Abstract
For the purposes of vaccination, persons with asymptomatic HIV infection and CD4+ cell counts of 200/μL to 500/μL are considered to have limited immune deficits and are generally candidates for immunization. HIV-infected persons with CD4+ cell counts less than 200/μL or history of an AIDS-defining illness should not receive live-attenuated viral or bacterial vaccines because of the risk of serious systemic disease and suboptimal response to vaccination. Available data indicate that immunization during antiretroviral therapy restores vaccine immunogenicity, improves the rate and persistence of immune responses, and reduces risk of vaccine-related adverse events, although vaccine responses often are suboptimal. Major issues for travelers to the developing world are vaccine-preventable illnesses (hepatitis A virus, yellow fever, and typhoid fever), traveler’s diarrhea, and malaria. This article summarizes a presentation by D. Scott Smith, MD, at the IAS–USA continuing medical education program held in San Francisco in April 2012.
An estimated 8% of travelers to the developing world require medical treatment during or after travel.1 Major disease risks include vaccine-preventable diseases (hepatitis A virus, yellow fever, and typhoid fever) as well as diarrheal illness and malaria. The Centers for Disease Control and Prevention (CDC) Yellow Book 2012 provides a review of considerations for vaccination of immunocompromised travelers.2 Following is a summary of these considerations in HIV-infected individuals.
Travelers With Limited Immunodeficiency
For the purposes of vaccination, persons with asymptomatic HIV infection and CD4+ cell counts of 200/μL to 500/μL are considered to have limited immune deficits. Most vaccines can elicit seroprotective levels of antibody in most HIV-infected patients in this category, although seroconversion rates and geometric mean titers of antibody in response to vaccines may be lower in HIV-infected individuals than in healthy people. Current CD4+ cell counts (increased by antiretroviral therapy), rather than their historical nadir counts, should be used to categorize immunologic status. In patients with CD4+ cell counts of 200μL to 500μL, the exact time at which reconstituted lymphocytes are fully functional is not well defined. To achieve a maximal vaccine response with minimal risk, many clinicians thus advise delaying immunization until 3 months after immune reconstitution, if urgency is not indicated.2 Transient increases in HIV RNA levels, which return quickly to baseline, have been observed after administration of several different vaccines in HIV-infected people. Although the clinical significance of such increases is not known, these increases do not preclude the use of any vaccine.
Travelers With Severe Immunodeficiency
HIV-infected persons with CD4+ cell counts less than 200/μL or history of an AIDS-defining illness should not receive live-attenuated viral or bacterial vaccines because of the risk that the vaccine could cause serious systemic disease.2 In addition, response to inactivated vaccines is suboptimal in these individuals. Thus, HIV-infected persons who have been immunized while CD4+ cell counts were less than 200/μL should be revaccinated at least 3 months after immune reconstitution with undetectable HIV RNA on antiretroviral therapy. Newly diagnosed, treatment-naive patients with CD4+ cell counts less than 200/μL should delay travel until CD4+ counts have been reconstituted with antiretroviral therapy. This delay will minimize risk of infection and avoid immune reconstitution illness during travel. Household contacts of severely immunocompromised patients may be given live-virus vaccines, such as yellow fever, measles-mumps-rubella, or varicella vaccines, but should not be given the live-attenuated influenza vaccine.2
There are few controlled studies on the effectiveness of vaccination in patients taking effective antiretroviral therapy. The available data indicate that antiretroviral treatment restores immune responsiveness to vaccines, improves the rate and persistence of immune responses, and reduces risk of vaccine-related adverse events. Despite effective antiretroviral therapy, vaccine responses in HIV-infected people often are suboptimal compared with response in HIV-seronegative individuals, although responses improve with higher and more frequent vaccine doses.
Vaccination for Hepatitis A Virus, Yellow Fever, and Typhoid Fever
Hepatitis A Virus
The CDC Advisory Committee on Immunization Practices has not made this an official recommendation, but it is generally agreed that HIV-infected persons should receive hepatitis A vaccination if their titers are negative, regardless of whether they are traveling. No revaccination is necessary.3
Yellow Fever
The mosquito that carries the organism that causes yellow fever, Aedes aegypti, is an aggressive daytime biter (unlike the malaria-transmitting Anopheles mosquito, which feeds at night) that can also transmit dengue and chikungunya viruses. The CDC’s Yellow Book2,4 gets its name in recognition of the health impact of yellow fever and its vaccine. The World Health Organization estimates that there are some 200,000 cases of yellow fever annually worldwide and 30,000 related deaths. Yellow fever is endemic in areas of South America (13% of reported cases) and Africa (87% of reported cases). Areas of South America and Africa where yellow fever vaccine is recommended are shown in Figure 1.
Figure 1.
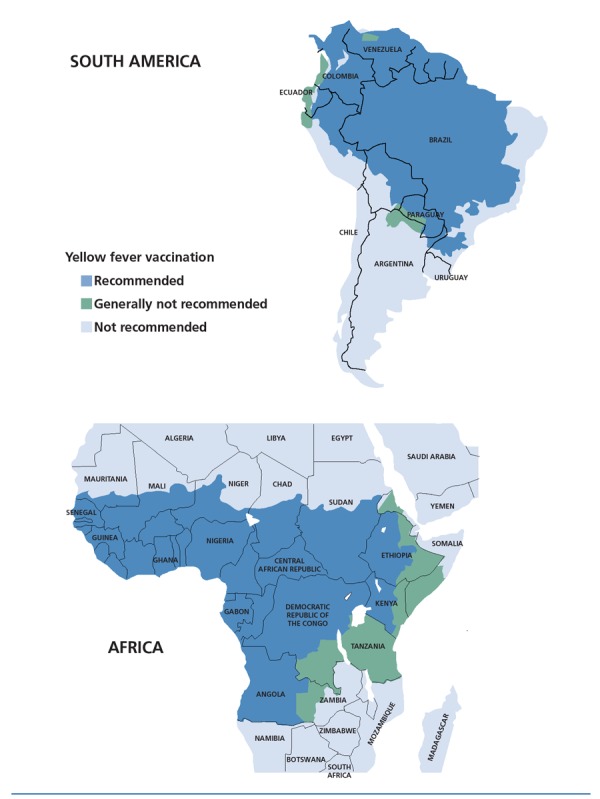
Areas in South America (top) and Africa (bottom) where yellow fever vaccination is recommended (blue), generally not recommended (green), and not recommended (light blue). Adapted from the Centers for Disease Control and Prevention Yellow Book.4
The yellow fever vaccine is a live-attenuated virus vaccine and thus is associated with risk of infectious complications in immunocompromised individuals. Major complications of yellow fever vaccination are vaccine-associated viscerotropic disease and vaccine-associated neurologic disease. These complications are reported to occur at rates of 0.4 and 0.8 cases per 100,000 doses distributed, although it is likely that cases are underreported.
Travelers with severe immune compromise, including those with symptomatic HIV infection and AIDS, should be strongly discouraged from travel to destinations that present a true risk for yellow fever. If travel to an area where yellow fever vaccine is recommended is unavoidable, these travelers should be carefully instructed in methods to avoid mosquito bites and be provided with a vaccination medical waiver.
Persons with limited immune deficits or asymptomatic HIV infection traveling to areas where yellow fever is endemic may be offered the vaccine and monitored closely for possible adverse effects. Since vaccine response may be suboptimal, persons receiving the vaccine are candidates for serologic testing 1 month after vaccination. Data from clinical and epidemiologic studies are insufficient at this time to evaluate the actual risk of severe adverse effects associated with yellow fever vaccine among recipients with limited immune deficits.
A recently reported study in 364 patients with HIV infection showed antibody response to the yellow fever vaccine in 93% of patients after a mean duration of 8.4 years after vaccination.5 The key determinant of antibody response was HIV RNA level at the time of vaccination; lower neutralizing antibody titers were associated with shorter duration of undetectable HIV RNA and higher HIV RNA level at immunization, with no correlation observed between CD4+ cell count and antibody response. The authors concluded that the key determinant of antibody response was the HIV replication status at immunization. No association was found between antibody response and CD4+ cell count. The CDC recommendation about yellow fever vaccination for only those with CD4+ cell counts greater than 200μ/L remains for now.
Vaccination “by pen” may be in order for some HIV-infected travelers. If international travel requirements— and not true exposure risk—are the only reasons to vaccinate a traveler with asymptomatic HIV infection or a limited immune deficit, the physician should provide a waiver letter. The exemption letter, signed by the physician, simply states “Yellow fever vaccine for ‘NAME’ is medically contraindicated because of the following condition: [age, pregnancy, immunocompromised status].” However, international health regulations do not allow an exemption from yellow fever vaccination for travel to a country that has a vaccination requirement for entry, even for medical reasons. Thus, travelers should be warned that vaccination waiver documents may not be accepted by some countries. If the waiver is rejected, the option of deportation might be preferable to receipt of vaccine at the destination. For countries that require vaccination for entry, travelers must have proof that the vaccine was administered at least 10 days prior to entry.
Typhoid Fever
Typhoid fever is most commonly caused by the gram-negative bacterium Salmonella typhi. It has an incubation period of 1 to 3 weeks, and its clinical presentation is characterized by high fever (with gradual increase), headache, fatigue, anorexia, dizziness, abdominal pain, nausea, and constipation or diarrhea. Diarrhea may become hemorrhagic or dysenteric. Transmission occurs through person-to-person contact or through contaminated food, drink, or water. Humans are the sole reservoir hosts. Typhoid can be contracted even when care is taken with food and water. Areas of high, intermediate, and low typhoid prevalence are shown in Figure 2.
Figure 2.
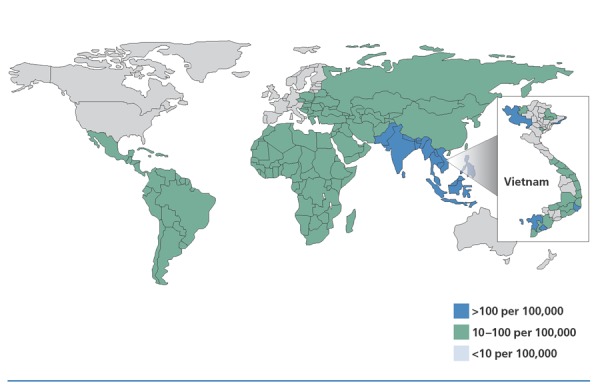
Incidence of typhoid fever per 100,000 persons. Country-specific mean annual incidence rates, some of which are estimates, are for 2000. Province-specific incidence rates for Vietnam are for children to 5 to 14 years of age, between 1999 and 2003 (inset). Adapted from DeRoeck et al.6
Two typhoid vaccines are available, both with approximately 50% to 80% efficacy. The oral live-attenuated vaccine requires 4 doses over 7 days and is protective for more than 5 years. The parenteral Vi capsular polysaccharide vaccine, which consists of a bacterial capsule of S typhi, is given as a single intramuscular dose and is protective for approximately 2 years. HIV-infected people with CD4+ cell counts below 200/μL can receive the parenteral vaccine—but not the oral vaccine (which is live). Although the parenteral vaccine is less immunogenic the more immunosuppressed the patient is, this vaccination can be offered (and is recommended) when a live vaccine is not appropriate and the travel destination would lead to significant typhoid exposure. Vaccination is recommended for anyone traveling for 3 weeks or more in endemic areas and anyone traveling for any duration in the Indian subcontinent or off usual tourist routes in endemic areas. Vaccination ideally should be given or started at least 2 weeks prior to exposure.
Diarrhea
Diarrheal illness accounts for 20% to 40% of reported disease in travelers.1 Traveler’s diarrhea may be caused by a large number of different foodborne and waterborne pathogens (a few common examples include Salmonella, Campylobacter, Giardia, and Cryptosporidium species), and can be severe or become chronic in immunocompromised people. Enteroaggregative Escherichia coli is an emerging enteric pathogen that can also cause persistent diarrhea in HIV-infected people. A meta-analysis of randomized trials examining the effect of modifying behaviors to avoid contracting diarrheal illness indicates no preventive impact.
Bacteria account for most cases of traveler’s diarrhea. The most common antibiotic treatment is ciprofloxacin (500 mg twice daily for 1-3 days). Ciprofloxacin (or another fluoroquinolone) or azithromycin (1 g once daily for 3 days) is used for illness contracted in Southeast Asia. Because Campylobacter infection is common in Thailand and fluoroquinolone resistance has been reported in 70% to 90% of cases, azithromycin is the treatment of choice there. There are high resistance rates to trimethoprim-sulfamethoxazole worldwide, and it is rarely used for treatment of diarrheal illness outside of that contracted in Mexico or Central America. Rifaximin is an effective drug, but it is relatively expensive and requires twice-daily dosing.
There are few concerns over interaction of antibiotic treatment with antiretroviral drugs. Fluoroquinolones have no clinically significant interactions with HIV protease inhibitors (PIs), with nucleoside analogue reverse transcriptase inhibitors (nRTIs), or with nonnucleoside analogue RTIs (NNRTIs). Interactions between macrolide antibiotics and antiretroviral drugs include increased clarithromycin levels with ritonavir, atazanavir, and lopinavir; decreased zidovudine levels with clarithromycin; and potential interactions between clarithromycin and efavirenz or nevirapine. Azithromycin appears to pose little risk of drug interactions with antiretroviral drugs. There are no data available on potential interactions between rifaximin and antiretroviral drugs; rifaximin acts in the gut lumen, with little systemic exposure.
Antibiotic prophylaxis for traveler’s diarrhea is not recommended, because it poses risk of adverse effects, may contribute to drug resistance, and may contribute to poor judgment in terms of exposure (eg, among adventurous eaters). Such risks should be weighed against the potential outcome of prompt, early self-treatment. Data on prophylactic use of probiotics are inconclusive. Bismuth subsalicylate is effective as prophylaxis. This agent has antisecretory, antiinflammatory, and antibacterial effects, and has been found to be 40% to 65% protective and to reduce the number of stools and duration of illness by 50%. Bismuth subsalicylate decreases antibiotic absorption, and must be taken 6 hours before or after an antibiotic dose. It may also cause blackening of the tongue and stools and has been associated with risk of tinnitus. Because bismuth subsalicylate contains aspirin, it must be avoided by persons who have aspirin allergy; it also should be avoided by those taking warfarin. As prophylaxis, bismuth subsalicylate should be taken at a dose of 2 tablets 4 times a day (eg, before each meal and at bedtime).
In addition to antibiotic treatment, traveler’s diarrhea can be safely treated with antimotility agents (eg, synthetic opiates such as loperamide or diphenoxylate), and oral rehydration therapy.
Malaria
A study published in 2006 indicated that for travelers returning with fever, malaria was the cause in 62% of cases from sub-Saharan Africa; 13% to 14% of cases from Central America, South America, Southeast Asia, and South Central Asia; and less than 1% of cases from the Caribbean.1 As with immunocompetent travelers, immunocompromised travelers to malaria-endemic areas should receive counseling about ways to avoid mosquito bites (eg, bed netting, insect repellants, permethrin-impregnated clothing). They should also be prescribed appropriate drugs for malaria prophylaxis. However, it must be stressed that HIV infection may be associated with more serious malarial disease and that malaria increases HIV RNA level and may thus exacerbate HIV disease progression. Further, drugs used in malaria prophylaxis may interact with antiretroviral drugs and there is a general lack of data on safety and efficacy of antimalarial regimens in patients taking antiretroviral therapy.
In areas where malaria is chloroquine-sensitive, weekly chloroquine is the first choice for prophylaxis. In areas with chloroquine resistance, weekly mefloquine, daily doxycycline, daily atovaquone-proguanil, or daily primaquine are options. Advantages of mefloquine include the weekly schedule and moderate cost; disadvantages include the potential for neuropsychiatric adverse effects and the need to take it 1 to 2 weeks before and 4 weeks after exposure. Advantages of doxycycline include low cost and preventive effects against diarrhea, leptospirosis, and Rickettsia species infections. Doxycycline’s disadvantages include the need to take it daily, associated photosensitivity, the potential for gastrointestinal upset and vaginal candidiasis, and the need to take it 1 day before and 4 weeks after exposure. Advantages of atovaquone-proguanil include its safety and the need to take it only 1 day before and 7 days after exposure; disadvantages include higher cost, the potential for headache, gastrointestinal upset, insomnia, and the need to take it daily and with food. Advantages of primaquine include low cost and the need to take the drug only 1 day before and 3 days after exposure; disadvantages include the need to measure glucose-6-phosphate dehydrogenase levels prior to taking the drug, reduced efficacy compared with other options, and the need to take the drug daily.
Potential interactions between antiretroviral drugs and antimalarial drugs are shown in Table 1. Because no clinically significant interactions are expected between tetracyclines and PIs or NNRTIs, doxycycline might be a reasonable choice for malaria prophylaxis in a patient on antiretroviral therapy. Atovaquone-proguanil is a reasonable option for patients whose antiretroviral regimen includes nelfinavir or nevirapine. Although atovaquone is not expected to have substantial interaction with commonly used nRTIs, no data are available on potential interactions between proguanil and nRTIs. Few data are available on potential interactions between antimalarial drugs and HIV entry inhibitors or HIV integrase strand transfer inhibitors.
Table 1.
Potential Interactions Between Antiretroviral and Antimalarial Drugs*
| HIV Protease Inhibitors | Nucleoside Analogue Reverse Transcriptase Inhibitors | Nonnucleoside Analogue Reverse Transcriptase Inhibitors | |
|---|---|---|---|
| Mefloquine | Potential interaction with all protease inhibitors | No data available | Decreased mefloquine levels with efavirenz and nevirapine |
| Atovaquone- Proguanil | Atovaquone: potential interactions with indi- navir, ritonavir, lopinavir, | Atovaquone: no clinically significant interactions expected | Atovaquone: potential interaction with efavirenz |
| atazanavir, darunavir, tipranavir | Proguanil: no data available | Proguanil: potential interaction with | |
| Proguanil: potential interactions with ritonavir, lopinavir | efavirenz | ||
| Doxycycline | No clinically significant interactions expected | No data available | No clinically significant interactions expected |
| Chloroquine | Potential interaction with ritonavir | No data available | No clinically significant interactions expected |
| Primaquine | No clear data | No data available | No data available |
Known potential interactions within an HIV drug class are noted in the table. Currently there are no known drug combinations with absolute contraindications to coadministration. Adapted from the Centers for Disease Control and Prevention (CDC) Yellow Book.2
In summary, HIV-infected patients are traveling more because of the great health gains observed from antiretroviral regimens over the last decade. It is crucial in this population to ensure safe travel using the array of interventions available including vaccines and antiinfective medicines to prevent commonly observed and serious infections. We have reviewed safe choices for optimizing this prevention, paying attention to the specific HIV regimen and CD4+ cell counts with respect to choosing vaccines as well as minimizing risks for malaria and diarrheal disease.
References
- 1.Freedman DO, Weld LH, Kozarsky PE, et al. Spectrum of disease and relation to place of exposure among ill returned travelers. N Engl J Med. 2006;354(2):119-130. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC). Immunocompromised travelers: approach to the immunocompromised traveler. http://wwwnc.cdc.gov/travel/yellowbook/2012/chapter-8-advising-travelers-with-specific-needs/immunocompromised-travelers.htm. Accessed July 18, 2012.
- 3.Weissman S, Feucht C, Moore BA. Response to hepatitis A vaccine in HIV-positive patients. J Viral Hepat. 2006;13(2):81-86. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC). Yellow Book: Yellow Fever Vaccine Maps. http://wwwnc.cdc.gov/travel/yellowbook/2012/chapter-3-infectious-diseases-related-to-travel/yellow-fever.htm#2853. Accessed August 1, 2012.
- 5.Pacanowski J, Lacombe K, Campa P, et al. Plasma HIV-RNA is the key determinant of long-term antibody persistence after Yellow fever immunization in a cohort of 364 HIV-infected patients. JAIDS. 2012;59(4):360-367. [DOI] [PubMed] [Google Scholar]
- 6.DeRoeck D, Jodar L, Clemens J. Putting typhoid vaccination on the global health agenda. N Engl J Med. 2007;357(11):1069-1071. [DOI] [PubMed] [Google Scholar]


