Now that [18F] fluorodeoxyglucose positron emission tomography (FDG-PET) has become an established imaging tool in oncology, it is attracting interest in the field of infectious diseases.1 Several studies have used FDG-PET to examine the pathophysiology of HIV infection as well as other conditions such as lipodystrophic syndrome and HIV-related neurocognitive disorders.2 In clinical practice, FDG-PET has been proposed to assess fever of unknown origin3 or with lymphoproliferative disorders such as Castleman disease in individuals with HIV infection.4
Castleman disease has heterogeneous manifestations ranging from asymptomatic disease to recurrent episodes of widespread lymphadenomegaly with systemic symptoms.5 Its incidence is not known, but the estimated number of cases in the United States ranges from 30,000 to 100,000.6 Castleman disease traditionally has been classified as unicentric and multicentric disease,7 but more recently histopathogenic taxonomy has been preferred.8 The 4 types of Castleman disease according to this classification are in the box.
Box. Classification of Castleman Disease by Histopathogenic Taxonomy.
Hyaline-vascular (HV) type, which usually has a unicentric presentation, involving a single node or a localized group of nodes, lacks systemic signs or symptoms, and generally has a benign course
Plasma-cell (PC) type, which is more commonly multicentric, presents systemic symptoms and abnormal laboratory findings, and behaves more aggressively
Plasmablastic (PB) type, which occurs in immunosuppressed patients, is related to human herpesvirus 8 (HHV8) infection, and presents as mainly multicentric, with systemic symptoms and a poor outcome
Not otherwise specified type, with a multicentric presentation but with few systemic symptoms
Case Presentation
Recently 2 patients attending the outpatient clinic at Niguarda Cà Granda Hospital in Milan, Italy, developed multicentric Castleman disease. Each underwent the same laboratory and imaging assessments, which elicited some different results. Although combined FDG-PET/computed tomography (CT) scans in HIV-seropositive patients with multicentric Castleman disease can demonstrate widespread nodal and spleen abnormalities that improve with remission,9 FDG-PET/CT tested negative in the first patient and positive in the second. The clinical features and laboratory values in these patients are described in Table 1. The 2 cases were compared in an effort to explain these different results.
Table 1.
Clinical Features and Laboratory Values
| Patient 1 | Patient 2 | |
|---|---|---|
| Year of HIV diagnosis | 2005 | 2009 |
| HIV risk group | MSM | MSM |
| CD4+ cells/μL (%) at HIV diagnosis | 487 (17%) | 285 (16%) |
| HIV RNA copies/mL at HIV diagnosis | 61,074 | 1,180,316 |
| Nadir CD4+ cells/μL (%) | 219 (15%) | 196 (22%) |
| Zenith HIV RNA copies/mL | 534,955 | 1,180,316 |
| Antiretroviral regimen | Emtricitabine/tenofovir+ atazanavir/ritonavir | Emtricitabine/tenofovir+ atazanavir/ritonavir |
| Months of antiretroviral treatment at diagnosis of multicentric Castleman disease | 58 | 19 |
| Status at the time of multicentric Castleman disease diagnosis | ||
| CD4+ cells/μL (%) | 895 (28%) | 171 (21%) |
| HIV RNA copies/mL | <40 | 110 |
| HHV8 DNA copies/mL | 38 | 958,962 |
| HHV8 Ab (lytic antigen) | 256 | 4,096 |
| HHV8 Ab (latency antigen) | 256 | 512 |
| KS lesions site (number) | Skin (10) | None |
| Maximum diameter of enlarged lymph nodes (mm) | ||
| • Submandibular (15) | • Neck (25) | |
| • Neck (20) | • Supra- and | |
| • Subclavian (20) | subclavian (28) | |
| • Axilla (20) | • Axilla (40) | |
| • Mediastinum (15) | • Abdomen (coeliac, para-aortic, iliac) (34) | |
| • Abdomen (coeliac, para-aortic, iliac) (20) | • Groin (20) | |
| • Groin (15) | ||
| Spleen diameter (cm) | 15 | 18 |
| Histopathogenic type | Hyaline-vascular | Plasmablastic |
HHV8 DNA was assessed with real-time polymerase chain reaction in plasma; antibodies to IgG anti-HHV8 lytic/latency antigens detected by immunofluorescence assay.
MSM indicates men who have sex with men; Ab, antibody; HHV8, human herpesvirus 8; lgG, immunoglobulin G; KS, Kaposi sarcoma.
Patient 1
This patient was a 40-year-old man who tested HIV-seropositive in 2005. At the beginning of 2010, he started complaining of fever, and had diffuse lymphadenopathy and florid Kaposi sarcoma (KS) skin lesions. Systemic symptoms had spontaneously resolved but recurred during the year with progressive worsening. A first lymph node biopsy was performed in June 2010, but it did not result in a diagnosis. In August 2010, a total body CT scan documented superficial and deep enlarged lymph nodes in the patient’s neck, axilla, mediastinum, and abdomen. Subsequently, a FDG-PET/CT was performed, but it did not show any lesions with increased metabolic activity (Figure 1). A second lymph node biopsy was performed, which showed a mantle zone hyperplasia with skin-onion features but CD31- and human herpesvirus 8 (HHV8)−negative immunohistochemistry. At the beginning of 2011, a pathologist with experience in Castleman disease reviewed the slides from the second biopsy, which had been performed at the time the FDG-PET/CT was performed, and made a diagnosis of hyaline-vascular (HV)-type Castleman disease with a weakly HHV8-positive immunohistochemistry. The patient underwent systemic chemotherapy with 4 cycles of etoposide plus rituximab, followed by 4 cycles of rituximab alone. At a 12-month follow-up, he had full remission of his disease.
Figure 1.
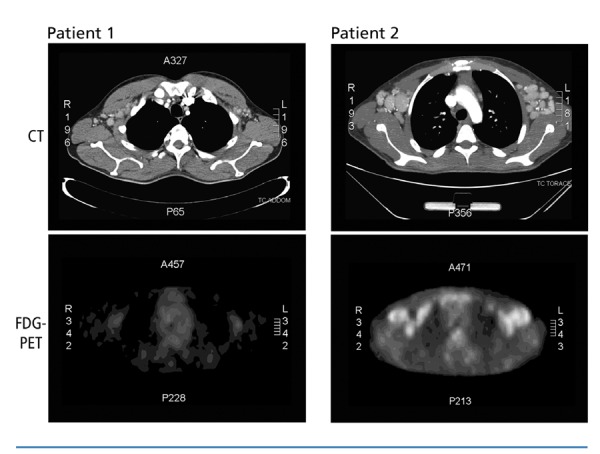
Computed tomography (CT) scans (upper panels) and [18F] fluorodeoxyglucose positron emission tomography (FDG-PET) scans (lower panels) on patients 1 and 2. CT scans show enlarged axillary lymph nodes in both patients. FDG-PET scan on patient 1 (lower left panel) does not show increased metabolic activity. FDG-PET scan on patient 2 has substantial pathologic accumulation of FDG.
Patient 2
Patient 2 was a 21-year-old man who first tested HIV-seropositive in 2009. At the end of 2010, he started complaining of fever, and had diffuse lymphadenopathy, splenomegaly, and a high serum C-reactive protein level. Systemic symptoms had resolved spontaneously but recurred during the year with progressive worsening. In June 2011, a total body CT scan documented enlarged lymph nodes in the patient’s neck, axilla, abdomen, and groin, and an enlarged spleen 18 cm in diameter.
Subsequently, a FDG-PET/CT scan confirmed increased metabolic activity (2). A lymph node biopsy, performed in July 2011, documented chronic lymphadenitis with intrafollicular dendritic cell expansion. The immunohistochemistry was negative for HHV8, CD3, CD5, CD20, CD21, CD30, CD79α, BCL2, BCL6, and MIB1. At that time he had a sudden clinical worsening that led to a life-threatening multiorgan impairment. A second lymph node biopsy, performed in August 2011, showed a retained architecture, with HHV8-positive follicles of variable size, involuted germinal centers, mantle zone hyperplasia with skin-onion features, plasma cells (CD138+, MUM1p+), proliferating (Ki-67–positive), and activated plasma blasts (CD30+).
A diagnosis of plasmablastic (PB)-type Castleman disease was made, and the patient started systemic chemotherapy with rituximab. After the first dose, he developed a splenic infarct and underwent a splenectomy. Six cycles of rituximab were administered and he showed rapid clinical improvement. At a 3-month follow-up visit, the patient still had abdominal lymphadenopathy and a low HHV8 viral load. At 9 months, the patient was in good clinical condition, HHV8 viral load was undetectable, and a new FDG-PET/CT scan did not show any signs of disease activity.
Discussion
These 2 patients had quite different HIV virologic and immunologic status. Patient 1 had a high CD4+ count with undetectable viral load. Patient 2 had a very low CD4+ cell count and had detectable HIV RNA, suggesting HIV as an ancillary cause of diffuse nodal enlargement that might jeopardize the assessment of the real extent of Castleman disease. Further, there were other major differences between these 2 cases of multicentric Castleman disease. In clinical course, patient 1 had less aggressive disease. In terms of correlation with HHV8, patient 1 had a low viral load and antibody titers. As far as presence of KS, patient 1 had 10 skin lesions compared with none in patient 2. Histopathogenic type was HV in patient 1 and PB in patient 2. Such observations emphasize that HV- and PB-type Castleman disease have very different features. This could be a reason for the different FDG-PET/CT results. HV-type is generally unicentric,10 although multicentric disease has been reported.11 HV-type is not thought to be related to HHV8;12 its pathogenesis is still unknown, even if vascular endothelial growth factor may have an important role.13
FDG-PET/CT has been described as a reliable tool in detecting Castleman disease,14-16 but data on its usefulness for HV-type differ. Reddy and Graham showed that FDG-PET effectively revealed a thoracic HV-type mass,17 and Murphy and colleagues found that FDG-PET detected a pelvic HV-type mass, but only with a modest accumulation of FDG.18 Barker and colleagues evaluated the role of FDG-PET/CT in the management of PB-type and found that although FDG-PET/CT might be more sensitive than CT alone in detecting multicentric Castleman disease, it is less reliable in monitoring disease activity after treatment.19
The utility of FDG-PET/CT in the management of HIV-associated Castleman disease has lights and shadows. Although HV-type Castleman disease is uncommon in HIV-infected patients, this possible diagnosis, when indicated by FDG-PET/CT result, should be taken into account. Additionally, FDG-PET/CT has limited value in ruling out other causes of fever and lymphadenopathy in HIV, such as lymphoma or an opportunistic infection. In particular, there are no data on whether FDG-PET/CT can detect different metabolic activities of the recently described KS-associated herpesvirus (KSHV) inflammatory cytokine syndrome (KICS), whose clinical, biochemical, and virologic features are similar to Castleman disease but whose histopathologic findings are not.20,21
Despite the relatively low incidence of Castleman disease, its aggressive and life-threatening course warrants an optimization of diagnostic tools. So far, FDG-PET/CT use for diagnosing Castleman disease has been reported in only a small number of patients.4 Data defining sensitivity and specificity of FDG-PET/CT for Castleman disease diagnosis are lacking. FDG-PET/CT might have an important role, but it should not replace biopsy for diagnosis of Castleman disease. Instead, FDG-PET/CT may help in choosing which gland to biopsy in cases in which a previous biopsy failed to confirm a diagnosis of Castleman disease.22
References
- 1.Meller J, Sahlmann CO, Scheel AK. 18F-FDG PET and PET/CT in fever of unknown origin. J Nucl Med. 2007;48(1):35-45. [PubMed] [Google Scholar]
- 2.Sathekge M, Goethals I, Maes A, van de Wiele C. Positron emission tomography in patients suffering from HIV-1 infection. Eur J Nucl Med Mol Imaging. 2009;36(7):1176-1184. [DOI] [PubMed] [Google Scholar]
- 3.Castaigne C, Tondeur M, de Wit S, Hildebrand M, Clumeck N, Dusart M. Clinical value of FDG-PET/CT for the diagnosis of human immunodeficiency virus-associated fever of unknown origin: a retrospective study. Nucl Med Commun. 2009;30(1):41-47. [DOI] [PubMed] [Google Scholar]
- 4.van Rhee F, Stone K, Szmania S, Barlogie B, Singh Z. Castleman disease in the 21st century: an update on diagnosis, assessment, and therapy. Clin Adv Hematol Oncol. 2010;8(7):486-498. [PubMed] [Google Scholar]
- 5.Reddy D, Mitsuyasu R. HIV-associated multicentric Castleman disease. Curr Opin Oncol. 2011;23(5):475-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore DF, Preti A, Tran SM. Prognostic implications following an indeterminate diagnostic work-up of lymphoma. Blood. 1996;88(Suppl 1):229a. [Google Scholar]
- 7.Hsi ED. Castleman disease. In: Hsi ED, ed. Hematopathology: A Volume in Foundations in Diagnostic Pathology Series, 1 e. Philadelphia: Churchill Livingstone Elsevier; 2007:150-155. [Google Scholar]
- 8.Cronin DM, Warnke RA. Castleman disease: an update on classification and the spectrum of associated lesions. Adv Anat Pathol. 2009;16(4):236-246. [DOI] [PubMed] [Google Scholar]
- 9.Polizzotto M, Millo C, Uldrick T, et al. 18-Fluoro-D-deoxyglucose PET in the diagnosis and monitoring of KSHV-MCD: correlation with clinical, inflammatory and virologic parameters. [Abstract 862.] 18th Conference on Retroviruses and Opportunistic Infections (CROI). February 27-March 2, 2011; Boston, MA. [Google Scholar]
- 10.Menke DM, Camoriano JK, Banks PM. Angiofollicular lymph node hyperplasia: a comparison of unicentric, multicentric, hyaline vascular, and plasma cell types of disease by morphometric and clinical analysis. Mod Pathol. 1992;5(5):525-530. [PubMed] [Google Scholar]
- 11.Skelton HG, Smith KJ. Extranodal multicentric Castleman’s disease with cutaneous involvement. Mod Pathol. 1998;11(1):93-98. [PubMed] [Google Scholar]
- 12.Nayler SJ, Taylor L, Cooper K. HHV-8 is not associated with follicular dendritic cell tumours. Mol Pathol. 1998;51(3):168-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McClain KL, Natkunam Y, Swerdlow SH. Atypical cellular disorders. Hematology Am Soc Hematol Educ Program. 2004;2004(1):283-296. [DOI] [PubMed] [Google Scholar]
- 14.Kunishima S, Taniguchi H, Koh T, Yamaguchi A, Yamagishi H. F-18 fluorodeoxyglucose positron emission tomography in mesenterial Castleman’s lymphoma. Clin Nucl Med. 2001;26(9):789-790. [DOI] [PubMed] [Google Scholar]
- 15.Blockmans D, Maes A, Stroobants S, Bobbaers H, Mortelmans L. FDG positron emission tomographic scintigraphy can reveal Castleman’s disease as a cause of inflammation. Clin Nucl Med. 2001;26(11):975-976. [DOI] [PubMed] [Google Scholar]
- 16.Enomoto K, Nakamichi I, Hamada K, et al. Unicentric and multicentric Castleman’s disease. Br J Radiol. 2007;80(949):e24-e26. [DOI] [PubMed] [Google Scholar]
- 17.Reddy MP, Graham MM. FDG positron emission tomographic imaging of thoracic Castleman’s disease. Clin Nucl Med. 2003;28(4):325-326. [DOI] [PubMed] [Google Scholar]
- 18.Murphy SP, Nathan MA, Karwal MW. FDG-PET appearance of pelvic Castleman’s disease. J Nucl Med. 1997;38(8):1211-1212. [PubMed] [Google Scholar]
- 19.Barker R, Kazmi F, Stebbing J, et al. FDG-PET/CT imaging in the management of HIV-associated multicentric Castleman’s disease. Eur J Nucl Med Mol Imaging. 2009;36(4):648-652. [DOI] [PubMed] [Google Scholar]
- 20.Uldrick TS, Wang V, O’Mahony D, et al. An interleukin-6-related systemic inflammatory syndrome in patients co-infected with Kaposi sarcoma-associated herpesvirus and HIV but without Multicentric Castleman disease. Clin Infect Dis. 2010;51(3):350-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polizzotto MN, Uldrick TS, Hu D, Yarchoan R. Clinical Manifestations of Kaposi Sarcoma Herpesvirus Lytic Activation: Multicentric Castleman Disease (KSHV-MCD) and the KSHV Inflammatory Cytokine Syndrome. Front Microbiol. 2012;3:73. Epub 2012 Mar 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bower M. How I treat HIV-associated multicentric Castleman disease. Blood. 2010;116(22):4415-4421. Epub 2010 Aug 5. [DOI] [PubMed] [Google Scholar]


