Abstract
Prolonged survival in HIV infection is accompanied by an increased frequency of non-HIV-related comorbidities. A number of age-related comorbidities occur earlier in HIV-infected patients than in individuals without HIV infection. This ”accelerated aging” appears to be largely related to chronic inflammation, chronic immune activation, and immunosenescence in HIV infection. Levels of markers of inflammation and coagulopathy are elevated in HIV-infected patients, and elevations in markers such as high-sensitivity C-reactive protein, D-dimer, and interleukin 6 (IL-6) have been associated with increased risk for cardiovascular disease, opportunistic conditions, or all-cause mortality. In both HIV infection and aging, immunosenescence is marked by an increased proportion of CD28-, CD57+ memory CD8+ T cells with reduced capacity to produce interleukin 2 (IL-2), increased production of IL-6, resistance to apoptosis, and shortened telomeres. A number of AIDS Clinical Trials Group studies are under way to examine treatment aimed at reducing chronic inflammation and immune activation in HIV infection. This article summarizes a presentation by Judith A. Aberg, MD, at the IAS–USA live continuing medical education course held in New York City in October 2011.
Patients with HIV infection have had a dramatic increase in life expectancy with the use of potent antiretroviral therapy. However, life expectancy for many patients—particularly those with low CD4+ cell counts and those on salvage regimens—is still shorter than that for the general population. Among the data showing increased life expectancy in HIV infection are the findings in the ATHENA (AIDS Therapy Evaluation in the Netherlands) cohort (N = 4174) indicating that HIV-infected persons who are asymptomatic on antiretroviral therapy have an estimated life expectancy nearly identical to that of the general population. This model suggested that HIV-infected patients aged 25 years and asymptomatic at 24 weeks after starting antiretroviral therapy had an estimated 52.7 life-years remaining, compared with 53.1 life-years for 25-year-old persons in the general population.1 The modeled life expectancies of HIV-infected patients presenting for care at older ages and HIV-infected women were somewhat lower than those in the general population.
Increased survival in HIV-infected persons has been accompanied by an increase in comorbidities compared with the general population, although the precise prevalence and distribution of such comorbidities have yet to be defined. HIV treatment guidelines issued in 2010 generally recommended that patients with HIV infection start antiretroviral therapy at CD4+ cell counts of 500/μL, and that those with certain comorbid conditions begin therapy regardless of CD4+ count.2,* Although there is no threshold above which initiation of therapy is contraindicated, it is not yet known if initiating antiretroviral therapy earlier reduces (or increases, eg, via chronic toxicity from treatment) the frequency of comorbidities. Patients starting antiretroviral therapy at higher CD4+ cell counts do have persistent recovery of CD4+ counts, suggesting better preservation of immune function.
A study in the HOPS (HIV Outpatient Study) cohort of 1378 patients observed from 1996 to 2007 showed that median CD4+ cell count peaks were progressively higher (P < .001) in patients with higher CD4+ cell counts at treatment initiation (Figure 1).4 Multivariate analysis showed that compared with patients with initial CD4+ cell counts of 350/μL or above, mortality was 4.6 times more likely in patients with initial CD4+ cell counts below 50/μL and 2.6 times more likely in those with initial counts of 50/μL to 199/μL. A lower CD4+ cell count at initiation of treatment was also associated with increased risk of mortality from non–AIDS-related causes, suggesting that earlier initiation of therapy and preservation of CD4+ cell count might influence the frequency and nature of comorbidities in HIV infection.
Figure 1.
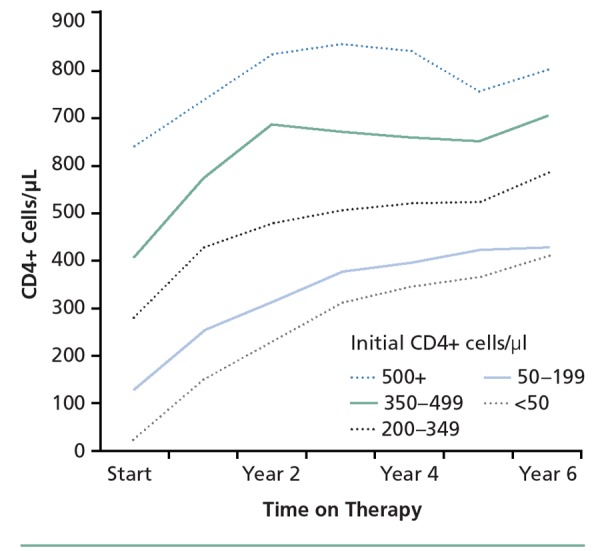
Median CD4+ cell count during first 6 years of antiretroviral therapy according to initial CD4+ cell count category. Adapted from Palella et al.4
A number of age-related morbidities occur at earlier ages in individuals with HIV infection than in the general population. As discussed further below, this phenomenon of “accelerated aging” may reflect chronic inflammation in HIV infection. Given the observation of earlier onset of age-related morbidities in HIV infection, it is questionable whether primary care practice guidelines for the general population are applicable to the HIV-infected population. For example, there is some evidence that HIV-infected patients develop colorectal cancer precursor lesions and more advanced disease at an earlier age than do persons in the general population,5 raising the question of whether screening for colorectal cancer should begin earlier (eg, at age 40 years rather than 50 years) in HIV-infected patients. It remains to be determined whether primary care guidelines specific to the HIV-infected population might reduce the frequency of comorbidities in this population.
Aging and HIV Infection
Considerable attention has been given to the idea that patients with HIV infection age more rapidly than HIV-uninfected individuals, as suggested by the occurrence of age-related comorbidities at earlier ages in those with HIV infection. This phenomenon appears to reflect, in large part, the increased risk for a number of disease states stemming from chronic inflammation and immunosenescence, both of which occur in HIV infection.
Studies in women with systemic lupus erythematosus have shown that they have a dramatically increased risk of myocardial infarction (MI) compared with age-matched controls in the general population. Yet few would say that patients with lupus are at increased risk for MI because they are aging faster. Instead, it can be said that patients with lupus have a chronic inflammatory disease that puts them at risk for comorbidities. Patients with HIV infection have begun to think of themselves as aging faster—it may be better if practitioners, when confronted with this message, explain that HIV infection is a chronic inflammatory condition that may be associated with comorbidities. Patients should be informed that there appears to be a higher prevalence of comorbidities at all ages among persons with HIV infection. Treatment of lupus reduces inflammation, as demonstrated by observation of validated biomarkers of inflammation, and reduces risk for cardiovascular disease. It is hoped that treatment of HIV infection will have similar results.
To emphasize the distinction between chronic inflammation and aging, age-related hearing loss (a hallmark of aging) can be considered.6 Hearing impairment was recently assessed in MACS (Multicenter AIDS Cohort Study) and WIH (Women’s Interagency HIV Study), involving 334 men with a median age of 54 years, 46% of whom had HIV infection, and 178 women with a median age of 45 years, 77% of whom had HIV infection. Testing for hearing impairment via distortionproduct otoacoustic emissions showed that risk factors for impairment were a 10-year increase in age, male sex, and nonblack race. HIV infection was not a risk factor for hearing loss, and neither were antiretroviral therapy, nadir CD4+ cell count, nor HIV RNA level. In fact, greater hearing loss was detected in persons without HIV infection.
Comorbidities in HIV Infection: Inflammation and “Inflamm-Aging”
Chronic adverse effects of antiretroviral therapy, HIV infection itself, traditional risk factors, or a combination of all of these contribute to increased risk of coronary heart disease in HIV-infected patients, as well as increased frequency of a number of metabolic abnormalities and other comorbidities. Osteoporosis and hypogonadism occur at an earlier age in HIV-infected persons. There is no reason to expect that antiretroviral therapy provides protection from development of malignancies at the ages at which they are seen in the general population, including esophageal, lung, rectal (human papilloma virus–related), renal, and liver cancers. In fact, lung cancer is more prevalent in HIV-infected persons than in the general population after adjustment for smoking, with any potential interaction between HIV and this malignancy remaining undefined.7 As noted previously, colorectal cancer precursor lesions and more advanced disease are more prevalent at a younger age in HIV-infected individuals.
It is clear that the vast majority of deaths in HIV-infected patients in developed countries are currently not caused by AIDS-defining illnesses. In a study reported in 2008 that examined mortality in patients from 3 randomized HIV clinical trials, AIDS-defining illnesses accounted for only 10% of mortality. Non–AIDS-defining malignancies accounted for 21% of mortality and unknown causes accounted for 18%; other specific causes, including cardiovascular disease, liver disease (excluding malignancy), non–AIDS-defining infection, suicide, trauma-related or accidental causes, and drug overdose or acute intoxication, each accounted for 9% or less of mortality.8
There are also data to indicate that, compared with the general population, HIV-infected patients have greater rates of mortality per given level of comorbidity as classified by the Charlson Comorbidity Index (CCI). The CCI is a scale that was initially developed for use in longitudinal studies to classify comorbid conditions that alter the risk for 1-year mortality after hospitalization, with a greater number of points indicating greater severity of the condition. For example, an MI is assigned a value of 1 point, whereas diabetes with end-organ damage is 2 points. Lymphoma is 2 points whereas a metastatic solid tumor is 6 points. In a recently reported study, Lohse and colleagues adapted the CCI to determine if survival from time of HIV diagnosis in HIV-infected persons differed from that in age- and sex-matched HIV-uninfected persons with identical CCI scores.9 Survival was statistically significantly reduced among HIV-infected persons with CCI scores of 0, 1, or 2 at diagnosis, suggesting that the impact of preexisting conditions may be worsened in HIV infection (Figure 2).
Figure 2.
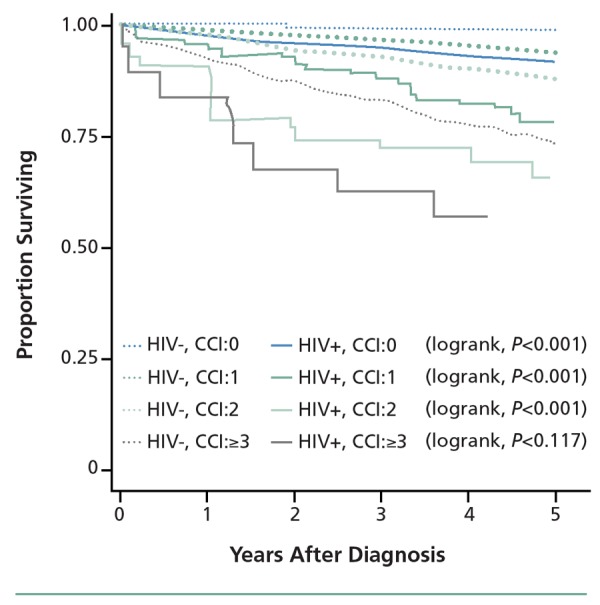
Survival after HIV diagnosis in HIV-infected patients (HIV+) and matched HIV-uninfected controls (HIV-) according to Charlson Comorbidity Index (CCI) score at time of diagnosis. Adapted from Lohse et al.9
Role of Inflammation and Immunosenescence
Inflammation can be described as part of the body’s complex biological response to harmful stimuli such as pathogens and cell damage. Franceschi and colleagues coined the term “inflamm-aging” to describe the interplay between inflammation and aging, mediated by proinflammatory cytokines and other proinflammatory and procoagulant factors, in such conditions as atherosclerosis, Alzheimer’s disease, type 2 diabetes, osteoporosis, arthritis, cerebrovascular disease, chronic pulmonary disease, and thromboembolic disorders.10,11
HIV infection does indeed share numerous clinical similarities with aging, including an increased incidence of cardiovascular disease, malignancy, infection, chronic viral reactivation, sarcopenia and osteopenia, neurocognitive decline, and frailty. HIV infection results in T-cell activation and immunosenescence, which is characteristic of aging. In both HIV infection and aging, immunosenescence is marked by an increased proportion of CD28-, CD57+ memory CD8+ T cells with reduced capacity to produce interleukin 2 (IL-2), increased production of interleukin 6 (IL-6), resistance to apoptosis, and shortened telomeres. Up to half of peripheral CD8+ T cells are activated in HIV-infected patients; less than 10% are activated in healthy persons without HIV infection. One study of HIV-infected persons with a median age of 56 years and good immune reconstitution and viral suppression showed that they had T-cell characteristics similar to those of a group of HIV-uninfected subjects with a median age of 88 years.12
A very similar phenomenon of “inflamm-aging” has been observed in persons with cytomegalovirus (CMV) infection. CMV-seropositive persons older than 65 years have a much greater expansion of CD28- cells than age-matched CMV-seronegative control subjects, with many of these cells representing the oligoclonal expansion of CMV-specific T cells. Although the clinical significance of these findings remains unclear, it has been observed that older CMV-seropositive persons are less likely to respond to vaccines than age-matched CMV-seronegative persons,13 and that CMV-associated immune system changes are predictive of early mortality among older persons.14
Several studies have shown increased levels of biomarkers of inflammation in HIV-infected patients and an association of these elevated levels with increased risk for poorer outcomes and all-cause mortality. A case-control study in the SMART (Strategies for Management of Antiretroviral Therapy) trial population measured levels of the biomarkers high-sensitivity C-reactive protein (hs-CRP), IL-6, and D-dimer in patients with an opportunistic infection (OI) event. Levels of these markers were statistically significantly increased at the last measurement before OI onset, compared with levels at matched time points in control patients without an opportunistic infection.15 Median CD4+ cell count was statistically significantly lower and plasma HIV RNA level was statistically significantly higher among patients with an OI event. The association of OI occurrence with elevated hs-CRP and IL-6 remained statistically significant on multivariate analysis, indicating an effect independent of low CD4+ cell count and elevated viral load.
Another study compared levels of inflammatory markers in patients in the SMART trial with levels in a longterm cohort of non–HIV-infected subjects without coronary heart disease who were being observed for atherosclerosis (MESA, Multi-Ethnic Study for Atherosclerosis). The study showed that after adjustment for age, sex, race, and factors such as dyslipidemia and smoking (which were more prevalent among HIV-infected patients), HIV-infected patients exhibited an approximately 50% higher hs-CRP level, 150% higher IL-6 level, 90% higher D-dimer level, and 25% higher cystatin-C level.16
Another nested case-control study in the SMART population examined the association of inflammatory markers with all-cause mortality, with adjustment for numerous potential cofactors including age, race, antiretroviral therapy, HIV RNA level, CD4+ cell count, body mass index, total and high-density lipoprotein (HDL) cholesterol, smoking, diabetes, hepatitis B virus (HBV) and hepatitis C virus (HCV) coinfection, and use of lipid-lowering and antihypertension medication. On this analysis, the adjusted odds ratios (ORs) for mortality when comparing the highest quartile of values for each biomarker with the lowest quartile were 3.1 for hs-CRP (P = .02), 3.1 for amyloid A (P = .05), 12.4 for IL-6 (P<.0001), and 41.2 for D-dimer (P < .0001).17 The SMART trial included a group receiving continuous antiretroviral therapy and a treatment interruption group. Among patients in the treatment interruption group who had plasma HIV RNA levels of 400 copies/mL or below at the time of antiretroviral therapy interruption, D-dimer levels increased by 27% (P<.001). Among patients not receiving antiretroviral therapy at study entry, initiation of therapy was associated with a 22% decrease in D-dimer level (P < .001).
A case-control study in the FIRST (Flexible Initial Retroviral Suppressive Therapies) trial population of antiretroviral therapy–naive patients examined the association between pretreatment biomarker levels and risk for AIDS or death. Pretreatment patients who developed AIDS or who died had statistically significantly elevated median levels of D-dimer (OR, 2.4; P < .01), CRP (OR, 2.1; P < .01), IL-6 (OR, 1.8; P = .01), IL-10 (OR, 1.5; P = .02), and hyaluronate (OR, 1.7; P < .01), with an increase in IL-8 approaching statistical significance (OR, 1.5; P = .08). Pretreatment interferon gamma (γ) and tumor necrosis factor alpha (TNF-α) levels were not statistically significantly increased in case patients.18
A summary of the relationships between inflammatory and coagulopathy markers and adverse outcomes in randomized controlled trials in HIV-infected subjects indicates that in addition to being associated with mortality, elevated D-dimer is associated with increased risk of cardiovascular disease; hs-CRP and IL-6 are associated with cardiovascular disease and opportunistic disease, and soluble CD14 (sCD14) is associated with microbial translocation.19 Antiretroviral therapy has been found to reduce D-dimer levels and may reduce IL-6 levels, but has not been found to reduce hs-CRP levels, and its effect on sCD14 is unknown. It has been observed that although antiretroviral therapy reduces levels of some biomarkers, these levels still remain elevated compared with levels in HIV-uninfected persons.
Studies of Modulation of Immune Activation and Inflammation in HIV Infection
Although we are beginning to identify inflammation markers that appear to be associated with risk of poor outcome in HIV infection and are observing changes in some of these markers with antiretroviral therapy, it is still unclear how patients should be treated on the basis of this information or whether these markers should be used to assess potential risk reduction. Studies examining these issues are under way.
ACTG (AIDS Clinical Trial Group) A5275 is evaluating modulation of immune activation with statin therapy (atorvastatin) in HIV-infected patients without hyperlipidemia.20 In addition to lowering low-density lipoprotein (LDL) cholesterol, the blocking of HMG-CoA reductase by statins reduces activation of guanosine triphosphate (GTP)-binding proteins (Ras and Rho) that act as molecular “switches” and regulate transcription of inflammatory response genes. Statins have been shown to reduce CD8+ T cell activation and reduce expression of IL-6, hs-CRP, D-dimer, sCD14, and TNF-α, with reductions in these biomarkers observed in numerous other settings including sepsis, pneumonia, influenza, chronic obstructive pulmonary disease, hepatocellular carcinoma, and cardiovascular disease.
In the JUPITER (Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin) study,21,22 treatment with rosuvastatin statistically significantly reduced mortality and risk of venous thrombotic disease in apparently healthy subjects with elevated hs-CRP (> 2 mg/dL) and “normal” LDL cholesterol (< 130 mg/dL). It should be noted that the protective effects of statin therapy in this trial were only weakly correlated with the reductions in hs-CRP and LDL cholesterol, leaving it unclear precisely how statins are producing such benefits in this setting.
In addition to ACTG A5275, a number of other trials are examining immune activation, immunosenescence, and inflammation in HIV infection. Effects of rifamixin and sevelamer (which binds lipopolysaccharide) on microbial translocation are being examined, as are anticytokine or immunomodulatory effects of chloroquine, vitamin D, statin therapy, and HDL cholesterol–raising therapy with niacin or fibrates. Other potential strategies that warrant investigation include attempts to reduce inflammatory effects on end organs with such agents as aspirin, methotrexate, colchicine, fish oil, or a polypill regimen including a statin and low-dose warfarin or some combination of antiinflammatories.
Summary
In summary, although cohort studies demonstrate that patients who are virologically suppressed on antiretroviral therapy are still at greater risk of developing comorbidities at all ages, the precise mechanism has not been identified. Immune activation in HIV-infected patients suppressed on antiretroviral therapy has been attributed to endotoxemia resulting from a compromise in immunity caused by destruction of T cells in gut-associated lymphoid tissue and by residual HIV viremia. Lichtfuss and colleagues have also shown that natural killer cells remain activated in virologically suppressed patients on antiretroviral therapy and that defective natural killer cell antibody-dependent cell-mediated cytotoxity signaling is not restored by therapy, therefore contributing to such risk.23 However, studies that clearly demonstrate a cause and effect that the latent virus is responsible for this increased risk of comorbidities are not available nor are there data to confirm that targeting inflammation and reducing markers of immune activation will, in fact, reduce this risk.
References
- 1.van Sighem AI, Gras LA, Reiss P, Brinkman K, De Wolf F, ATHENA Cohort Study Group. Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals. AIDS. 2010;24(10):1527-1535. [DOI] [PubMed] [Google Scholar]
- 2.Thompson MA, Aberg JA, Cahn P, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA. 2010;304(3):321-333. [DOI] [PubMed] [Google Scholar]
- 3.Thompson MS, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA. 2012;308(4):387-402. [DOI] [PubMed] [Google Scholar]
- 4.Palella F, Armon C, Buchacz K, et al. CD4 at HAART initiation predicts long-term CD4 responses and mortality from AIDS and non-AIDS causes in the HIV outpatients study. [Abstract 983.] 17th Conference on Retroviruses and Opportunistic Infections (CROI). February 16-19, 2010; San Francisco, CA. [Google Scholar]
- 5.Bini EJ, Green B, Poles MA. Screening colonoscopy for the detection of neoplastic lesions in asymptomatic HIV-infected subjects. Gut. 2009;58(8):1129-1134. [DOI] [PubMed] [Google Scholar]
- 6.Torre III P, Hoffman H, Springer G, et al. Cochlear function among Multicenter AIDS Cohort Study (MACS) and Women's Interagency HIV Study (WIHS) participants. [Abstract TUPE138.] 6th IAS Conference on HIV Pathogenesis, Treatment and Prevention. July 17-20, 2011; Rome, Italy. [Google Scholar]
- 7.Kirk GD, Merlo C, O’Driscoll P, et al. HIV infection is associated with an increased risk for lung cancer, independent of smoking. Clin Infect Dis. 2007;45(1):103-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lifson AR, Belloso WH, Carey C, et al. Determination of the underlying cause of death in three multicenter international HIV clinical trials. HIV Clin Trials. 2008;9(3):177-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lohse N, Gerstoft J, Kronborg G, et al. Comorbidity acquired before HIV diagnosis and mortality in persons infected and uninfected with HIV: a Danish population-based cohort study. JAIDS. 2011;57(4):334-339. [DOI] [PubMed] [Google Scholar]
- 10.Franceschi C, Bonafè M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244-254. [DOI] [PubMed] [Google Scholar]
- 11.De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflammation markers predicting frailty and mortality in the elderly. Exp Mol Pathol. 2006;80(3):219-227. [DOI] [PubMed] [Google Scholar]
- 12.Desai S, Ronquillo R, Usuga X, et al. Immune senescence, activation, and abnormal T cell homeostasis despite effective HAART, a hallmark of early aging in HIV disease. [Abstract 381.] 16th Conference on Retroviruses and Opportunistic Infections (CROI). February 8-11, 2009; Montreal, Canada. [Google Scholar]
- 13.Trzonkowski P, Mysliwska J, Szmit E, et al. Association between cytomegalovirus infection, enhanced proinflammatory response and low level of anti-hemagglutinins during the anti-influenza vaccination—an impact of immunosenescence. Vaccine. 2003;21(25-26):3826-3836. [DOI] [PubMed] [Google Scholar]
- 14.Hadrup SR, Strindhall J, Kollgaard T, et al. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J Immunol. 2006;176(4):2645-2653. [DOI] [PubMed] [Google Scholar]
- 15.Rodger AJ, Fox Z, Lundgren JD, et al. Activation and coagulation biomarkers are independent predictors of the development of opportunistic disease in patients with HIV infection. J Infect Dis. 2009;200(6):973-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neuhaus J, Jacobs DR, Jr., Baker JV, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201(12):1788-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5(10):e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boulware DR, Hullsiek KH, Puronen CE, et al. Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J Infect Dis. 2011;203(11):1637-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nixon DE, Landay AL. Biomarkers of immune dysfunction in HIV. Curr Opin HIV AIDS. 2010;5(6):498-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.AIDS Clinical Trials Group Network. Trials open to enrollment. https://actgnetwork.org/trials_open_enrollment. Accessed June 14, 2012.
- 21.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195-2207. [DOI] [PubMed] [Google Scholar]
- 22.Ridker PM, Danielson E, Fonseca FA, et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009;373(9670):1175-1182. [DOI] [PubMed] [Google Scholar]
- 23.Lichtfuss GF, Cheng WJ, Farsakoglu, et al. Virologically suppressed HIV patients show activation of NK cells and persistent innate immune activation. J Immunol. 2012; 189(3):1491-9. [DOI] [PubMed] [Google Scholar]


