tissue are approximately 10 to 100 times higher than those in cervicovaginal tissue, which may help explain the difference in efficacy results between men and women even when adherence rates appear similar.5 Understandably, the wide range of effectiveness estimates from phase III trials has resulted in confusion among practitioners and patients. Although subject to inherent limitations, models and subset analyses based on these data provide some clarity regarding the level of adherence needed to achieve high protective effectiveness. A modeled analysis of data from the iPrEx trial suggests that a 99% rate of preventive effectiveness (confidence interval [CI], 96%-99%) in cases of rectal exposure is achievable in men when PrEP with tenofovir/emtri-citabine is taken 7 days per week as prescribed.13 An analysis of women in the Partners PrEP trial who had plasma tenofovir levels suggestive of steady state daily dosing indicated that 6 to 7 daily doses of tenofovir/emtricitabine per week could achieve a 94% rate of effectiveness (CI, -17% to 100%) in cases of vaginal exposure.14,15
Figure 2.
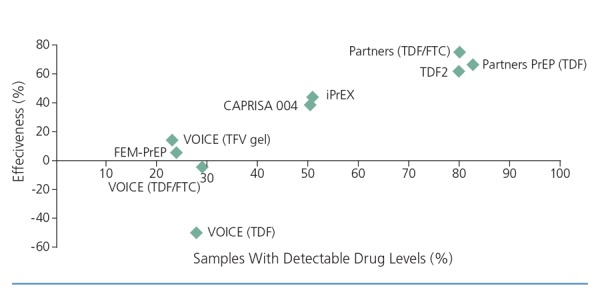
Relationship between effectiveness and adherence in preexposure prophylaxis (PrEP) and microbicide trials (Pearson correlation, 0.86; P = .003). CAPRISA indicates Centre for the AIDS Programme of Research in South Africa; FEM-PrEP, Preexposure Prophylaxis Trial for HIV Prevention Among African Women; FTC; emtricitabine; iPrEx, Chemoprophylaxis for HIV Prevention in Men; TDF, tenofovir disoproxil fumarate; TFV, tenofovir; VOICE, Vaginal and Oral Interventions to Control the Epidemic. Adapted from AIDS Vaccine Advocacy Coalition (AVAC).36
PrEP Uptake
PrEP has not been widely adopted despite strong evidence of its preventive effectiveness. Some of the delay in uptake may be caused by competing priorities and limited resources. However, PrEP raises concerns that undoubtedly contribute to its slow uptake. There is concern that PrEP will lead to decreased condom use, increased numbers of sexual partners, and increased numbers of sexually transmitted infections (STIs), including HIV infections—so-called risk compensation. There are also concerns about emergence of resistant virus that could compromise subsequent dosing options, about how quickly protection is achieved and how durable it is after cessation of treatment, and about safety, particularly in diverse populations that are not well represented in phase III clinical trials. More data are needed to determine the best settings and contexts in which to administer PrEP, and the best ways to measure and maximize adherence to prescribed PrEP regimens; to assess if less than daily dosing of PrEP with tenofovir/emtricitabine can confer protection and to whom PrEP should be targeted; to determine if better options are coming; and, perhaps most importantly, to determine if PrEP can be made available to and be used by individuals at the greatest risk.
Risk Compensation
With risk compensation there is the concern that PrEP users will increase their risk behaviors in the setting of imperfect preventive effectiveness, thus increasing the risk of HIV infection at the individual and population levels. Modeling of the potential effects of risk compensation in resource-limited settings showed that increases in risk behaviors at higher levels of preventive effectiveness of PrEP could maintain substantial reductions in numbers of new infections at the population level; however, an increase in risk behaviors would be expected to be associated with a population-level increase in new HIV infections at lower levels of preventive effectiveness.16
Primary analyses of data from phase III trials of PrEP did not show evidence of risk compensation, a finding that is likely attributable to the provision of combination HIV prevention interventions regardless of randomized medication assignment and to the use of placebos in the trials’ designs. Studies of other prevention modalities (eg, antiretroviral therapy as prevention, vaccines, and voluntary male circumcision) have yielded inconsistent data on risk compensation, including increases in risk behaviors in some vaccine trials, trials of voluntary male circumcision, and studies of antiretroviral therapy as prevention.17-19 In addition, the occurrence of risk compensation is suggested by increases in rates of STIs, particularly syphilis, among HIV-infected MSM since the widespread adoption of antiretroviral therapy. Although early data from open-label extensions of phase III trials of PrEP have generally supported decreases in risk behaviors when PrEP is used in the context of combination prevention services, a secondary analysis of the Partners PrEP study showed that an overall decrease in risk behaviors during the blinded phase of the trial was followed by an increased trajectory of risk behaviors—measured as number of nonprimary sexual partners—after determination of PrEP efficacy and unblinding of treatment assignment to study participants.20 Further, it was reported at an October 2014 forum on PrEP sponsored by the San Francisco Department of Public Health that in a subset of a large PrEP cohort in Northern California, 45% of individuals reported reduced condom use in association with PrEP use.
Viral Resistance
Perhaps the most contentious issue in the field of PrEP is the potential for emergence of viral resistance when HIV seroconversion occurs in the context of PrEP use. Various models have yielded conflicting predictions, ranging from doubling of or greater rates of transmitted resistance to an extremely limited impact on circulating resistant species.21,22
Data from phase III trials suggest that HIV seroconversion with resistant virus is quite rare. PrEP use, in the context of the monthly HIV testing performed in the studies, conferred sufficient protection to prevent HIV acquisition or was used sufficiently infrequently such as to be permissive of HIV acquisition without maintaining sufficient drug levels to select for resistant quasispecies of virus. Analyses of trial populations indicated the presence of the tenofovir-resistant mutation K65R or K70E or the emtricitabine-resistant mutation M184V/I in 0 of 36 participants who seroconverted in the active arm of the iPrEx study, in 4 of 51 participants in the active arm of the Partners PrEP study, in 0 of 10 participants in the active arm of the TDF2 study, in 4 of 33 participants in the active arm of the FEM-PrEP study, and in 1 of 113 participants in the active arm of the VOICE study. In these trials, there was a total of 9 (3.7%) cases of resistance among 243 serocoverters, or 5 (2.0%) cases when transmitted resistance was excluded; this total is equivalent to seroconversion with resistant quasispecies in 0.06% of persons exposed to tenofovir-based PrEP.7,9-12,23-25 The M184V/I mutation was the most common and was associated with resistance to emtricitabine and lamivudine.
However, now that clinical protocols will allow for greater intervals between HIV tests, it will be important to monitor rates of occult HIV acquisition in the absence of PrEP use followed by resumption of PrEP use prior to occult infection diagnosis. Data suggest that administration of PrEP with tenofovir/emtricitabine in the setting of occult primary (acute) HIV infection carries an extremely high risk of generating resistance—more than 25% in aggregate across randomized studies. For this reason, clinical exoneration of acute or primary HIV infection, or use of viral load screening prior to PrEP initiation in high-risk patients is prudent.
Onset and Offset of Effect
Modeling using pharmacokinetic data that correlate tenofovir levels in peripheral blood mononuclear cells (PBMCs) with risk reduction observed in iPrEx study participants with similar levels of tenofovir in PBMCs estimates that a 99% risk reduction is achieved after approximately 5 daily doses of tenofovir/emtricitabine and that a greater than 90% risk reduction persists up to 7 days after stopping drug from steady state.26 After 7 days, protection would be expected to drop off precipitously. These estimates are based on daily dosing and apply to rectal HIV exposures only. Vaginal exposures have not been similarly modeled, although levels thought to be protective in cervicovaginal tissues, if in fact such levels are the crucial parameter for protection, are not achieved until after approximately 3 weeks of daily dosing with tenofovir/emtricitabine.
Safety
In general, tenofovir/emtricitabine is well tolerated in uninfected individuals. Three broad categories of adverse events are notable. A gastrointestinal “start-up syndrome” was observed in up to 18% of participants in 3 of the 5 phase III PrEP trials mentioned above but was usually self-limited and did not commonly result in PrEP discontinuation. Nephrotoxicity, an expected complication based on experience with tenofovir-based antiretroviral regimens in HIV-infected populations, was observed at grade 2 or higher in only 0.2% of nearly 5500 participants (no observed cases of Fanconi syndrome) randomly assigned to receive tenofovir/emtricitabine; all cases resolved after withdrawal of tenofovir/emtricitabine. Loss of up to 1.5% of bone mineral density (BMD) was observed during PrEP with tenofovir/emtricitabine, with some reversal of the trend observed after withdrawal of the drug. The observed changes in BMD were not associated with increased fracture risk.
These data represent adverse event rates in the setting of imperfect adherence to PrEP and relatively short follow-up periods. Optimal adherence could result in a greater frequency of adverse events. Evaluation of adverse event profiles of PrEP in diverse populations, some of which may already have a disposition toward renal or bone complications, will be important.
Supporting Adherence
Given that maximal protection is provided by daily dosing of tenofovir/emtricitabine, support for adherence is an important component of PrEP services. The most common approach to adherence support has been next-step counseling, which is a brief, theory-based intervention that uses a manualized intervention derived from motivational interviewing.27 In 2014, the CDC distilled adherence support down to 3 topics for ease of clinician delivery: asking how patients have remembered to take past medications, asking if patients have had any difficulty taking their pills, and asking about what has been most helpful for reminding patients to take their medications.28
Investigational techniques to promote adherence include customized text messaging, such as a platform currently being evaluated by the California Collaborative Treatment Group (CCTG), and smart devices, such as an electronic pill case that provides an opportunity for real-time monitoring of dose taking and for interventions when doses are missed. Additional technologies, such as “smart” pill bottles that have a variety of adherence support strategies built in to them are in development.
A research group in Los Angeles, California, is currently completing enrollment of a demonstration project that will use real-time measurement of plasma tenofovir levels to support adherence among 375 MSM and transgender women at 2 community-based sites. Undetectable plasma levels will be used as a trigger for escalation of adherence support. This study will include a substudy of vitamin D and calcium supplementation in an attempt to mitigate loss of BMD.
Implementation and Scale-Up
Cost-effectiveness models of PrEP in a variety of populations emphasize that cost-effectiveness is greatest when PrEP is targeted to those with the highest risk of HIV acquisition. Buchbinder and colleagues showed that optimal targeting of PrEP—defined as targeting of populations with the lowest number needed to treat per HIV infection averted—for MSM and transgender women in the iPrEx study, was aimed toward those who reported engaging in condomless receptive anal intercourse in the past 3 months, having an STI in the past 6 months, or using cocaine in the past month.29 In the iPrEx Open-Label Extension (OLE) study, 76% of the participants opted to take open-label PrEP at some point during follow-up, and higher-risk individuals were more likely to opt to take PrEP, suggesting good intervention targeting.30 Participants whose tenofovir levels suggested adherence at an average of 4 or more doses per week had no seroconversions, with adherence again being higher in higher-risk individuals. Consistent with the comprehensive package of prevention services provided as part of the iPrEx OLE study, risk behavior decreased over time for all participants, whether or not they were taking tenofovir/emtricitabine as PrEP.
In the 3-city US PrEP Demonstration Project study, which evaluated individuals who were self-referred or referred by a practitioner for PrEP in community settings in San Francisco, California, Washington, DC, and Miami, Florida, 60% of referred individuals were interested in enrolling in the study and taking tenofovir/emtricitabine-based PrEP.31,32 Of these individuals, between 80% and 100% maintained maximally protective mean adherence levels of at least 4 doses per week over 48 weeks of observation.
With regard to PrEP uptake outside the clinical trial setting, modeling performed by Grant and colleagues at the San Francisco AIDS Foundation has shown that based on known rates of HIV diagnoses, viral suppression, serosorting behavior, and uptake and persistence of PrEP among MSM and transgender women in San Francisco, uptake of PrEP is approximately one-third of that needed to achieve a 70% reduction in annual HIV infections.33
At present, there is no consensus among practitioners and policymakers regarding the optimal setting for deploying PrEP in clinical practice. Krakower and colleagues refer to "the purview paradox" to describe the differing perspectives of HIV and primary care practitioners regarding the responsibility of PrEP prescribing.34 Primary care practitioners are more likely to encounter at-risk individuals before HIV acquisition and often have the best longitudinal relationships with patients, but they may be less comfortable with the management of antiretroviral medications and their adverse effects and with the testing necessary to evaluate acute and chronic HIV disease. There may also be competing priorities in primary, acute, and preventive care that make a complex and possibly uncomfortable discussion with patients regarding sexual practices seem impractical. HIV practitioners, on the other hand, are familiar with antiretroviral drugs and their management, but may be less likely to encounter patients before seroconversion. In addition, HIV practitioners often have overburdened practices and often see patients in locations identified and stigmatized as catering to individuals already living with HIV or AIDS.
Although the optimal setting for PrEP delivery remains to be defined, there is considerable progress in attempts to create suitable infrastructure. Local jurisdictions are developing lists of practitioners who prescribe PrEP, such as that solicited by the Commissioner of Health in New York City. Consumers and practitioners are using the Internet to crowdsource practitioner referrals, troubleshoot operational impasses, and share novel science: a moderated “PrEP Facts” Facebook page has been created to serve as a trusted mechanism for such innovation. Community-based organizations have developed informational materials for PrEP users, including tips and strategies for educating practitioners on PrEP use and monitoring. The University of California San Francisco Clinicians Consultation Center has expanded its scope from PEP and prevention of mother-to-child transmission to include PrEP, and the Mississippi Department of Public Health has debuted a PrEP information hotline. The CDC and the World Health Organization have issued formal clinical guidance for practitioners regarding PrEP.28,35 Some community-based organizations have created user-friendly materials to further streamline clinical processes for practitioners who may find formal guidelines overwhelming. New York City has developed academic detailing methods and has already detailed 900 clinical practices to educate providers, and has also developed implementation seminars to educate clinics on the operational details of providing PrEP services.
Despite evidence of cost-effectiveness when PrEP is appropriately targeted, PrEP users in the United States still struggle to piece together coverage of medication costs and associated testing and services. Private insurance will often provide coverage for PrEP in name; however, some plans carry large deductibles or very high copays. A number of resources exist to help with these problems (eg, patient assistance and copay support programs), but substantial self-efficacy and consumer ownership are still required to corral the required documentation and navigate processes. Some clinics are beginning to provide navigation assistance for PrEP use. For example, Washington state has implemented the first drug assistance program for PrEP in the United States. Although the program covers drug costs, it does not cover care, instead linking users to the Affordable Care Act and other insurance options for longitudinal care. New York state has instituted a different model, developing a care support program for PrEP but leveraging existing mechanisms to fund drug costs.
Expanding PrEP Options
With regard to therapeutic options, the field of PrEP is still in its infancy. Options being investigated include the use of maraviroc alone or in combination with the individual components of tenofovir/emtricitabine, which is currently in phase II safety evaluation in men and women. Long-acting injectable formulations would be an exciting advance in the field of PrEP. Long-acting rilpivirine, currently in phase II evaluation in the HIV Prevention Trials Network (HPTN) 076 study in women, is administered every 8 weeks, and cabotegravir, an investigational integrase strand transfer inhibitor that can be administered quarterly, is also in phase II safety evaluation in the HPTN 077 and ECLAIR studies. Monoclonal antibodies such as VRC01 have additional promise for HIV prevention and are moving into proof-of-concept trials.
Conclusion
PrEP is highly effective when taken as prescribed, and the most at-risk populations should be targeted. More data are needed regarding PrEP use in vulnerable and at-risk populations, including transgender individuals and at-risk women. Additional data and guidance on the use of PrEP for peri-contraception coverage are also needed. PrEP should be implemented in close partnership with communities. No single intervention is likely to end the HIV epidemic, and PrEP scale-up is a global health imperative as part of combined prevention efforts.
The 1930s saw a burgeoning syphilis epidemic, fueled by public stigma surrounding syphilis testing and treatment and by concerns that syphilis treatment would increase sexual risk behaviors. The Works Progress Administration commissioned a “living newspaper,” a public service message produced as a work of theater called Spirochete, the aim of which was to draw attention to and normalize the need for routine syphilis testing and treatment. As hormonal treatments for amenorrhea and dysmenorrhea became used for family planning in the 1950s, such was the stigma surrounding individuals seeking hormonal contraception that the New York Times only acknowledged its FDA approval with a 136-word article buried on page 75 of the May 10, 1960, issue, which included a caveat on the morality of such an indication for use.
Practitioners should not be on the wrong side of history by allowing individuals to be stigmatized by seeking to use PrEP to avoid HIV infection and its attendant lifelong treatment. Placing control in the hands of uninfected individuals is a crucial advance, the power of which should not be overestimated.
References
References
- 1. Joint United Nations Programme on HIV/AIDS (UNAIDS). The gap report. http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Gap_report_en.pdf. Accessed on May 4, 2015.
- 2. US Food and Drug Administration. FDA approves first drug for reducing the risk of sexually acquired HIV infection. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm312210.htm. Accessed on April 27, 2015.
- 3.Garcia-Lerma JG, Otten RA, Qari SH, et al. Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir. PLoS Med. 2008;5(2):e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castillo-Mancilla JR, Meditz A, Wilson C, et al. Reduced immune activation during tenofovir-emtricitabine therapy in HIV-negative individuals. J Acquir Immune Defic Syndr. 2015;68(5):495;501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patterson KB, Prince HA, Kraft E, et al. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med. 2011;3(112):112re4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Lerma JG, Cong M, Mitchell J, et al. Intermittent prophylaxis with oral Truvada protects macaques from rectal SHIV infection. Sci Transl Med. 2010;2(14):1;8. [DOI] [PubMed] [Google Scholar]
- 7.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587;2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grant RM, Lama J, Glidden D, iPrEx Study Team. Pre-exposure chemprophylaxis for prevention of HIV among trans-women and MSM: iPrEx Study [Abstract 92]. 18th Conference on Retroviruses and Opportunistic Infections (CROI) February 27-March 3, 2011; Boston, Massachusetts. [Google Scholar]
- 9. Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367(5):411;422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367(5): 423;434. [DOI] [PubMed] [Google Scholar]
- 11.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399;410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372(6): 509;518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4(151):151ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cottrell ML, Yang KH, Prince HMA, et al. Predicting effective truvada® PrEP dosing strategies with a novel PK-PD model incorporating tissue active metabolites and endogenous nucleotides (EN). AIDS Res Hum Retroviruses. 2014;30(S1):A60. [Google Scholar]
- 15.Donnell D, Baeten JM, Bumpus NN, et al. HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. J Acquir Immune Defic Syndr. 2014;66(3): 340;348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbas UL, Anderson RM, Mellors JW. Potential impact of antiretroviral chemoprophylaxis on HIV-1 transmission in resource-limited settings. PLoS One. 2007; 2(9):e875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stolte IG, Dukers NH, Geskus RB, Coutinho RA, de Wit JB. Homosexual men change to risky sex when perceiving less threat of HIV/AIDS since availability of highly active antiretroviral therapy: a longitudinal study. AIDS. 2004;18(2):303;309. [DOI] [PubMed] [Google Scholar]
- 18.Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369:657-666. [DOI] [PubMed] [Google Scholar]
- 19.Bartholow BN, Buchbinder S, Celum C, et al. HIV sexual risk behavior over 36 months of follow-up in the world's first HIV vaccine efficacy trial. J Acquir Immune Defic Syndr. 2005;39(1):90;101. [DOI] [PubMed] [Google Scholar]
- 20.Mugwanya KK, Donnell D, Celum C, et al. Sexual behaviour of heterosexual men and women receiving antiretroviral preexposure prophylaxis for HIV prevention: a longitudinal analysis. Lancet Infect Dis. 2013;13(12):1021;1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Supervie V, Garcia-Lerma JG, Heneine W, Blower S. HIV, transmitted drug resistance, and the paradox of preexposure prophylaxis. Proc Natl Acad Sci USA. 2010; 107(27):12381;12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van de Vijver DA, Nichols BE, Abbas UL, et al. Preexposure prophylaxis will have a limited impact on HIV-1 drug resistance in sub-Saharan Africa: a comparison of mathematical models. AIDS. 2013;27(18):2943;2951. [DOI] [PubMed] [Google Scholar]
- 23.Liegler T, Abdel-Mohsen M, Bentley LG, et al. HIV-1 drug resistance in the iPrEx preexposure prophylaxis trial. J Infect Dis. 2014;210(8):1217;1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehman DA, Baeten JM, McCoy CO, et al. Risk of drug resistance among persons acquiring HIV within a randomized clinical trial of single- or dual-agent preexposure prophylaxis. J Infect Dis. 2015;[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grant RM, Liegler T, Defechereux P, et al. Drug resistance and plasma viral RNA level after ineffective use of oral preexposure prophylaxis in women. AIDS. 2015;29(3):331;337. [DOI] [PubMed] [Google Scholar]
- 26.Seifert SM, Glidden DV, Meditz AL, et al. Dose response for starting and stopping HIV preexposure prophylaxis for men who have sex with men. Clin Infect Dis. 2015;60(5):804;810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amico KR, Mansoor LE, Corneli A, Torjesen K, Van Der Straten A. Adherence support approaches in biomedical HIV prevention trials: experiences, insights and future directions from four multisite prevention trials. AIDS Behav. 2013;17(6):2143;2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Centers for Disease Control and Prevention (CDC). Preexposure prophylaxis for the prevention of HIV infection in the United States - 2014: a clinical practice guideline. http://www.cdc.gov/hiv/pdf/guidelines/PrEPguidelines2014.pdf. Accessed on May 6, 2015.
- 29.Buchbinder SP, Glidden DV, Liu AY, et al. HIV pre-exposure prophylaxis in men who have sex with men and transgender women: a secondary analysis of a phase 3 randomised controlled efficacy trial. Lancet Infect Dis. 2014;14(6):468;475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grant RM, Anderson PL, McMahan V, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14(9):820;829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen S, Vittinghoff E, Anderson P, et al. Implementation of PrEP in STD and community health clinics: high uptake and drug concentrations among MSM in the Demo Project [Abstract 377]. 9th International Conference on HIV Treatment and Prevention Adherence June 8-10, 2014; Miami, Florida. [Google Scholar]
- 32.Cohen SE, Vittinghoff E, Bacon O, et al. High interest in preexposure prophylaxis among men who have sex with men at risk for HIV infection: baseline data from the US PrEP Demonstration Project. J Acquir Immune Defic Syndr. 2015;68(4): 439;448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grant RM, Liu A, Hecht J et al. Scale-up of preexposure prophylaxis in San Francisco to impact HIV incidence [Abstract 25]. 22nd Conference on Retroviruses and Opportunistic Infections (CROI) February 23-26, 2015; Seattle, Washington. [Google Scholar]
- 34.Krakower D, Ware N, Mitty JA, Maloney K, Mayer KH. HIV providers' perceived barriers and facilitators to implementing pre-exposure prophylaxis in care settings: a qualitative study. AIDS Behav. 2014;18(9):1712;1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. World Health Organization. Guidance on oral pre-exposure prophylaxis (PrEP) for serodiscordant couples, men and transgender women who have sex with men at high risk of HIV: recommendations for use in the context of demonstration projects. http://www.who.int/hiv/pub/guidance_prep/en/. Accessed on May 29, 2015. [PubMed]
- 36. AIDS Vaccine Advocacy Coalition (AVAC). AVAC report 2013: research and reality. http://www.avac.org/sites/default/files/resource-files/AVAC%20Report%202013_0.pdf. Accessed on June 18, 2015.


