Abstract
BACKGROUND
Nigella sativa and its derivatives have been reported to have anti-inflammatory and bronchodilator effects, but the effects have been evaluated in only a few clinical studies.
OBJECTIVES
To determine the effect of N sativa supplementation on inflammation of the airways and limitation of airflow in partly controlled asthma patients.
DESIGN
Single-blind, placebo-controlled, randomized study.
SETTING
Asthma and allergy clinic of a university hospital in eastern Saudi Arabia.
PATIENTS AND METHODS
Patients were divided into three groups. A control group (n=24) received the placebo, while NS-1 and NS-2 groups (n=26 each) received 1 and 2 g/day of N sativa, respectively, for 3 months along with maintenance inhaled therapy.
MAIN OUTCOME MEASURE(S)
Asthma control test (ACT) score, fractional exhaled nitric oxide (FeNO), peak expiratory flow (PEF) variability and other pulmonary function tests, IgE, serum cytokines, and frequency of exacerbations.
RESULTS
FEF25-75% and FEV1 (% predicted) increased significantly (P<.05) at both 6 and 12 weeks in the NS-2 group. PEF variability significantly improved in both NS-1 and NS-2 groups at 6 and 12 weeks as compared with the controls (P<.05). FeNO and serum IgE decreased significantly after 12 weeks in both the NS-1 and NS-2 groups vs baseline (P<.05). Both doses of N sativa produced a significant increase in the serum IFN-γ at 12 weeks vs baseline (P<.05) as well as a significant improvement in the ACT score at 6 and 12 weeks vs baseline (P<.001, <.01). Significantly fewer patients had exacerbations in the NS-1 group (P<.05).
CONCLUSION
N sativa supplementation with inhaled maintenance therapy improves some measures of pulmonary function and inflammation in partly controlled asthma.
LIMITATIONS
No bronchoalveolar lavage or sputum samples taken for measurement of asthma markers.
Asthma is a serious public health problem throughout the world, with a global prevalence ranging from 1% to 18%.1,2 All manifestations of asthma are now known to result from an underlying chronic inflammatory process mediated and orchestrated by products of certain immune cells.3–5 So far there are no effective preventive measures or cure for asthma, and the goal of current treatment is to achieve and maintain clinical control of the manifestations.1 Furthermore, current treatment does not modify the underlying loss of lung function and does not stop the progression of chronic inflammation and remodeling associated with asthma.6,7 The need for new effective, safe and tolerable treatment modalities for asthma is apparent.
Many herbs have been evaluated for their beneficial effects on asthma in human or animal models.8 Among them is Nigella sativa, which grows in the Middle East, Eastern Europe, Western and Middle Asia. N sativa has been used as a natural remedy for many ailments for over 2000 years.9,10 Several potential anti-asthmatic effects have been reported in studies conducted in humans as well as animals. They include relaxant effects on different smooth muscle preparations,11 anti-cholinergic effects,12 an inhibitory effect on histamine (H1) receptors,13 and a stimulatory effect on β-adrenergic receptors.14 Anti-inflammatory, immunomodulatory and anti-oxidant effects have also been documented for this herb.15
Only a few clinical studies have evaluated the effect of N sativa on asthmatic patients.16–18 A significant improvement in subjective feelings and pulmonary function tests were observed in those studies, but limitations of the studies were the small number of patients and lack of clear outcome measures on the effect on airway inflammation. The aim of this study was to evaluate the effects of N sativa supplementation on clinical outcome measures and indicators of airway inflammation in patients with partly controlled asthma.
PATIENTS AND METHODS
This participant-blinded, randomized and placebo-controlled clinical trial was carried out at the University of Dammam in the Eastern Province of Saudi Arabia and its affiliated teaching hospital in accordance with the principle of the Declaration of Helsinki.19 The trial was approved by KACST (King Abdulaziz City of Science & Technology) as well as by the Institutional Review Board of the university and it was registered in an international clinical trial registry, ISRCTN (BioMed Central (ISRCTN48853858). Written informed consent was obtained from all patients who volunteered for the trial. Previously diagnosed adult asthmatic patients (18–65 years old) were recruited from the pulmonary outpatient clinic of the university hospital. Asthma in all patients was diagnosed according to the criteria of the National Institute of Health (NIH).20
The inclusion criteria were a diagnosis of partly controlled asthma according to Global Initiative for Asthma (GINA) guidelines,1 non-smoking status and having prior treatment for at least 3 months with daily maintenance therapy of inhaled corticosteroids without any concomitant asthma medications except for short-acting β agonist (SABA) as needed. Exclusion criteria included use of additional asthma medications (e.g. leukotriene modifiers, oral steroids), severe exacerbation or hospitalization for asthma within one month prior to or during the study period, chronic diseases (e.g. diabetes mellitus, autoimmune diseases), compliance <90% of the assigned medication, pregnancy or lactation.
Partly controlled asthmatic patients who agreed to participate in the study were selected by non-probability (convenience) sampling. Patients who met inclusion criteria were allocated to three groups by random allocation. Based upon 5 years of records from the asthma clinic, it was anticipated that about 150 subjects with partly controlled asthma would be reporting in at least four asthma seasons (about 2 years). After observing the selection criteria and accommodating dropouts, this number was determined to be sufficient to derive a reasonable sample size with a close to normal distribution. The first patient who met selection criteria was coded as subject 1 and subsequent patients were numbered sequentially, up to subject 150. The RANDBETWEEN (1,150) command of Microsoft Excel 2010 (Microsoft Company, Redmond, WA, USA) was used to create a list of random numbers for the three study groups (control, NS-1 and NS-2). The lists were sorted in numerical order. In addition, keeping in mind time and cost constraints, recruitment was limited to a maximum 2 years (approximately four asthma seasons).
The initial screening visit to the clinic was followed by a one-week run-in period to determine eligibility for recruitment, as well as to establish the baseline values for the different study measurements. At the end of the run-in-period, patients attended an entry visit (visit-0) when they were assigned to the respective groups following the distribution order of random numbers generated (Figure 1). The control group received 1 capsule of placebo twice daily; the NS-1 group received 1 gram N sativa daily in two divided doses (500 mg cap twice daily), and the NS-2 group received 2 grams N sativa daily in two divided doses (2 capsules of 500 mg twice daily). Subsequently they were followed with two visits scheduled 6 weeks (visit 1) and 12 weeks (visit 2) after starting the treatment. Clinical assessment, spirometry, measurement of fractional exhaled nitric oxide (FeNO) and serum ‘total immunoglobulin E’ (IgE) were performed at all visits. Blood samples were taken to measure serum levels of cytokines during visit-0 and visit-2 only.
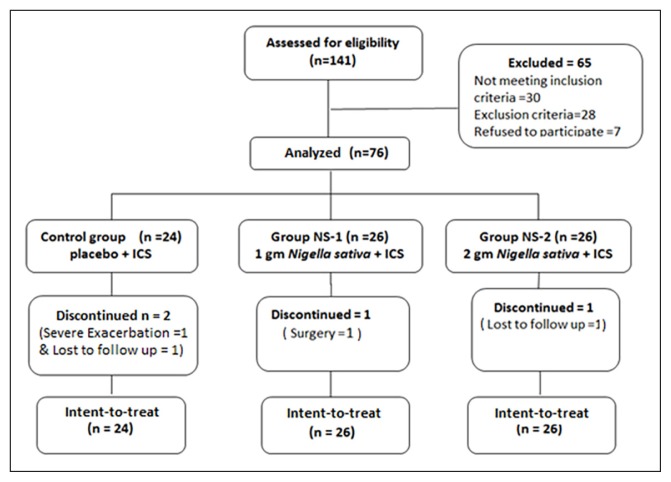
Figure 1.
Flow diagram of recruitment and dispensation of subjects. ICS=Inhaled corticosteroid
All the medications were taken orally for 12 weeks in addition to the maintenance inhaler therapy (mostly budosonide 400 μg daily and in some cases fluticasone propionate 500 μg daily into two divided doses). Nigella sativa (black seeds) in the form of 500 mg capsules of whole ground seeds (Bio Extracts (Pvt) Ltd, Sri Lanka), were used in this study. Charcoal powder in the form of capsules (260 mg) supplied by Arkopharma Pharmaceutical Laboratories Carros, France, was used as placebo. The participants were blinded; none knew whether he or she was receiving placebo or N sativa.
The degree of control of asthma symptoms was assessed using Asthma Control Test (ACT)21 which has been found to correlate well with GINA guidelines for control of asthma.22–24 Information on daytime symptoms, limitation of activities, nocturnal symptoms, need for reliever/rescue treatment, frequency of exacerbations and lung functions was recorded in a proforma at baseline. At each visit, the occurrence of moderate or severe exacerbations, defined according to the American Thoracic Society (ATS) and European Respiratory Society (ERS) criteria,25 were recorded. The percentage of patients with at least one exacerbation, over the whole treatment period, was calculated in each group.
Spirometry was carried out following ATS guidelines for standardization of spirometry,26,27 using a calibrated computerized pneumatograph spirometer (Vitalograph) that included software for a BTPS (body temperature and pressure saturated) correction factor. The predicted values of forced expiratory volume at one second (FEV1 % predicted), forced vital capacity (FVC), and forced expiratory flow (FEF25-75%) were calculated automatically based on age, gender, height and ethnicity. The procedure was fully explained to the patients before the test, and subjects were encouraged to perform at least three valid maneuvers during each measurement, with the best values used for analysis.
The participants were trained to measure their peak expiratory flow (PEF) at home using a handheld portable peak expiratory flow meter (Mini -Wright standard peak flow meter-Clement Clarke international limited- London, UK) provided free of cost at the start of study. PEF was measured twice daily during the week of the run-in-period preceding the start of the trial and was repeated during the week preceding every follow up visit. PEF was measured first thing in the morning and at night before medicines were taken. The participants were reminded by phone and readings were plotted in a PEF chart. An index value for PEF variability was calculated by obtaining the lowest morning reading (AM PEF) over the week, divided by the highest PEF reading in that week expressed as a percent. The higher the index, the lower the PEF variability, and control of asthma symptoms is better (and vice versa).1
Fractional exhaled nitric oxide (FeNO) was measured according to ATS/ERS guidelines28,29 using a small portable hand held NO analyzer (Niox Mino-Aerocrine AB, Solna, Sweden). This device has a measurement range of 5–300 ppb, and reproducibility of <3 ppb for measured values below 30 ppb and <10% for measured values ≥30 ppb. In addition, FeNO was measured before spirometry to avoid the effect of the spirometric maneuver on FeNO.
Eight milliliters of blood were collected in a plain tube from every patient by venipuncture. The blood was allowed to clot for at least half an hour at room temperature and then centrifuged at 4°C at 3000 rpm for 15 minutes. Serum was collected into small aliquots and stored at −80 °C for total IgE and cytokines analysis. Total IgE was measured using a fluoroenzyme immunoassay technique using ImmunoCAP total IgE (Phadia AB, Uppsala, Sweden). The cytokines (IL-4, IL-10, IL-17, IFN-γ and eotaxin) were measured in serum using specific quantitative sandwich ELISA technique using kits supplied by IBL company (Germany). Samples were analyzed in single measurements since the IBL cytokines assay has good precision in pretests with a stated intra-assay mean coefficient of variance (CV) of less than 10%.
Statistical analysis
Data were reported as arithmetic means and standard deviation for all variables except for cytokines where the data were transformed to logarithmic scale to assume normal distribution and reported as geometric mean. Analysis of variance (ANOVA) was used to compare the demographic and biometric characteristics of the patients. Comparison of proportions between groups was done using the chi-square (χ2) test. Analysis of covariance (ANCOVA) was used for between the groups comparison of pulmonary function tests, serum cytokines, FeNO and IgE. The covariate for ANCOVA was the respective baseline value, the test being compared as the dependent variable and the groups as fixed variable. Wilcoxon rank test and Kruskal Wallis tests were used for analysis of ACT score within and between groups, respectively. For within group comparisons the paired t test was used. Data were analyzed using Statistical Package of Social Science (SPSS) software version 16. P values of <.05 were considered significant. The intention to treat methodology was used for the number of participants finally analyzed with missing values being ascribed by last observation carried forward.
RESULTS
One hundred and forty-one partly controlled asthmatic patients participated in the study (Figure 1). Sixty-five participants were excluded. Before the start of the study, 7 of those patients withdrew when briefed about methodology for informed consent. Seventy-six patients were subsequently allocated to three groups by random allocation (Table 1). Of the 76 patients enrolled in the study, two patients from the control group discontinued because of severe exacerbations, one patient from group NS-1 discontinued because of an urgent (unrelated) surgery and one patient from group NS-2 was lost to follow. Table 2 summarizes the results of lung function tests for all patients in the three groups, at baseline, after 6 weeks and after 12 weeks of treatment. There were no significant differences between the three groups at baseline in any of the pulmonary function tests. Treatment with placebo for 6 or 12 weeks was not associated with statistically significant changes from baseline in any of the lung function tests. In the group treated with the high-dose N sativa (NS-2) at 6 and 12 weeks, FEV1 (% predicted), FEF25-75 and FEF25-75 (% predicted) were significantly higher than baseline values. In addition, the ratio of FEV1/FVC showed a significant increment at the 6 week measurement in NS-2 group, compared with the corresponding baseline values. PEF variability was significantly higher at 6 weeks and 12 weeks of treatment with the low dose N sativa (NS-1) and at 12 weeks treatment with the high dose N sativa (NS-2) compared to their corresponding baseline values. Furthermore, the improvement in PEF variability from baseline by both N sativa doses after 12 weeks of treatment was significantly higher than the corresponding placebo change.
Table 1.
Demographic and biometric characteristics of the patients.
| Variables* | Control Group (n=24) | Group NS-1 (n=26) | Group NS-2 (n=26) |
|---|---|---|---|
|
| |||
| Age (years) | 37.1 (11.2) | 37.5 (12.7) | 39.2 (13.6) |
| Male, n (%) | 9 (37.5) | 8 (30.8) | 9 (34.6) |
| Female, n (%) | 15 (62.5) | 18 (69.2) | 17 (65.4) |
| Height (m) | 1.58 (0.09) | 1.60 (0.1) | 1.60 (0.07) |
| Weight (kg) | 75.49 (15.5) | 77.12 (19.5) | 78.28 (15.2) |
| BMI (kg/m2) | 30.1 (6.0) | 30.6 (8.8) | 30.4 (5.2) |
| Asthma duration (years) | 13.3 (8.5) | 10.3 (8.9) | 13.1 (6.4) |
| Atopic asthma n (%) | 14 (58.3) | 12 (46.2) | 12 (46.2) |
Data is presented as (mean±SD) for all variables. No statistically significant differences between treatment groups. Control: Placebo group, NS-1: 1 g/day Nigella sativa, NS-2: 2 g/day Nigella sativa
Table 2.
Pulmonary function tests in patients treated with placebo, low-dose Nigella sativa (NS-1) and high-dose Nigella sativa (NS-2) at baseline, and after 6 and 12 weeks of treatment.
| Variable | Group | Baseline | 6 weeks | 12 weeks |
|---|---|---|---|---|
|
| ||||
| FEV1 (L) | Control group | 2.3 (0.6) | 2.3 (0.7) | 2.3 (0.6) |
| Group NS-1 | 2.5 (0.8) | 2.6 (0.8) | 2.6 (0.8) | |
| Group NS-2 | 2.3 (0.7) | 2.4 (0.8) | 2.4 (0.7) | |
| FEV1 (% predicted) | Control group | 81.1 (19.1) | 79.8 (20.6) | 80.8 (20.6) |
| Group NS-1 | 85.5 (17.3) | 86.8 (15.3) | 87.7 (15.8) | |
| Group NS-2 | 78.1 (21.4) | 83.8 (21.4)* | 85.5 (22.9)* | |
| FVC (L) | Control group | 3.0 (0.7) | 3.0 (0.7) | 3.0 (0.7) |
| Group NS-1 | 3.2 (1.0) | 3.2 (1.0) | 3.3 (1.0) | |
| Group NS-2 | 3.0 (0.9) | 3.1 (0.9) | 3.2 (0.9) | |
| FVC (% predicted) | Control group | 90.1 (13.7) | 88.8 (17.1) | 89.1 (13.7) |
| Group NS-1 | 92.8 (17.3) | 93.3 (16.8) | 94.8 (14.8) | |
| Group NS-2 | 88.7 (21.9) | 91.4 (14.8) | 93.0 (22.9) | |
| FEVI/FVC (%) | Control group | 76.2 (13.2) | 76.2 (11.8) | 77.2 (12.2) |
| Group NS-1 | 78.6 (9.1) | 79.1 (9.2) | 79.0 (9.1) | |
| Group NS-2 | 74.3 (9.7) | 77.4 (9.7)* | 76.7 (7.6) | |
| FEF 25-75 (L/sec) | Control group | 2.3 (1.3) | 2.2 (1.2) | 2.3 (1.5) |
| Group NS-1 | 2.5 (1.1) | 2.6 (1.2) | 2.7 (1.3) | |
| Group NS-2 | 1.9 (0.9) | 2.3 (1.2)** | 2.3 (1.0)** | |
| FEF 25-75 (% predicted) | Control group | 67.5 (38.7) | 66.2 (35.3) | 68.2 (40.7) |
| Group NS-1 | 74.5 (29.1) | 76.5 (30.1) | 77.3 (31.6) | |
| Group NS-2 | 56.3 (25.0) | 66.5 (31.6)** | 66.4 (27.5) | |
| PEF variability | Control group | 76.6 (7.3) | 78.3 (8.3) | 78.5 (8.8) |
| Group NS-1 | 73.5 (10.7) | 80.9 (9.7)** | 83.6 (8.7)*** | |
| Group NS-2 | 73.7 (11.2) | 75.3 (11.7) | 81.4 (8.7)** | |
Data are presented as mean and standard deviation. Control: Placebo group (n=24), NS-1: 1g/day Nigella sativa (n=26), NS-2: 2 g/day Nigella sativa (n=26), FEV1: Forced expiratory volume at one second, FVC: Forced vital capacity, FEF: Forced expiratory flow, PEF: Peak expiratory flow.
P<.05,
P<.01,
P<.01 for between groups comparison PEF variability at 12 weeks.
For ANCOVA Tests of between-subjects effects: F=5.016, P=.009, adjusted R-squared=.300. The Levene test indicated equality of variances for the PEF variability tests (P=.563). For within group analysis using paired t test FEV1 for group NS-2 at 6 weeks as well as 12 weeks and PEF variability for Group NS-1 at 6 weeks shows significant difference when compared with the baseline.
In levels of cytokines, no significant differences between the three groups were observed at the baseline (Table 3). The levels of IL-4, IL-10, IL-17A and the eotaxins did not change significantly in any of the three groups at any of the follow up visits. However, for both N sativa groups (NS-1 and NS-2), the serum level of IFN-γ at 12 weeks was significantly higher than at baseline.
Table 3.
Serum cytokine levels in the control (placebo) and Nigella sativa-treated groups at the baseline, and after 6 and 12 weeks of treatment.
| Cytokines | Group | Baseline | 12 weeks | P |
|---|---|---|---|---|
|
| ||||
| IL-4 (pg/mL) | Control group | 1.6 (5.7) | 1.6 (5.7) | .50 |
| Group NS-1 | 2.4 (6.7) | 2.3 (6.8) | .90 | |
| Group NS-2 | 2.2 (6.5) | 2.1 (6.4) | .96 | |
| IL-10 (pg/mL) | Control group | 2.2 (5.8) | 1.6 (6.3) | .07 |
| Group NS-1 | 2.4 (6.1) | 2.8 (6.3) | .20 | |
| Group NS-2 | 1.7 (6.6) | 1.5 (6.6) | .40 | |
| IL-17A (pg/mL) | Control group | 1.2 (6.4) | 1.0 (6.6) | .30 |
| Group NS-1 | 1.8 (7.2) | 2.9 (7.3) | .13 | |
| Group NS-2 | 1.9 (7.0) | 1.6 (7.4) | .34 | |
| IFN-γ (pg/mL) | Control group | 3.0 (5.5) | 2.6 (5.4) | .26 |
| Group NS-1 | 3.8 (5.8) | 4.7 (6.0) | .03* | |
| Group NS-2 | 2.8 (5.8) | 3.3 (6.0) | .03* | |
| Eotaxin (pg/mL) | Control group | 98.1 (238.6) | 100.6 (256.2) | .26 |
| Group NS-1 | 99.4 (104.5) | 100.9 (40.8) | .91 | |
| Group NS-2 | 96.2 (42.3) | 100.7 (30.1) | .42 | |
All variables are represented as geometric mean (SD), except eotaxin which is represented as arithmetic mean (SD).
Significant difference (P<.05).
IL=interleukin; IFN-γ=interferon gamma. NS: Nigella sativa, Control placebo group (n=24), NS-1: 1 g/day Nigella sativa (n=26), NS-2: 2 g/day Nigella sativa (n=26)
P<.05
For between groups comparison IFN-γ (interferon gamma) at 12 weeks, for ANCOVA of between subjects effects: F=4.287, P=.017, adjusted R-squared=0.560. The Levene test indicated equality of variances for IFN-γ tests (P=.321).
In fractional exhaled nitric oxide (FeNO) and serum levels of immunoglobulin E (IgE), no significant differences between the three groups at baseline were found for both FeNO and IgE (Table 4). The low dose of NS (NS-1) produced a significant reduction in FeNO at weeks 6 and 12, while the higher dose group (NS-2) showed significant reductions in serum IgE compared with baseline.
Table 4.
FeNO and serum Ig E in the control (placebo) and in the Nigella sativa-treated groups at the baseline, and after 6 and 12 weeks of treatment.
| Variable | Group | Baseline | 6 weeks | 12 weeks |
|---|---|---|---|---|
|
| ||||
| FeNO (ppb) | Control group | 34.9(32.8) | 38.2 (37.7) | 34.8 (26.9) |
| Group NS-1 | 23.0 (13.3) | 20.8 (11.2) | 18.1 (8.2)* | |
| Group NS-2 | 27.6 (30.6) | 24.4 (21.4) | 26.9 (29.1) | |
| IgE (kU/L) | Control group | 623.8 (802.5) | 579.6 (672.6) | 595.9 (752.5) |
| Group NS-1 | 453.5 (711.8) | 401.3 (621.6) | 391.5 (616.0) | |
| Group NS-2 | 393.8 (470.1) | 345.3 (392.6) | 322.0 (373.2)** | |
Data is presented as (mean ± SD), NS=Nigella sativa
P<.05,
P<.01;
FeNO: fractional exhaled nitric oxide, Ig E: immunoglobulin E; Control placebo group (n=24), NS-1: 1 g/day Nigella sativa (n=26), NS-2: 2 g/day Nigella sativa (n=26)
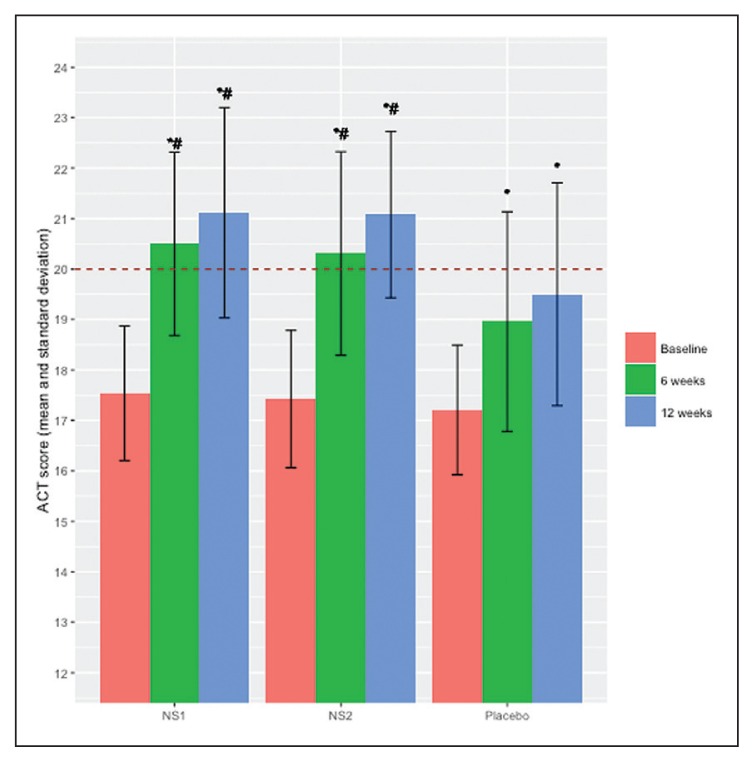
In ACT scores, no significant differences were found between the three groups at baseline and the scores for the three groups were below the cut-off value of 20, which separates controlled from uncontrolled asthmatic patients (Figure 2). ACT scores increased significantly from the respective baseline values after 6 and 12 weeks of treatment in placebo and both test groups. The increment in ACT score in the placebo group, however, remained below the cut-off value of 20, while both N sativa groups exceeded this barrier. Also, ACT scores at 6 weeks and 12 weeks of treatment with any of the two doses of N sativa were significantly higher than the corresponding values in the group treated with placebo (control group). Nine patients (37.5%) in the control group, 3 (11.5%) in the NS-1 group and 4 (15.4%) in the NS-2 group developed severe exacerbations during the follow-up period. The frequency of exacerbations in the NS-1 group was significantly less than the placebo group.
Figure 2.
ACT score (mean and standard deviation) within each treatment group at baseline and at 6 and 12 weeks (the horizontal line at the ACT score of 20 indicates the cut-off point score).*Significant difference (P<.001) compared to corresponding baseline value within each group. #Significant difference (P<.01) between groups in the mean ACT scores at both 6 and 12 weeks visits compared to the corresponding measurements in the placebo group. ACT=asthma control test, NS=Nigella sativa
DISCUSSION
N sativa has been studied for the control of bronchial asthma using in vitro methods,30 animal models,31 and clinical trials.17 These studies have reported several positive effects on different aspects of asthma control like clinical outcome, pulmonary function tests, cytokines, IgE and FeNO. However, to the best of our knowledge, the current study is the first in which all of these aspects have been studied simultaneously. Evaluation of these aspects together could increase the reliability of conclusions and elucidate a more mechanistic basis for therapeutic effect. A special feature of our study was an active effort to reinforce compliance through frequent contact with participants in between the visits. Thus we achieved a good compliance percentage among our patients (>90%). In general, N sativa was well tolerated and no side effects were reported by any of our participants.
Our results support two previous studies, which reported a favorable effect of N sativa on lung function tests in asthmatic patients.16,17 The improvement in lung function tests points to a possible bronchodilator effect of N sativa. However, our study also showed improvement in PEF variability with administration of N sativa, which better reflects bronchial hyper-responsiveness than other lung function tests. This finding indicates that N sativa supplementation specifically improves expiratory flow during the mid-part of vital capacity (FEF25-75%). FEF25-75% is known to reflect the function of the small airways32 and has recently been implicated in the pathogenesis of asthma.33
Our results suggest that N sativa might minimize the underlying inflammation. This might prevent progression of remodelling of bronchial tissue. This potential effect on remodeling is further supported by the finding that most of the effects of N sativa in this study are more remarkable after 12 weeks of continuous use of this herb. Remodelling is a long-term process and thus N sativa might be ameliorating the inflammatory reaction. We could not ascertain this as our study was not designed to assess actual remodelling due to the invasive procedures involved.
Our study is the first to show that administration of N sativa to patients with asthma is associated with a reduction in FeNO, an indicator of the inflammatory reaction underlying pathogenesis of bronchial asthma.29 However, it is not clear why the reduction in FeNO at the 1 g dose disappeared at the 2 g dose of NS. This observation seems to be in line with a recent in vitro study on the effect of an alcoholic extract N sativa on NO production in rat mix glial cells inflamed by lipopolysaccharide, which were treated with different doses of black seeds extract. The authors observed a significant reduction in NO production at lower doses of the extract, which was lost at higher doses.34 In addition, earlier studies on the effect of N sativa on other health disorders reported the same observation. A higher dose of N sativa cells (3 g/day) was less effective than a lower dose (2 g/day) in eradicating H pylori in patients with non-ulcer dyspepsia35 and in controlling blood glucose in diabetic patients.36 A possible explanation lies in the fact that the whole black seed contains more than 100 constituents, many of which have not been chemically identified nor have they been pharmacologically tested. Some of these ingredients of N sativa might have been maximally active against NO production at a 1 g/day dosage; the effect was minimized at higher doses.
Most aspects of the pathogenesis of bronchial asthma have been linked to an underlying chronic inflammatory process that is dependent on a balance between two groups of mediators, pro-inflammatory and anti-inflammatory. In our study we included IL-4 (produced by Th2) and IFN-γ (produced by Th1) as representative of these two groups. It appears that this balance can be tipped towards increased or decreased inflammation by certain cytokines. It has been suggested that IL-4 tips this balance towards increased inflammation while IFN-γ has the opposite effect.37 Interestingly, our study shows that IFN-γ is increased by both doses of N sativa, which might partially account for its anti-inflammatory effect. Other studies have shown that N sativa38 as well as thymoquinone (an active ingredient of NS),39 suppress the release of IL-4 and augment production of IFN-γ. This opens room for further study on a possible mechanism for the anti-asthmatic potential of N sativa—restoration of the balance between Th1/Th2 cytokines.
The reduction in serum IgE produced by the 2-g dose of NS supports a previous study in the mouse model of asthma.40 This reduction in serum Ig E level might be linked to the high levels of IFN-γ associated with both doses of N sativa used in our study. Noteworthy is the fact that IFN-γ is known to suppress the inflammatory reaction in asthma.41
The scores on the ACT improved in all groups, including those who received placebo. This placebo effect has been reported42 and is expected in a subjective test like ACT. The ACT score has been validated by multiple studies. It includes items on shortness of breath, night time waking, interference with activity, rescue treatment use and rating of asthma control by the patient. It correlates with the degree of airways inflammation reflected by FeNO43 and with the frequency of exacerbation and the degree of control of asthma.44
None of our subjects reported any unpleasant or untoward effects. We did not statistically evaluate other reported effects. Some of our patients who had co-existing allergic rhinitis reported a marked improvement in the allergic symptoms such as itching, running nose, sneezing and nasal blockage. Others reported an improvement in oral thrush (a common side effect of inhaled corticosteroid therapy (ICS). These observations point to the need for further studies to evaluate the use of N sativa in allergic rhinitis and as complementary therapy to ICS in asthma.
One limitation of this study was the difficulty in obtaining bronchoalveolar lavage or induced sputum samples for inflammatory cells and marker analysis. Both of these procedures are uncomfortable and have a significant risk of bronchospasm. Most patients refuse these once explained. Furthermore, the processing techniques for these samples are complicated with a limited success rate even in highly qualified centers. We compensated for this limitation by substituting these procedures with the non-invasive and novel measurement of fractional exhaled nitric oxide, which has a comparable reliability and specificity to invasive techniques.45,46
We conclude that addition of the N sativa to the controller ICS therapy improves the overall level of control and decreases exacerbations in partly controlled asthma.
Acknowledgment
This study was carried out with the support of a grant from KACST (King Abdul Aziz City for Science & Technology).
Footnotes
Conflict of interest
The authors report no conflict of interest.
ISRCTN registry: ISRCTN48853858 DOI 10.1186/ISRCTN48853858
REFERENCES
- 1.Global strategy for asthma management and prevention. Global intiative of asthma (GINA) Date last updated. 2011 May 4 [cited 2012 November 12]; Available from: www.ginasthma.org.
- 2.Braman SS. The global burden of asthma. Chest. 2006;130(1 Suppl):4S–12S. doi: 10.1378/chest.130.1_suppl.4S. [DOI] [PubMed] [Google Scholar]
- 3.Holgate ST. Pathogenesis of asthma. ClinExp Allergy. 2008;38(6):872–97. doi: 10.1111/j.1365-2222.2008.02971.x. [DOI] [PubMed] [Google Scholar]
- 4.O’Byrne PM. Cytokines or their antagonists for the treatment of asthma. Chest. 2006;130(1):244–50. doi: 10.1378/chest.130.1.244. [DOI] [PubMed] [Google Scholar]
- 5.Al-Muhsen S, Johnson JR, Hamid Q. Remodeling in asthma. J Allergy ClinImmunol. 2011;128(3):451–62. doi: 10.1016/j.jaci.2011.04.047. quiz 63–4. [DOI] [PubMed] [Google Scholar]
- 6.Barnes PJ. Current therapies for asthma. Promise and limitations. Chest. 1997;111(2 Suppl):17S–26S. doi: 10.1378/chest.111.2_supplement.17s. [DOI] [PubMed] [Google Scholar]
- 7.Chu EK, Drazen JM. Asthma: one hundred years of treatment and onward. Am J RespCrit Care Med. 2005;171(11):1202–8. doi: 10.1164/rccm.200502-257OE. [DOI] [PubMed] [Google Scholar]
- 8.Clark CE, Arnold E, Lasserson TJ, Wu T. Herbal interventions for chronic asthma in adults and children: a systematic review and meta-analysis. Prim Care Respir J. 2010;19(4):307–14. doi: 10.4104/pcrj.2010.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ali BH, Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytother Res. 2003;17(4):299–305. doi: 10.1002/ptr.1309. [DOI] [PubMed] [Google Scholar]
- 10.Khan MA. Chemical composition and medicinal properties of Nigella sativa Linn. Inflammopharmacol. 1999;7(1):15–35. doi: 10.1007/s10787-999-0023-y. [DOI] [PubMed] [Google Scholar]
- 11.Gilani AH, Aziz N, Khurram IM, Chaudhary KS, Iqbal A. Bronchodilator, spasmolytic and calcium antagonist activities of Nigella sativa seeds (Kalonji): a traditional herbal product with multiple medicinal uses. J Pak Med Assoc. 2001;51(3):115–20. [PubMed] [Google Scholar]
- 12.MHB, MS Bronchodilatory and anticholinergic effects of Nigella sativa on isolated guinea-pig tracheal chains. Iran J Med Sci. 1997;22:127–133. [Google Scholar]
- 13.Boskabady MH, Shiravi N. Inhibitory effect of Nigella sativa on histamine (H1) receptors of isolated guinea pig tracheal chains. Eur Respir J. 2000;16(supp 31):461s. [Google Scholar]
- 14.Boskabady MH, Kiani S, Jandaghi P. Stimulatory effect of Nigella sativa on 2-adronceptors of guinea pig tracheal chains. Med J Islam Rep Iran. 2004;18(2):153–8. [Google Scholar]
- 15.Salem ML. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int Immunopharmacol. 2005;5(13–14):1749–70. doi: 10.1016/j.intimp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Boskabady MH, Javan H, Sajady M, Rakhshandeh H. The possible prophylactic effect of Nigella sativa seed extract in asthmatic patients. Fundam Clin Pharmacol. 2007;21(5):559–66. doi: 10.1111/j.1472-8206.2007.00509.x. [DOI] [PubMed] [Google Scholar]
- 17.Boskabady MH, Mohsenpoor N, Takaloo L. Antiasthmatic effect of Nigella sativa in airways of asthmatic patients. Phytomedicine. 2010;17(10):707–13. doi: 10.1016/j.phymed.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Kalus U, Pruss A, Bystron J, Jurecka M, Smekalova A, Lichius JJ, et al. Effect of Nigella sativa (black seed) on subjective feeling in patients with allergic diseases. Phytother Res. 2003;17(10):1209–14. doi: 10.1002/ptr.1356. [DOI] [PubMed] [Google Scholar]
- 19.Williams JR. The Declaration of Helsinki and public health. Bulletin of the World Health Organization. 2008;86:650–651. doi: 10.2471/BLT.08.050955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Heart, Lung, and Blood Institute. National Asthma Education and Prevention Program. Expert panel report 3: Guidelines for Diagnosis and Management of Asthma. Jul, 2007. [cited 2012 October 8]; Available from: http://www.nhlbi.nih.gov/guidelines/asthma.
- 21.Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Al-Moamary MS, Al-Hajjaj MS, Idrees MM, Zeitouni MO, Alanezi MO, Al-Jahdali H, Al Dabbagh M. The Saudi Initiative for asthma. Ann Thorac Med. 2009;4(4):216–33. doi: 10.4103/1817-1737.56001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen VN, Chavannes N, Le LT, Price D. The Asthma Control Test (ACT) as an alternative tool to Global Initiative for Asthma (GINA) guideline criteria for assessing asthma control in Vietnamese outpatients. Prim Care Respir J. 2012;21(1):85–9. doi: 10.4104/pcrj.2011.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas M, Kay S, Pike J, Williams A, Rosenzweig JR, Hillyer EV, et al. The Asthma Control Test (ACT) as a predictor of GINA guideline-defined asthma control: analysis of a multinational cross-sectional survey. Prim Care Respir J. 2009;18(1):41–9. doi: 10.4104/pcrj.2009.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Resp Crit Care Med. 2009;180(1):59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- 26.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardization of spirometry, 1994 update. American thoracic society. Am J Respir Crit Care Med. 1995;152(3):1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 27.Miller MR, Hankinson JA, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Europ Resp J. 2005;26(2):319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 28.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–15. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171(8):912–30. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 30.Mahmood MS, Gilani AH, Khwaja A, Rashid A, Ashfaq MK. The in vitro effect of aqueous extract of Nigella sativa seeds on nitric oxide production. Phytother Res. 2003;17(8):921–4. doi: 10.1002/ptr.1251. [DOI] [PubMed] [Google Scholar]
- 31.Shahzad M, Yang X, Asim MR, Sun Q, Han Y, Zhang F, et al. Black seed oil ameliorates allergic airway inflammation by inhibiting T-cell proliferation in rats. Pulm Pharmacol Ther. 2009;22(1):37–43. doi: 10.1016/j.pupt.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi RE, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–68. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 33.Hamid Q. Pathogenesis of small airways in asthma. Respiration. 2012;84(1):4–11. doi: 10.1159/000339550. [DOI] [PubMed] [Google Scholar]
- 34.Alemi M, Sabouni F, Sanjarian F, Haghbeen K, Ansari S. Anti-inflammatory Effect of Seeds and Callus of Nigella sativa L. Extracts on Mix Glial Cells with Regard to Their Thymoquinone Content. AAPS Pharm Sci Tech. 2013;14(1):160–7. doi: 10.1208/s12249-012-9899-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salem EM, Yar T, Bamosa AO, Al-Quorain A, Yasawy MI, Alsulaiman RM, Randhawa MA. Comparative study of Nigella Sativa and triple therapy in eradication of Helicobacter Pylori in patients with non-ulcer dyspepsia. Saudi J Gastroenterol. 2010;16(3):207–14. doi: 10.4103/1319-3767.65201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bamosa AO, Kaatabi H, Lebdaa FM, Elq AM, Al-Sultanb A. Effect of Nigella sativa seeds on the glycemic control of patients with type 2 diabetes mellitus. Indian J Physiol Pharmacol. 2010;54(4):344–54. [PubMed] [Google Scholar]
- 37.Packard KA, Khan MM. Effects of histamine on Th1/Th2 cytokine balance. Int Immunopharmacol. 2003;3(7):909–20. doi: 10.1016/S1567-5769(02)00235-7. [DOI] [PubMed] [Google Scholar]
- 38.Boskabady MH, Keyhanmanesh R, Khameneh S, Doostdar Y, Khakzad MR. Potential immunomodulation effect of the extract of Nigella sativa on ovalbumin sensitized guinea pigs. J Zhejiang Uni Sci B. 2011;12(3):201–9. doi: 10.1631/jzus.B1000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keyhanmanesh R, Boskabady MH, Khamneh S, Doostar Y. Effect of thymoquinone on the lung pathology and cytokine levels of ovalbumin-sensitized guinea pigs. Pharmacol Rep. 2010;62(5):910–6. doi: 10.1016/s1734-1140(10)70351-0. [DOI] [PubMed] [Google Scholar]
- 40.Balaha MF, Tanaka H, Yamashita H, Abdel Rahman MN, Inagaki N. Oral Nigella sativa oil ameliorates ovalbumin-induced bronchial asthma in mice. Int Immunopharmacol. 2012;14(2):224–31. doi: 10.1016/j.intimp.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 41.Chung F. Anti-inflammatory cytokines in asthma and allergy: interleukin-10, interleukin-12, interferon-gamma. Mediators Inflamm. 2001;10(2):51–9. doi: 10.1080/09629350120054518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beecher HK. The powerful placebo. J Am Med Assoc. 1955 Dec 24;159(17):1602–6. doi: 10.1001/jama.1955.02960340022006. [DOI] [PubMed] [Google Scholar]
- 43.Rosias PPR, Dompeling E, Dentener MA, Pennings HJ, Hendriks HJE, Van Iersel MPA, et al. Childhood asthma: Exhaled markers of airway inflammation, asthma control score, and lung function tests. Pediatric Pulmonology. 2004;38(2):107–14. doi: 10.1002/ppul.20056. [DOI] [PubMed] [Google Scholar]
- 44.Koolen BB, Pijnenburg MW, Brackel HJ, Landstra AM, van den Berg NJ, Merkus PJ, et al. Comparing Global Initiative for Asthma (GINA) criteria with the Childhood Asthma Control Test (C-ACT) and Asthma Control Test (ACT) Euro Resp J. 2011 Sep;38(3):561–6. doi: 10.1183/09031936.00173710. [DOI] [PubMed] [Google Scholar]
- 45.Payne DN, Adcock IM, Wilson NM, Oates T, Scallan M, Bush A. Relationship between exhaled nitric oxide and mucosal eosinophilic inflammation in children with difficult asthma, after treatment with oral prednisolone. Am J Resp Crit Care Med. 2001;164(8 Pt 1):1376–81. doi: 10.1164/ajrccm.164.8.2101145. [DOI] [PubMed] [Google Scholar]
- 46.Lex C, Ferreira F, Zacharasiewicz A, Nicholson AG, Haslam PL, Wilson NM, et al. Airway eosinophilia in children with severe asthma: predictive values of noninvasive tests. Am J Resp Crit Care Med. 2006;174(12):1286–91. doi: 10.1164/rccm.200603-352OC. [DOI] [PubMed] [Google Scholar]